Lupus Nephritis in Children: Novel Perspectives
Abstract
:1. Introduction
2. Epidemiology
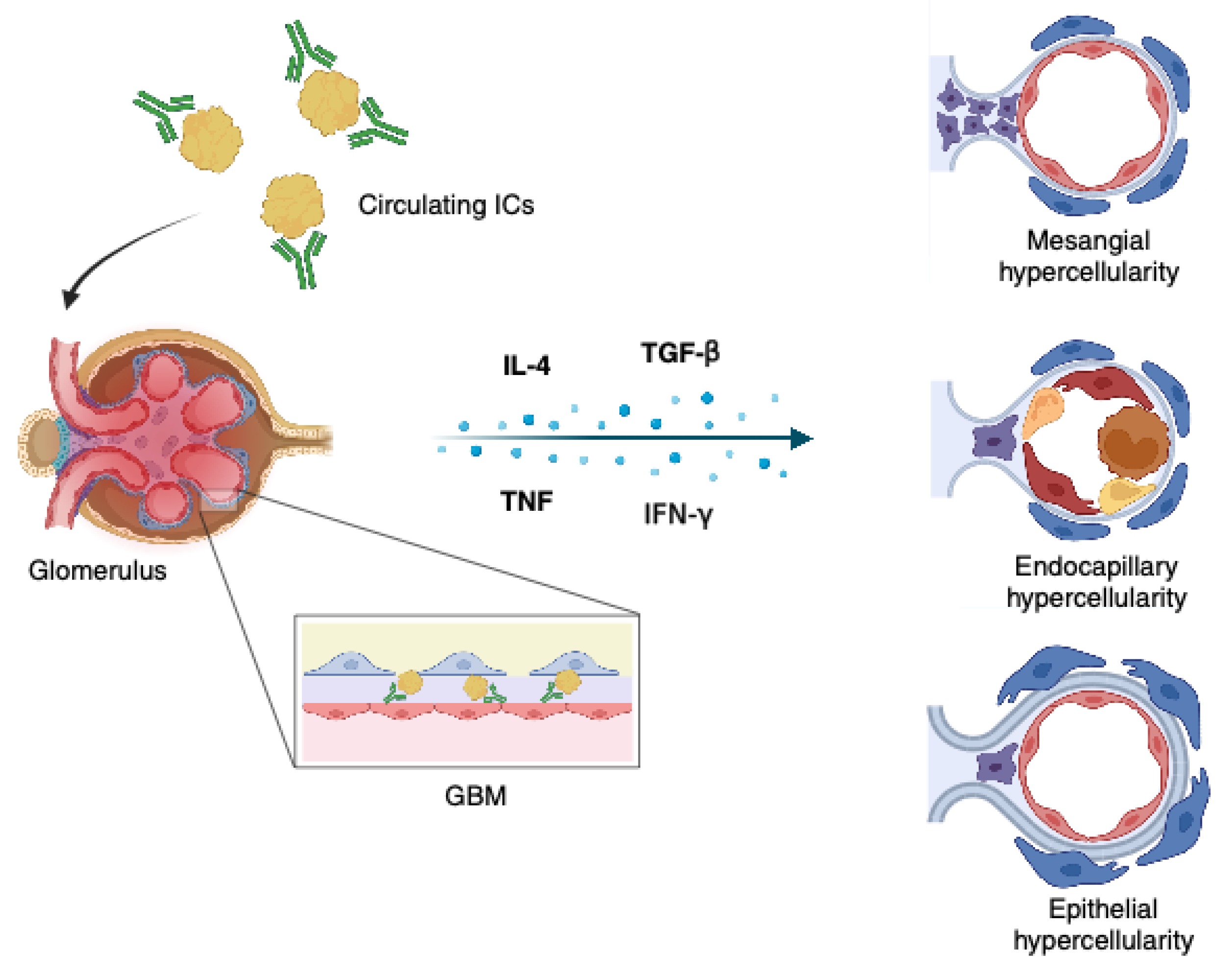
3. Pathogenesis
4. Pathology
5. Diagnosis
6. Treatment
6.1. General Treatment
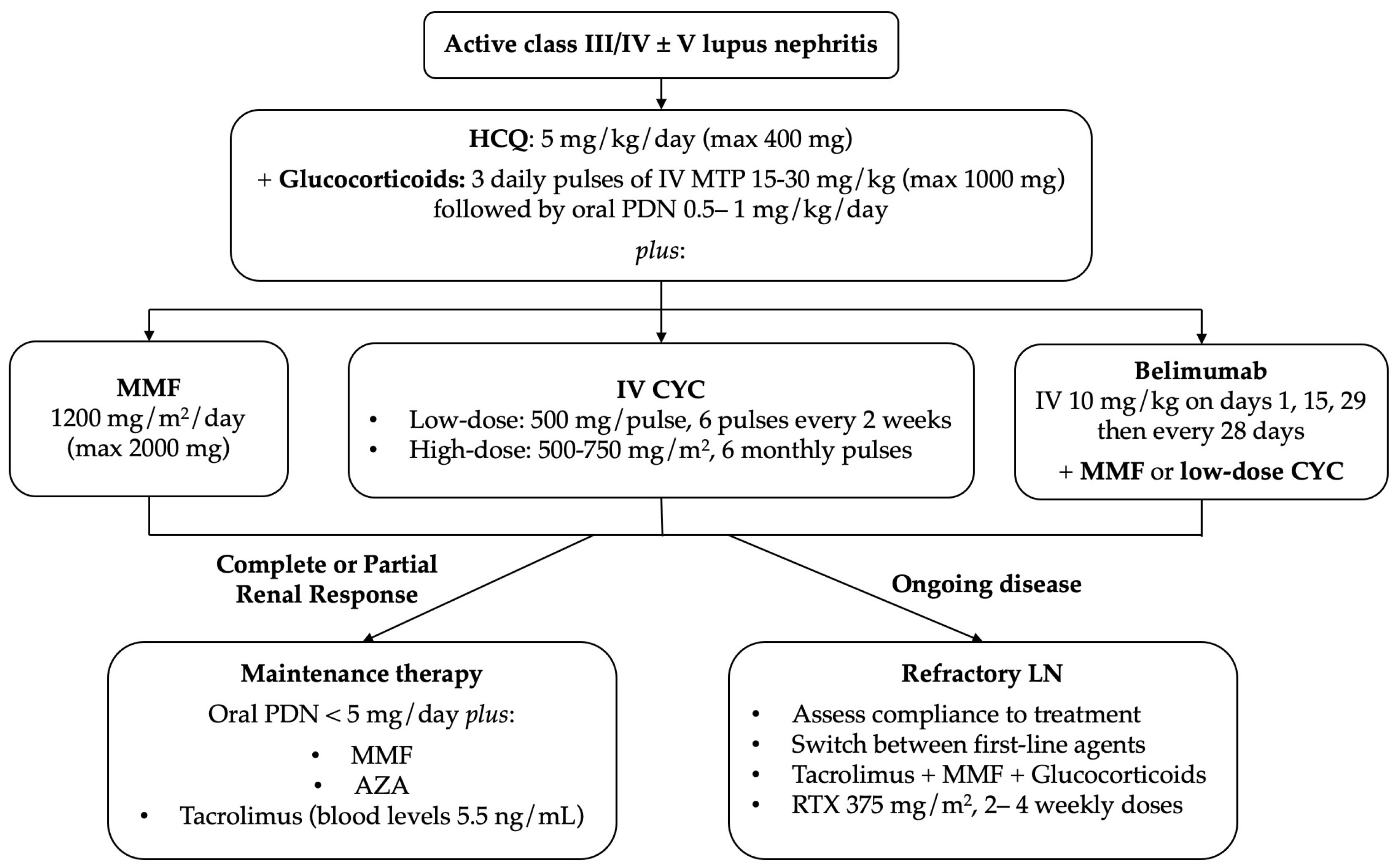
6.2. Immunosuppressive Treatment
6.2.1. Goals of Treatment
6.2.2. Initial Treatment
6.2.3. Subsequent Treatment
6.2.4. Refractory Disease and Relapse
6.3. Emerging Therapies
7. Follow-Up and Prognosis
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsokos, G.C. Systemic Lupus Erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Malattia, C.; Martini, A. Paediatric-Onset Systemic Lupus Erythematosus. Best Pract. Res. Clin. Rheumatol. 2013, 27, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Tarr, T.; Dérfalvi, B.; Gyori, N.; Szántó, A.; Siminszky, Z.; Malik, A.; Szabó, A.J.; Szegedi, G.; Zeher, M. Similarities and Differences between Pediatric and Adult Patients with Systemic Lupus Erythematosus. Lupus 2015, 24, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Wenderfer, S.E.; Chang, J.C.; Davies, A.G.; Luna, I.Y.; Scobell, R.; Sears, C.; Magella, B.; Mitsnefes, M.; Stotter, B.R.; Dharnidharka, V.R.; et al. Using a Multi-Institutional Pediatric Learning Health System to Identify Systemic Lupus Erythematosus and Lupus Nephritis: Development and Validation of Computable Phenotypes. Clin. J. Am. Soc. Nephrol. 2022, 17, 65–74. [Google Scholar] [CrossRef]
- Hiraki, L.T.; Feldman, C.H.; Liu, J.; Alarcõn, G.S.; Fischer, M.A.; Winkelmayer, W.C.; Costenbader, K.H. Prevalence, Incidence, and Demographics of Systemic Lupus Erythematosus and Lupus Nephritis from 2000 to 2004 among Children in the US Medicaid Beneficiary Population. Arthritis Rheum. 2012, 64, 2669–2676. [Google Scholar] [CrossRef]
- Fiorot, F.J.; Islabão, A.G.; Pereira, R.M.; Terreri, M.T.; Saad-Magalhães, C.; Novak, G.V.; Molinari, B.C.; Sakamoto, A.P.; Aikawa, N.E.; Campos, L.M.; et al. Disease Presentation of 1312 Childhood-Onset Systemic Lupus Erythematosus: Influence of Ethnicity. Clin Rheumatol. 2019, 38, 2857–2863. [Google Scholar] [CrossRef]
- Vazzana, K.M.; Daga, A.; Goilav, B.; Ogbu, E.A.; Okamura, D.M.; Park, C.; Sadun, R.E.; Smitherman, E.A.; Stotter, B.R.; Dasgupta, A.; et al. Principles of Pediatric Lupus Nephritis in a Prospective Contemporary Multi-Center Cohort. Lupus 2021, 30, 1660–1670. [Google Scholar] [CrossRef]
- Blair, E.; Langdon, K.; McIntyre, S.; Lawrence, D.; Watson, L. Survival and Mortality in Cerebral Palsy: Observations to the Sixth Decade from a Data Linkage Study of a Total Population Register and National Death Index. BMC Neurol. 2019, 19, 111. [Google Scholar] [CrossRef]
- Hiraki, L.T.; Benseler, S.M.; Tyrrell, P.N.; Hebert, D.; Harvey, E.; Silverman, E.D. Clinical and Laboratory Characteristics and Long-Term Outcome of Pediatric Systemic Lupus Erythematosus: A Longitudinal Study. J. Pediatr. 2008, 152, 550–556. [Google Scholar] [CrossRef]
- Watson, L.; Leone, V.; Pilkington, C.; Tullus, K.; Rangaraj, S.; McDonagh, J.E.; Gardner-Medwin, J.; Wilkinson, N.; Riley, P.; Tizard, J.; et al. Disease Activity, Severity, and Damage in the UK Juvenile-Onset Systemic Lupus Erythematosus Cohort. Arthritis Rheum. 2012, 64, 2356–2365. [Google Scholar] [CrossRef]
- Demir, S.; Gülhan, B.; Özen, S.; Çelĕgen, K.; Batu, E.D.; Taş, N.; Orhan, D.; Bilginer, Y.; Düzova, A.; Ozaltin, F.; et al. Long-Term Renal Survival of Paediatric Patients with Lupus Nephritis. Nephrol. Dial. Transplant. 2022, 37, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Brunner, H.I. Biomarkers and Updates on Pediatrics Lupus Nephritis. Rheum. Dis. Clin. N. Am. 2013, 39, 833–853. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Jackson, S.W. Recent Advances in Immunotherapies for Lupus Nephritis. Pediatr. Nephrol. 2023, 38, 1001–1012. [Google Scholar] [CrossRef]
- Kamphuis, S.; Silverman, E.D. Prevalence and Burden of Pediatric-Onset Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2010, 6, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Pineles, D.; Valente, A.; Warren, B.; Peterson, M.; Lehman, T.; Moorthy, L.N. Worldwide Incidence and Prevalence of Pediatric Onset Systemic Lupus Erythematosus. Lupus 2011, 20, 1187–1192. [Google Scholar] [CrossRef]
- Lahita, R.G. The Role of Sex Hormones in Systemic Lupus Erythematosus. Curr. Opin. Rheumatol. 1999, 11, 352–356. [Google Scholar] [CrossRef]
- Oliver, J.E.; Silman, A.J. Why Are Women Predisposed to Autoimmune Rheumatic Diseases? Arthritis Res. Ther. 2009, 11, 252. [Google Scholar] [CrossRef]
- Hiraki, L.T.; Benseler, S.M.; Tyrrell, P.N.; Harvey, E.; Hebert, D.; Silverman, E.D. Ethnic Differences in Pediatric Systemic Lupus Erythematosus. J. Rheumatol. 2009, 36, 2539–2546. [Google Scholar] [CrossRef]
- Petri, M. Epidemiology of Systemic Lupus Erythematosus. Best Pract. Res. Clin. Rheumatol. 2002, 16, 847–858. [Google Scholar] [CrossRef]
- Farhat, S.C.L.; Yariwake, V.Y.; Veras, M.M.; Braga, A.L.F.; Maluf, A.E.; Silva, C.A. Inhaled Ultrafine Particles, Epigenetics and Systemic Autoimmune Rheumatic Diseases. Autoimmun. Rev. 2020, 19, 102640. [Google Scholar] [CrossRef]
- Conde, P.G.; Farhat, L.C.; Braga, A.L.F.; Sallum, A.E.M.; Farhat, S.C.L.; Silva, C.A. Are Prematurity and Environmental Factors Determinants for Developing Childhood-Onset Systemic Lupus Erythematosus? Mod. Rheumatol. 2018, 28, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Goulart, M.F.G.; Alves, A.G.F.; Farhat, J.; Braga, A.L.F.; Pereira, L.A.A.; de Faria Coimbra Lichtenfels, A.J.; de Arruda Campos, L.M.; da Silva, C.A.A.; Elias, A.M.; Farhat, S.C.L. Influence of Air Pollution on Renal Activity in Patients with Childhood-Onset Systemic Lupus Erythematosus. Pediatr. Nephrol. 2020, 35, 1247–1255. [Google Scholar] [CrossRef]
- Trindade, V.C.; Carneiro-Sampaio, M.; Bonfa, E.; Silva, C.A. An Update on the Management of Childhood-Onset Systemic Lupus Erythematosus. Paediatr. Drugs 2021, 23, 331–347. [Google Scholar] [CrossRef] [PubMed]
- James, J.A.; Kaufman, K.M.; Farris, A.D.; Taylor-Albert, E.; Lehman, T.J.A.; Harley, J.B. An Increased Prevalence of Epstein-Barr Virus Infection in Young Patients Suggests a Possible Etiology for Systemic Lupus Erythematosus. J. Clin. Investig. 1997, 100, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Voigt, S.; Tagawa, T.; Albanese, M.; Chen, Y.F.A.; Chen, Y.; Fachko, D.N.; Pich, D.; Göbel, C.; Skalsky, R.L.; et al. Multiple Viral MicroRNAs Regulate Interferon Release and Signaling Early during Infection with Epstein-Barr Virus. mBio 2021, 12, e03440-20. [Google Scholar] [CrossRef] [PubMed]
- Pyfrom, S.; Paneru, B.; Knox, J.J.; Cancro, M.P.; Posso, S.; Buckner, J.H.; Anguera, M.C. The Dynamic Epigenetic Regulation of the Inactive X Chromosome in Healthy Human B Cells Is Dysregulated in Lupus Patients. Proc. Natl. Acad. Sci. USA 2021, 118, e2024624118. [Google Scholar] [CrossRef]
- Yu, B.; Qi, Y.; Li, R.; Shi, Q.; Satpathy, A.T.; Chang, H.Y. B Cell-Specific XIST Complex Enforces X-Inactivation and Restrains Atypical B Cells. Cell 2021, 184, 1790–1803.e17. [Google Scholar] [CrossRef]
- Deafen, D.; Escalante, A.; Weinrib, L.; Horwitz, D.; Bachman, B.; Roy-Burman, P.; Walker, A.; Mack, T.M. A Revised Estimate of Twin Concordance in Systemic Lupus Erythematosus. Arthritis Rheum. 1992, 35, 311–318. [Google Scholar] [CrossRef]
- Crow, M.K. Pathogenesis of Systemic Lupus Erythematosus: Risks, Mechanisms and Therapeutic Targets. Ann. Rheum. Dis. 2023, 82, 999–1014. [Google Scholar] [CrossRef]
- Almlöf, J.C.; Nystedt, S.; Mechtidou, A.; Leonard, D.; Eloranta, M.L.; Grosso, G.; Sjöwall, C.; Bengtsson, A.A.; Jönsen, A.; Gunnarsson, I.; et al. Contributions of de Novo Variants to Systemic Lupus Erythematosus. Eur. J. Hum. Genet. 2021, 29, 184–193. [Google Scholar] [CrossRef]
- Ha, E.; Bae, S.C.; Kim, K. Recent Advances in Understanding the Genetic Basis of Systemic Lupus Erythematosus. Semin. Immunopathol. 2022, 44, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Lee, J.T.; Rahmberg, A.R.; Kumar, G.; Mi, T.; Scharer, C.D.; Boss, J.M. A Super Enhancer Controls Expression and Chromatin Architecture within the MHC Class II Locus. J. Exp. Med. 2020, 217, e20190668. [Google Scholar] [CrossRef] [PubMed]
- Costa-Reis, P.; Sullivan, K.E. Monogenic Lupus: It’s All New! Curr. Opin. Immunol. 2017, 49, 87–95. [Google Scholar] [CrossRef]
- Demirkaya, E.; Sahin, S.; Romano, M.; Zhou, Q.; Aksentijevich, I. New Horizons in the Genetic Etiology of Systemic Lupus Erythematosus and Lupus-Like Disease: Monogenic Lupus and Beyond. J. Clin. Med. 2020, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Yasutomo, K.; Horiuchi, T.; Kagami, S.; Tsukamoto, H.; Hashimura, C.; Urushihara, M.; Kuroda, Y. Mutation of DNASE1 in People with Systemic Lupus Erythematosus. Nat. Genet. 2001, 28, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayouf, S.M.; Sunker, A.; Abdwani, R.; Al Abrawi, S.; Almurshedi, F.; Alhashmi, N.; Al Sonbul, A.; Sewairi, W.; Qari, A.; Abdallah, E.; et al. Loss-of-Function Variant in DNASE1L3 Causes a Familial Form of Systemic Lupus Erythematosus. Nat. Genet. 2011, 43, 1186–1188. [Google Scholar] [CrossRef]
- Batlle-Masó, L.; Mensa-Vilaró, A.; Solís-Moruno, M.; Marquès-Bonet, T.; Arostegui, J.I.; Casals, F. Genetic Diagnosis of Autoinflammatory Disease Patients Using Clinical Exome Sequencing. Eur. J. Med. Genet. 2020, 63, 103920. [Google Scholar] [CrossRef]
- Botto, M.; Dell’Agnola, C.; Bygrave, A.E.; Thompson, E.M.; Cook, H.T.; Petry, F.; Loos, M.; Pandolfi, P.P.; Walport, M.J. Homozygous C1q Deficiency Causes Glomerulonephritis Associated with Multiple Apoptotic Bodies. Nat. Genet. 1998, 19, 56–59. [Google Scholar] [CrossRef]
- Macedo, A.C.L.; Isaac, L. Systemic Lupus Erythematosus and Deficiencies of Early Components of the Complement Classical Pathway. Front. Immunol. 2016, 7, 55. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, Y.; Chung, E.K.; Zhou, B.; Kitzmiller, K.J.; Savelli, S.L.; Nagaraja, H.N.; Birmingham, D.J.; Tsao, B.P.; Rovin, B.H.; et al. Phenotypes, Genotypes and Disease Susceptibility Associated with Gene Copy Number Variations: Complement C4 CNVs in European American Healthy Subjects and Those with Systemic Lupus Erythematosus. Cytogenet. Genome Res. 2008, 123, 131–141. [Google Scholar] [CrossRef]
- Joseph, S.; George, N.I.; Green-Knox, B.; Treadwell, E.L.; Word, B.; Yim, S.; Lyn-Cook, B. Epigenome-Wide Association Study of Peripheral Blood Mononuclear Cells in Systemic Lupus Erythematosus: Identifying DNA Methylation Signatures Associated with Interferon-Related Genes Based on Ethnicity and SLEDAI. J. Autoimmun. 2019, 96, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Marion, M.C.; Ramos, P.S.; Bachali, P.; Labonte, A.C.; Zimmerman, K.D.; Ainsworth, H.C.; Heuer, S.E.; Robl, R.D.; Catalina, M.D.; Kelly, J.A.; et al. Nucleic Acid-Sensing and Interferon-Inducible Pathways Show Differential Methylation in MZ Twins Discordant for Lupus and Overexpression in Independent Lupus Samples: Implications for Pathogenic Mechanism and Drug Targeting. Genes 2021, 12, 1898. [Google Scholar] [CrossRef] [PubMed]
- Javierre, B.M.; Fernandez, A.F.; Richter, J.; Al-Shahrour, F.; Ignacio Martin-Subero, J.; Rodriguez-Ubreva, J.; Berdasco, M.; Fraga, M.F.; O’Hanlon, T.P.; Rider, L.G.; et al. Changes in the Pattern of DNA Methylation Associate with Twin Discordance in Systemic Lupus Erythematosus. Genome Res. 2010, 20, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Lilue, J.; Stavrou, S.; Moran, E.A.; Ross, S.R. AIM2-Like Receptors Positively and Negatively Regulate the Interferon Response Induced by Cytosolic DNA. mBio 2017, 8, e00944-17. [Google Scholar] [CrossRef] [PubMed]
- Coit, P.; Ortiz-Fernandez, L.; Lewis, E.E.; McCune, W.J.; Maksimowicz-McKinnon, K.; Sawalha, A.H. A Longitudinal and Transancestral Analysis of DNA Methylation Patterns and Disease Activity in Lupus Patients. JCI Insight 2020, 5, e143654. [Google Scholar] [CrossRef]
- Fava, A.; Rao, D.A.; Mohan, C.; Zhang, T.; Rosenberg, A.; Fenaroli, P.; Belmont, H.M.; Izmirly, P.; Clancy, R.; Trujillo, J.M.; et al. Urine Proteomics and Renal Single-Cell Transcriptomics Implicate Interleukin-16 in Lupus Nephritis. Arthritis Rheumatol. 2022, 74, 829–839. [Google Scholar] [CrossRef]
- Anders, H.J. Pseudoviral Immunity—A Novel Concept for Lupus. Trends Mol. Med. 2009, 15, 553–561. [Google Scholar] [CrossRef]
- An, J.; Minie, M.; Sasaki, T.; Woodward, J.J.; Elkon, K.B. Antimalarial Drugs as Immune Modulators: New Mechanisms for Old Drugs. Annu. Rev. Med. 2017, 68, 317–330. [Google Scholar] [CrossRef]
- Corzo, C.A.; Varfolomeev, E.; Setiadi, A.F.; Francis, R.; Klabunde, S.; Senger, K.; Sujatha-Bhaskar, S.; Drobnick, J.; Do, S.; Suto, E.; et al. The Kinase IRAK4 Promotes Endosomal TLR and Immune Complex Signaling in B Cells and Plasmacytoid Dendritic Cells. Sci. Signal 2020, 13, eaaz1053. [Google Scholar] [CrossRef]
- Eloranta, M.L.; Rönnblom, L. Cause and Consequences of the Activated Type I Interferon System in SLE. J. Mol. Med. 2016, 94, 1103–1110. [Google Scholar] [CrossRef]
- Lindau, D.; Mussard, J.; Rabsteyn, A.; Ribon, M.; Kötter, I.; Igney, A.; Adema, G.J.; Boissier, M.C.; Rammensee, H.G.; Decker, P. TLR9 Independent Interferon α Production by Neutrophils on NETosis in Response to Circulating Chromatin, a Key Lupus Autoantigen. Ann. Rheum. Dis. 2014, 73, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Sagar, D.; Hanna, R.N.; Lightfoot, Y.L.; Mistry, P.; Smith, C.K.; Manna, Z.; Hasni, S.; Siegel, R.M.; Sanjuan, M.A.; et al. Low-Density Granulocytes Activate T Cells and Demonstrate a Non-Suppressive Role in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Bonanni, A.; Petretto, A.; Vaglio, A.; Pratesi, F.; Santucci, L.; Migliorini, P.; Bertelli, R.; Galetti, M.; Belletti, S.; et al. Neutrophil Extracellular Traps Profiles in Patients with Incident Systemic Lupus Erythematosus and Lupus Nephritis. J. Rheumatol. 2020, 47, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Feng, Y.; Wang, Y.; Wang, J.; Zhang, Z.; Liang, J.; Xu, J. Correlation between Circulating Interleukin-18 Level and Systemic Lupus Erythematosus: A Meta-Analysis. Sci. Rep. 2021, 11, 4707. [Google Scholar] [CrossRef]
- Jesus, A.A.; Liphaus, B.L.; Silva, C.A.; Bando, S.Y.; Andrade, L.; Coutinho, A.; Carneiro-Sampaio, M. Complement and Antibody Primary Immunodeficiency in Juvenile Systemic Lupus Erythematosus Patients. Lupus 2011, 20, 1275–1284. [Google Scholar] [CrossRef]
- Tesser, A.; De Carvalho, L.M.; Sandrin-Garcia, P.; Pin, A.; Pastore, S.; Taddio, A.; Roberti, L.R.; De Paula Queiroz, R.G.; Ferriani, V.P.L.; Crovella, S.; et al. Higher Interferon Score and Normal Complement Levels May Identify a Distinct Clinical Subset in Children with Systemic Lupus Erythematosus. Arthritis Res. Ther. 2020, 22, 91. [Google Scholar] [CrossRef]
- Wallace, D.J.; Furie, R.A.; Tanaka, Y.; Kalunian, K.C.; Mosca, M.; Petri, M.A.; Dörner, T.; Cardiel, M.H.; Bruce, I.N.; Gomez, E.; et al. Baricitinib for Systemic Lupus Erythematosus: A Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet 2018, 392, 222–231. [Google Scholar] [CrossRef]
- Tsokos, G.C.; Lo, M.S.; Reis, P.C.; Sullivan, K.E. New Insights into the Immunopathogenesis of Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef]
- Paroli, M.; Caccavale, R.; Fiorillo, M.T.; Spadea, L.; Gumina, S.; Candela, V.; Paroli, M.P. The Double Game Played by Th17 Cells in Infection: Host Defense and Immunopathology. Pathogens 2022, 11, 1547. [Google Scholar] [CrossRef]
- Abraham, R.; Durkee, M.S.; Ai, J.; Veselits, M.; Casella, G.; Asano, Y.; Chang, A.; Ko, K.; Oshinsky, C.; Peninger, E.; et al. Specific in Situ Inflammatory States Associate with Progression to Renal Failure in Lupus Nephritis. J. Clin. Investig. 2022, 132, e155350. [Google Scholar] [CrossRef]
- Bocharnikov, A.V.; Keegan, J.; Wacleche, V.S.; Cao, Y.; Fonseka, C.Y.; Wang, G.; Muise, E.S.; Zhang, K.X.; Arazi, A.; Keras, G.; et al. PD-1hiCXCR5- T Peripheral Helper Cells Promote B Cell Responses in Lupus via MAF and IL-21. JCI Insight 2019, 4, e130062. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.D.; Reiff, A.; Yang, H.T.; Migone, T.S.; Ward, C.D.; Marzan, K.; Shaham, B.; Wee, C.P.; Garza, J.; Bernstein, B.; et al. B Lymphocyte Stimulator Expression in Pediatric Systemic Lupus Erythematosus and Juvenile Idiopathic Arthritis Patients. Arthritis Rheum. 2009, 60, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- Satterthwaite, A.B. TLR7 Signaling in Lupus B Cells: New Insights into Synergizing Factors and Downstream Signals. Curr. Rheumatol. Rep. 2021, 23, 80. [Google Scholar] [CrossRef] [PubMed]
- Nowling, T.K.; Gilkeson, G.S. Mechanisms of Tissue Injury in Lupus Nephritis. Arthritis Res. Ther. 2011, 13, 250. [Google Scholar] [CrossRef]
- Anders, H.J.; Fogo, A.B. Immunopathology of Lupus Nephritis. Semin. Immunopathol. 2014, 36, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mendoza, G.; Sansón, S.P.; Rodríguez-Castro, S.; Crispín, J.C.; Rosetti, F. Mechanisms of Tissue Injury in Lupus Nephritis. Trends Mol. Med. 2018, 24, 364–378. [Google Scholar] [CrossRef]
- Anders, H.J.; Weening, J.J. Kidney Disease in Lupus Is Not Always “Lupus Nephritis”. Arthritis Res. Ther. 2013, 15, 108. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef]
- Groot, N.; De Graeff, N.; Avcin, T.; Bader-Meunier, B.; Brogan, P.; Dolezalova, P.; Feldman, B.; Kone-Paut, I.; Lahdenne, P.; Marks, S.D.; et al. European Evidence-Based Recommendations for Diagnosis and Treatment of Childhood-Onset Systemic Lupus Erythematosus: The SHARE Initiative. Ann. Rheum. Dis. 2017, 76, 1788–1796. [Google Scholar] [CrossRef]
- Marks, S.D.; Sebire, N.J.; Pilkington, C.; Tullus, K. Clinicopathological Correlations of Paediatric Lupus Nephritis. Pediatr. Nephrol. 2007, 22, 77–83. [Google Scholar] [CrossRef]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society Classification for Lupus Nephritis: Clarification of Definitions, and Modified National Institutes of Health Activity and Chronicity Indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.H.; Roberts, I.S.D.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA Nephropathy 2016: An Update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.M.; Rietveld, A.; Van Den Brand, J.A.J.G.; Berden, J.H.M. Segmental and Global Subclasses of Class IV Lupus Nephritis Have Similar Renal Outcomes. J. Am. Soc. Nephrol. 2012, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Krassanairawiwong, K.; Charoenpitakchai, M.; Supasyndh, O.; Satirapoj, B. Revised ISN/RPS 2018 Classification of Lupus Renal Pathology Predict Clinical Remission. Int. Urol. Nephrol. 2021, 53, 1391–1398. [Google Scholar] [CrossRef]
- Hachiya, A.; Karasawa, M.; Imaizumi, T.; Kato, N.; Katsuno, T.; Ishimoto, T.; Kosugi, T.; Tsuboi, N.; Maruyama, S. The ISN/RPS 2016 Classification Predicts Renal Prognosis in Patients with First-Onset Class III/IV Lupus Nephritis. Sci. Rep. 2021, 11, 1525. [Google Scholar] [CrossRef]
- Patel, P.; de Guzman, M.; Hicks, M.J.; Maliakkal, J.G.; Rheault, M.N.; Selewski, D.T.; Twombley, K.; Misurac, J.M.; Tran, C.L.; Constantinescu, A.R.; et al. Utility of the 2018 Revised ISN/RPS Thresholds for Glomerular Crescents in Childhood-Onset Lupus Nephritis: A Pediatric Nephrology Research Consortium Study. Pediatr. Nephrol. 2022, 37, 3139–3145. [Google Scholar] [CrossRef]
- Wu, L.H.; Yu, F.; Tan, Y.; Qu, Z.; Chen, M.H.; Wang, S.X.; Liu, G.; Zhao, M.H. Inclusion of Renal Vascular Lesions in the 2003 ISN/RPS System for Classifying Lupus Nephritis Improves Renal Outcome Predictions. Kidney Int 2013, 83, 715–723. [Google Scholar] [CrossRef]
- Bomback, A.S.; Markowitz, G.S. Lupus Podocytopathy: A Distinct Entity. Clin. J. Am. Soc. Nephrol. 2016, 11, 199–205. [Google Scholar] [CrossRef]
- Freedman, B.I.; Langefeld, C.D.; Andringa, K.K.; Croker, J.A.; Williams, A.H.; Garner, N.E.; Birmingham, D.J.; Hebert, L.A.; Hicks, P.J.; Segal, M.S.; et al. End-Stage Renal Disease in African Americans with Lupus Nephritis Is Associated with APOL1. Arthritis Rheumatol. 2014, 66, 390–396. [Google Scholar] [CrossRef]
- Gomes, R.C.; Silva, M.F.; Kozu, K.; Bonfá, E.; Pereira, R.M.; Terreri, M.T.; Magalhães, C.S.; Sacchetti, S.B.; Marini, R.; Fraga, M.; et al. Features of 847 Childhood-Onset Systemic Lupus Erythematosus Patients in Three Age Groups at Diagnosis: A Brazilian Multicenter Study. Arthritis Care Res. 2016, 68, 1736–1741. [Google Scholar] [CrossRef]
- Novak, G.V.; Molinari, B.C.; Ferreira, J.C.; Sakamoto, A.P.; Terreri, M.T.; Pereira, R.M.R.; Saad-Magalhães, C.; Aikawa, N.E.; Campos, L.M.; Len, C.A.; et al. Characteristics of 1555 Childhood-Onset Lupus in Three Groups Based on Distinct Time Intervals to Disease Diagnosis: A Brazilian Multicenter Study. Lupus 2018, 27, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.M.; Alarcõn, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Levinsky, Y.; Broide, M.; Kagan, S.; Goldberg, O.; Scheuerman, O.; Tal, R.; Tirosh, I.; Butbul, Y.; Furst, D.E.; Harel, L.; et al. Performance of 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in a Paediatric Population—A Multicentre Study. Rheumatology 2021, 60, 5142–5148. [Google Scholar] [CrossRef]
- Munhoz, G.A.; Aikawa, N.E.; Silva, C.A.; Pasoto, S.G.; Pedrosa, T.N.; Seguro, L.P.C.; Bonfa, E.; Borba, E.F. Short-Term Accrual 2019 European League Against Rheumatism/American College of Rheumatology Domains and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage in Lupus Patients with and without Nephritis at Disease Onset. J. Clin. Rheumatol. 2023, 29, 190–195. [Google Scholar] [CrossRef]
- Stotter, B.R.; Cody, E.; Gu, H.; Daga, A.; Greenbaum, L.A.; Duong, M.D.; Mazo, A.; Goilav, B.; Boneparth, A.; Kallash, M.; et al. Acute Kidney Injury Requiring Kidney Replacement Therapy in Childhood Lupus Nephritis: A Cohort Study of the Pediatric Nephrology Research Consortium and Childhood Arthritis and Rheumatology Research Alliance. Pediatr. Nephrol. 2023, 38, 1653–1665. [Google Scholar] [CrossRef]
- Pinheiro, S.V.B.; Dias, R.F.; Fabiano, R.C.G.; Araujo, S.D.A.; Silva, A.C.S.E. Pediatric Lupus Nephritis. J. Bras. Nefrol. 2019, 41, 252–265. [Google Scholar] [CrossRef]
- Ding, J.Y.C.; Ibañez, D.; Gladman, D.D.; Urowitz, M.B. Isolated Hematuria and Sterile Pyuria May Indicate Systemic Lupus Erythematosus Activity. J. Rheumatol. 2015, 42, 437–440. [Google Scholar] [CrossRef]
- Malvar, A.; Pirruccio, P.; Alberton, V.; Lococo, B.; Recalde, C.; Fazini, B.; Nagaraja, H.; Indrakanti, D.; Rovin, B.H. Histologic versus Clinical Remission in Proliferative Lupus Nephritis. Nephrol. Dial. Transplant. 2017, 32, 1338–1344. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Cheema, K.; Anders, H.J.; Aringer, M.; Bajema, I.; Boletis, J.; Frangou, E.; Houssiau, F.A.; Hollis, J.; et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) Recommendations for the Management of Lupus Nephritis. Ann. Rheum. Dis. 2020, 79, S713–S723. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Vercelloni, P.G.; Quaglini, S.; Gatto, M.; Gianfreda, D.; Sacchi, L.; Raffiotta, F.; Zen, M.; Costantini, G.; Urban, M.L.; et al. Changing Patterns in Clinical-Histological Presentation and Renal Outcome over the Last Five Decades in a Cohort of 499 Patients with Lupus Nephritis. Ann. Rheum. Dis. 2018, 77, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, J.F.; Alon, U.S. The Teenager with Asymptomatic Proteinuria: Think Orthostatic First. Clin. Pediatr. 2011, 50, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Mina, R.; Von Scheven, E.; Ardoin, S.P.; Eberhard, B.A.; Punaro, M.; Ilowite, N.; Hsu, J.; Klein-Gitelman, M.; Moorthy, L.N.; Muscal, E.; et al. Consensus Treatment Plans for Induction Therapy of Newly Diagnosed Proliferative Lupus Nephritis in Juvenile Systemic Lupus Erythematosus. Arthritis Care Res. 2012, 64, 375–383. [Google Scholar] [CrossRef]
- Gualano, B.; Bonfa, E.; Pereira, R.M.R.; Silva, C.A. Physical Activity for Paediatric Rheumatic Diseases: Standing up against Old Paradigms. Nat. Rev. Rheumatol. 2017, 13, 368–379. [Google Scholar] [CrossRef]
- Liu, J.; Song, W.; Cui, D. Relationship between Blood Lipid Profiles and Risk of Lupus Nephritis in Children. Int. J. Clin. Pract. 2022, 2022, 6130774. [Google Scholar] [CrossRef]
- Lopes, S.R.M.; Gormezano, N.W.S.; Gomes, R.C.; Aikawa, N.E.; Pereira, R.M.R.; Terreri, M.T.; Magalhães, C.S.; Ferreira, J.C.; Okuda, E.M.; Sakamoto, A.P.; et al. Outcomes of 847 Childhood-Onset Systemic Lupus Erythematosus Patients in Three Age Groups. Lupus 2017, 26, 996–1001. [Google Scholar] [CrossRef]
- Groot, N.; Heijstek, M.W.; Wulffraat, N.M. Vaccinations in Paediatric Rheumatology: An Update on Current Developments. Curr. Rheumatol. Rep. 2015, 17, 46. [Google Scholar] [CrossRef]
- Avar Aydin, P.O.; Shan, J.; Brunner, H.I.; Mitsnefes, M.M. Blood Pressure Control over Time in Childhood-Onset Systemic Lupus Erythematous. Lupus 2018, 27, 657–664. [Google Scholar] [CrossRef]
- Jones, J.T.; Cunningham, N.; Kashikar-Zuck, S.; Brunner, H.I. Pain, Fatigue, and Psychological Impact on Health-Related Quality of Life in Childhood-Onset Lupus. Arthritis Care Res. 2016, 68, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.; Cisternas, M.G.; Smilek, D.E.; Straub, L.; Houssiau, F.A.; Cervera, R.; Rovin, B.H.; MacKay, M. Predictors of Long-Term Renal Outcome in Lupus Nephritis Trials: Lessons Learned from the Euro-Lupus Nephritis Cohort. Arthritis Rheumatol. 2015, 67, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Tamirou, F.; D’Cruz, D.; Sangle, S.; Remy, P.; Vasconcelos, C.; Fiehn, C.; Del Mar Ayala Guttierez, M.; Gilboe, I.M.; Tektonidou, M.; Blockmans, D.; et al. Long-Term Follow-up of the MAINTAIN Nephritis Trial, Comparing Azathioprine and Mycophenolate Mofetil as Maintenance Therapy of Lupus Nephritis. Ann. Rheum. Dis. 2016, 75, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Tamirou, F.; Lauwerys, B.R.; Dall’Era, M.; Mackay, M.; Rovin, B.; Cervera, R.; Houssiau, F.A.; Abramowicz, D.; Atzeni, F.; Blockmans, D.; et al. A Proteinuria Cut-off Level of 0.7 g/Day after 12 Months of Treatment Best Predicts Long-Term Renal Outcome in Lupus Nephritis: Data from the MAINTAIN Nephritis Trial. Lupus Sci. Med. 2015, 2, e000123. [Google Scholar] [CrossRef] [PubMed]
- Pons-Estel, G.J.; Alarcón, G.S.; McGwin, G.; Danila, M.I.; Zhang, J.; Bastian, H.M.; Reveille, J.D.; Vilá, L.M. Protective Effect of Hydroxychloroquine on Renal Damage in Patients with Lupus Nephritis: LXV, Data from a Multiethnic US Cohort. Arthritis Rheum. 2009, 61, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Gheet, F.S.; Dawoud, H.E.S.; El-Shahaby, W.A.; Elrifaey, S.M.; Abdelnabi, H.H. Hydroxychloroquine in Children with Proliferative Lupus Nephritis: A Randomized Clinical Trial. Eur. J. Pediatr. 2023, 182, 1685–1695. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.Y.; Melles, R.B.; Mieler, W.F.; Lum, F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Y.; Wang, S.; Chen, H.; Liu, Z.; Zeng, C.; Zhang, H.; Liu, Z. Clinical-Morphological Features and Outcomes of Lupus Podocytopathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 585–592. [Google Scholar] [CrossRef]
- Hu, W.X.; Chen, Y.H.; Bao, H.; Liu, Z.Z.; Wang, S.F.; Zhang, H.T.; Liu, Z.H. Glucocorticoid with or without Additional Immunosuppressant Therapy for Patients with Lupus Podocytopathy: A Retrospective Single-Center Study. Lupus 2015, 24, 1067–1075. [Google Scholar] [CrossRef]
- Austin, H.A.; Klippel, J.H.; Balow, J.E.; Le Riche, N.G.H.; Steinberg, A.D.; Plotz, P.H.; Decker, J.L. Therapy of Lupus Nephritis. Controlled Trial of Prednisone and Cytotoxic Drugs. N. Engl. J. Med. 1986, 314, 614–619. [Google Scholar] [CrossRef]
- Chan, T.M.; Li, F.K.; Tang, C.S.O.; Wong, R.W.S.; Fang, G.X.; Ji, Y.L.; Lau, C.S.; Wong, A.K.M.; Tong, M.K.L.; Chan, K.W.; et al. Efficacy of Mycophenolate Mofetil in Patients with Diffuse Proliferative Lupus Nephritis. Hong Kong-Guangzhou Nephrology Study Group. N. Engl. J. Med. 2000, 343, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Appel, G.B.; Contreras, G.; Dooley, M.A.; Ginzler, E.M.; Isenberg, D.; Jayne, D.; Li, L.S.; Mysler, E.; Sánchez-Guerrero, J.; Solomons, N.; et al. Mycophenolate Mofetil versus Cyclophosphamide for Induction Treatment of Lupus Nephritis. J. Am. Soc. Nephrol. 2009, 20, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Houssiau, F.A.; Vasconcelos, C.; D’Cruz, D.; Sebastiani, G.D.; De Ramon Garrido, E.; Danieli, M.G.; Abramovicz, D.; Blockmans, D.; Mathieu, A.; Direskeneli, H.; et al. Immunosuppressive Therapy in Lupus Nephritis: The Euro-Lupus Nephritis Trial, a Randomized Trial of Low-Dose versus High-Dose Intravenous Cyclophosphamide. Arthritis Rheum. 2002, 46, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Gourley, M.F.; Austin, H.A.; Scott, D.; Yarboro, C.H.; Vaughan, E.M.; Muir, J.; Boumpas, D.T.; Klippel, J.H.; Balow, J.E.; Steinberg, A.D. Methylprednisolone and Cyclophosphamide, Alone or in Combination, in Patients with Lupus Nephritis. A Randomized, Controlled Trial. Ann. Intern. Med. 1996, 125, 549–557. [Google Scholar] [CrossRef]
- Illei, G.G.; Austin, H.A.; Crane, M.; Collins, L.; Gourley, M.F.; Yarboro, C.H.; Vaughan, E.M.; Kuroiwa, T.; Danning, C.L.; Steinberg, A.D.; et al. Combination Therapy with Pulse Cyclophosphamide plus Pulse Methylprednisolone Improves Long-Term Renal Outcome without Adding Toxicity in Patients with Lupus Nephritis. Ann. Intern. Med. 2001, 135, 248–257. [Google Scholar] [CrossRef]
- Chen, Y.E.; Korbert, S.M.; Katz, R.S.; Schwartz, M.M.; Lewis, E.J.; Roberts, J.L.; Schwartz, M.M.; Rodby, R.A.; Corwin, H.L.; Lachin, J.M.; et al. Value of a Complete or Partial Remission in Severe Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2008, 3, 46–53. [Google Scholar] [CrossRef]
- Yap, D.Y.H.; Ma, M.K.M.; Mok, M.M.Y.; Tang, C.S.O.; Chan, T.M. Long-Term Data on Corticosteroids and Mycophenolate Mofetil Treatment in Lupus Nephritis. Rheumatology 2013, 52, 480–486. [Google Scholar] [CrossRef]
- Massari, P.; Duro-Garcia, V.; Girón, F.; Hernández, E.; Juárez, F.; Castro, C.; Toledo, M. Safety Assessment of the Conversion from Mycophenolate Mofetil to Enteric-Coated Mycophenolate Sodium in Stable Renal Transplant Recipients. Transplant. Proc. 2005, 37, 916–919. [Google Scholar] [CrossRef]
- Tamirou, F.; Husson, S.N.; Gruson, D.; Debiève, F.; Lauwerys, B.R.; Houssiau, F.A. Brief Report: The Euro-Lupus Low-Dose Intravenous Cyclophosphamide Regimen Does Not Impact the Ovarian Reserve, as Measured by Serum Levels of Anti-Müllerian Hormone. Arthritis Rheumatol. 2017, 69, 1267–1271. [Google Scholar] [CrossRef]
- McKinley, A.; Park, E.; Spetie, D.; Hackshaw, K.V.; Nagaraja, S.; Hebert, L.A.; Rovin, B.H. Oral Cyclophosphamide for Lupus Glomerulonephritis: An Underused Therapeutic Option. Clin. J. Am. Soc. Nephrol. 2009, 4, 1754–1760. [Google Scholar] [CrossRef]
- Rovin, B.H.; Solomons, N.; Pendergraft, W.F.; Dooley, M.A.; Tumlin, J.; Romero-Diaz, J.; Lysenko, L.; Navarra, S.V.; Huizinga, R.B.; Adzerikho, I.; et al. A Randomized, Controlled Double-Blind Study Comparing the Efficacy and Safety of Dose-Ranging Voclosporin with Placebo in Achieving Remission in Patients with Active Lupus Nephritis. Kidney Int. 2019, 95, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.; Solomons, N.; Federico, R.; Truman, M. Comparison of Standard of Care Treatment with a Low Steroid and Mycophenolate Mofetil Regimen for Lupus Nephritis in the ALMS and AURA Studies. Lupus 2019, 28, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Heshin-Bekenstein, M.; Trupin, L.; Yelin, E.; von Scheven, E.; Yazdany, J.; Lawson, E.F. Longitudinal Disease- and Steroid-Related Damage among Adults with Childhood-Onset Systemic Lupus Erythematosus. Semin. Arthritis Rheum. 2019, 49, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Jongvilaikasem, P.; Rianthavorn, P. Longitudinal Growth Patterns and Final Height in Childhood-Onset Systemic Lupus Erythematosus. Eur. J. Pediatr. 2021, 180, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Austin, H.A.; Illei, G.G.; Braun, M.J.; Balow, J.E. Randomized, Controlled Trial of Prednisone, Cyclophosphamide, and Cyclosporine in Lupus Membranous Nephropathy. J. Am. Soc. Nephrol. 2009, 20, 901–911. [Google Scholar] [CrossRef]
- Ginzler, E.M.; Dooley, M.A.; Aranow, C.; Kim, M.Y.; Buyon, J.; Merrill, J.T.; Petri, M.; Gilkeson, G.S.; Wallace, D.J.; Weisman, M.H.; et al. Mycophenolate Mofetil or Intravenous Cyclophosphamide for Lupus Nephritis. N. Engl. J. Med. 2005, 353, 2219–2228. [Google Scholar] [CrossRef]
- Hugle, B.; Silverman, E.D.; Tyrrell, P.N.; Harvey, E.A.; Hébert, D.; Benseler, S.M. Presentation and Outcome of Paediatric Membranous Non-Proliferative Lupus Nephritis. Pediatr. Nephrol. 2015, 30, 113–121. [Google Scholar] [CrossRef]
- Baskin, E.; Ozen, S.; Çakar, N.; Bayrakci, U.S.; Demirkaya, E.; Bakkaloglu, A. The Use of Low-Dose Cyclophosphamide Followed by AZA/MMF Treatment in Childhood Lupus Nephritis. Pediatr. Nephrol. 2010, 25, 111–117. [Google Scholar] [CrossRef]
- Benseler, S.M.; Bargman, J.M.; Feldman, B.M.; Tyrrell, P.N.; Harvey, E.; Hebert, D.; Silverman, E.D. Acute Renal Failure in Paediatric Systemic Lupus Erythematosus: Treatment and Outcome. Rheumatology 2009, 48, 176–182. [Google Scholar] [CrossRef]
- Lau, K.K.; Ault, B.H.; Jones, D.P.; Butani, L. Induction Therapy for Pediatric Focal Proliferative Lupus Nephritis: Cyclophosphamide versus Mycophenolate Mofetil. J. Pediatr. Health Care 2008, 22, 282–288. [Google Scholar] [CrossRef]
- Buratti, S.; Szer, I.S.; Spencer, C.H.; Bartosh, S.; Reiff, A. Mycophenolate Mofetil Treatment of Severe Renal Disease in Pediatric Onset Systemic Lupus Erythematosus. J. Rheumatol. 2001, 28, 2103–2108. [Google Scholar] [PubMed]
- Pereira, T.; Abitbol, C.L.; Seeherunvong, W.; Katsoufis, C.; Chandar, J.; Freundlich, M.; Zilleruelo, G. Three Decades of Progress in Treating Childhood-Onset Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2011, 6, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Houssiau, F.A.; D’Cruz, D.; Sangle, S.; Remy, P.; Vasconcelos, C.; Petrovic, R.; Fiehn, C.; Garrido, E.D.R.; Gilboe, I.M.; Tektonidou, M.; et al. Azathioprine versus Mycophenolate Mofetil for Long-Term Immunosuppression in Lupus Nephritis: Results from the MAINTAIN Nephritis Trial. Ann. Rheum. Dis. 2010, 69, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.A.; Jayne, D.; Ginzler, E.M.; Isenberg, D.; Olsen, N.J.; Wofsy, D.; Eitner, F.; Appel, G.B.; Contreras, G.; Lisk, L.; et al. Mycophenolate versus Azathioprine as Maintenance Therapy for Lupus Nephritis. N. Engl. J. Med. 2011, 365, 1886–1895. [Google Scholar] [CrossRef]
- Karasawa, K.; Uchida, K.; Kodama, M.; Moriyama, T.; Nitta, K. Long-Term Effects of Tacrolimus for Maintenance Therapy of Lupus Nephritis: A 5-Year Retrospective Study at a Single Center. Rheumatol. Int. 2018, 38, 2271–2277. [Google Scholar] [CrossRef]
- Yumura, W.; Suganuma, S.; Uchida, K.; Moriyama, T.; Otsubo, S.; Takei, T.; Naito, M.; Koike, M.; Nitta, K.; Nihei, H. Effects of Long-Term Treatment with Mizoribine in Patients with Proliferative Lupus Nephritis. Clin. Nephrol. 2005, 64, 28–34. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, C.; Dai, M.; Wang, S.; Xu, J.; Dai, L.; Li, Z.; He, L.; Zhu, X.; Sun, L.; et al. Leflunomide versus Azathioprine for Maintenance Therapy of Lupus Nephritis: A Prospective, Multicentre, Randomised Trial and Long-Term Follow-up. Ann. Rheum. Dis. 2022, 81, 1549–1555. [Google Scholar] [CrossRef]
- Mathian, A.; Pha, M.; Haroche, J.; Cohen-Aubart, F.; Hié, M.; Pineton De Chambrun, M.; Du Boutin, T.H.; Miyara, M.; Gorochov, G.; Yssel, H.; et al. Withdrawal of Low-Dose Prednisone in SLE Patients with a Clinically Quiescent Disease for More than 1 Year: A Randomised Clinical Trial. Ann. Rheum. Dis. 2020, 79, 339–346. [Google Scholar] [CrossRef]
- Condon, M.B.; Ashby, D.; Pepper, R.J.; Cook, H.T.; Levy, J.B.; Griffith, M.; Cairns, T.D.; Lightstone, L. Prospective Observational Single-Centre Cohort Study to Evaluate the Effectiveness of Treating Lupus Nephritis with Rituximab and Mycophenolate Mofetil but No Oral Steroids. Ann. Rheum. Dis. 2013, 72, 1280–1286. [Google Scholar] [CrossRef]
- Arends, S.; Grootscholten, C.; Derksen, R.H.W.M.; Berger, S.P.; De Sévaux, R.G.L.; Voskuyl, A.E.; Bijl, M.; Berden, J.H.M. Long-Term Follow-up of a Randomised Controlled Trial of Azathioprine/Methylprednisolone versus Cyclophosphamide in Patients with Proliferative Lupus Nephritis. Ann. Rheum. Dis. 2012, 71, 966–973. [Google Scholar] [CrossRef]
- Fernandes das Neves, M.; Irlapati, R.V.P.; Isenberg, D. Assessment of Long-Term Remission in Lupus Nephritis Patients: A Retrospective Analysis over 30 Years. Rheumatology 2015, 54, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Jourde-Chiche, N.; Costedoat-Chalumeau, N.; Baumstarck, K.; Loundou, A.; Bouillet, L.; Burtey, S.; Caudwell, V.; Chiche, L.; Couzi, L.; Daniel, L.; et al. Weaning of Maintenance Immunosuppressive Therapy in Lupus Nephritis (WIN-Lupus): Results of a Multicentre Randomised Controlled Trial. Ann. Rheum. Dis. 2022, 81, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.; Stone, D.; Levesque, V.; Cisternas, M.; Wofsy, D. Identification of Biomarkers That Predict Response to Treatment of Lupus Nephritis with Mycophenolate Mofetil or Pulse Cyclophosphamide. Arthritis Care Res. 2011, 63, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Costedoat-Chalumeau, N.; Pouchot, J.; Guettrot-Imbert, G.; Le Guern, V.; Leroux, G.; Marra, D.; Morel, N.; Piette, J.C. Adherence to Treatment in Systemic Lupus Erythematosus Patients. Best Pract. Res. Clin. Rheumatol. 2013, 27, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; To, C.H.; Yu, K.L.; Ho, L.Y. Combined Low-Dose Mycophenolate Mofetil and Tacrolimus for Lupus Nephritis with Suboptimal Response to Standard Therapy: A 12-Month Prospective Study. Lupus 2013, 22, 1135–1141. [Google Scholar] [CrossRef]
- Kasitanon, N.; Boripatkosol, P.; Louthrenoo, W. Response to Combination of Mycophenolate Mofetil, Cyclosporin A and Corticosteroid Treatment in Lupus Nephritis Patients with Persistent Proteinuria. Int. J. Rheum. Dis. 2018, 21, 200–207. [Google Scholar] [CrossRef]
- Choi, C.B.; Won, S.; Bae, S.C. Outcomes of Multitarget Therapy Using Mycophenolate Mofetil and Tacrolimus for Refractory or Relapsing Lupus Nephritis. Lupus 2018, 27, 1007–1011. [Google Scholar] [CrossRef]
- Jesus, D.; Rodrigues, M.; da Silva, J.A.P.; Inês, L. Multitarget Therapy of Mycophenolate Mofetil and Cyclosporine A for Induction Treatment of Refractory Lupus Nephritis. Lupus 2018, 27, 1358–1362. [Google Scholar] [CrossRef]
- Alshaiki, F.; Obaid, E.; Almuallim, A.; Taha, R.; El-haddad, H.; Almoallim, H. Outcomes of Rituximab Therapy in Refractory Lupus: A Meta-Analysis. Eur. J. Rheumatol. 2018, 5, 118–126. [Google Scholar] [CrossRef]
- Watson, L.; Beresford, M.W.; Maynes, C.; Pilkington, C.; Marks, S.D.; Glackin, Y.; Tullus, K. The Indications, Efficacy and Adverse Events of Rituximab in a Large Cohort of Patients with Juvenile-Onset SLE. Lupus 2015, 24, 10–17. [Google Scholar] [CrossRef]
- Peterknecht, E.; Keasey, M.P.; Beresford, M.W. The Effectiveness and Safety of Biological Therapeutics in Juvenile-Onset Systemic Lupus Erythematosus (JSLE): A Systematic Review. Lupus 2018, 27, 2135–2145. [Google Scholar] [CrossRef]
- Chan, E.Y.-H.; Wong, S.W.; Lai, F.F.Y.; Ho, T.W.; Tong, P.C.; Lai, W.M.; Ma, A.L.T.; Yap, D.Y.H. Long-Term Outcomes with Rituximab as Add-on Therapy in Severe Childhood-Onset Lupus Nephritis. Pediatr. Nephrol. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, H.; Spronk, P.; de Boer, G.; Limburg, P.; Kallenberg, C.; Derksen, R.; Wolters-Dicke, J.; Gmelig-Meyling, F.; Kater, L.; Hermans, J. Prevention of Relapses in Systemic Lupus Erythematosus. Lancet 1995, 345, 1595–1599. [Google Scholar] [CrossRef]
- Tseng, C.E.; Buyon, J.P.; Kim, M.; Belmont, H.M.; Mackay, M.; Diamond, B.; Marder, G.; Rosenthal, P.; Haines, K.; Ilie, V.; et al. The Effect of Moderate-Dose Corticosteroids in Preventing Severe Flares in Patients with Serologically Active, but Clinically Stable, Systemic Lupus Erythematosus: Findings of a Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum. 2006, 54, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.Y.H.; Kwan, L.P.Y.; Ma, M.K.M.; Mok, M.M.Y.; Chan, G.C.W.; Chan, T.M. Preemptive Immunosuppressive Treatment for Asymptomatic Serological Reactivation May Reduce Renal Flares in Patients with Lupus Nephritis: A Cohort Study. Nephrol. Dial. Transplant. 2019, 34, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Hunsicker, L.G.; Lan, S.-P.; Rohde, R.D.; Lachin, J.M. A Controlled Trial of Plasmapheresis Therapy in Severe Lupus Nephritis. The Lupus Nephritis Collaborative Study Group. N. Engl. J. Med. 1992, 326, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; van Vollenhoven, R.F.; Aranow, C.; Wagner, C.; Gordon, R.; Zhuang, Y.; Belkowski, S.; Hsu, B. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Treatment with Sirukumab (CNTO 136) in Patients with Active Lupus Nephritis. Arthritis Rheumatol. 2016, 68, 2174–2183. [Google Scholar] [CrossRef]
- Wofsy, D. Treatment of Lupus Nephritis with Abatacept: The Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol. 2014, 66, 3096–3104. [Google Scholar] [CrossRef]
- Furie, R.; Nicholls, K.; Cheng, T.T.; Houssiau, F.; Burgos-Vargas, R.; Chen, S.L.; Hillson, J.L.; Meadows-Shropshire, S.; Kinaszczuk, M.; Merrill, J.T. Efficacy and Safety of Abatacept in Lupus Nephritis: A Twelve-Month, Randomized, Double-Blind Study. Arthritis Rheumatol. 2014, 66, 379–389. [Google Scholar] [CrossRef]
- Grootscholten, C.; Ligtenberg, G.; Hagen, E.C.; Van Den Wall Bake, A.W.L.; De Glas-Vos, J.W.; Bijl, M.; Assmann, K.J.; Bruijn, J.A.; Weening, J.J.; Van Houwelingen, H.C.; et al. Azathioprine/Methylprednisolone versus Cyclophosphamide in Proliferative Lupus Nephritis. A Randomized Controlled Trial. Kidney Int. 2006, 70, 732–742. [Google Scholar] [CrossRef]
- Tunnicliffe, D.J.; Palmer, S.C.; Henderson, L.; Masson, P.; Craig, J.C.; Tong, A.; Singh-Grewal, D.; Flanc, R.S.; Roberts, M.A.; Webster, A.C.; et al. Immunosuppressive Treatment for Proliferative Lupus Nephritis. Cochrane Database Syst. Rev. 2018, 6, CD002922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qi, C.; Zha, Y.; Chen, J.; Luo, P.; Wang, L.; Sun, Z.; Wan, J.; Xing, C.; Wang, S.; et al. Leflunomide versus Cyclophosphamide in the Induction Treatment of Proliferative Lupus Nephritis in Chinese Patients: A Randomized Trial. Clin. Rheumatol. 2019, 38, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Furie, R.; Latinis, K.; Looney, R.J.; Fervenza, F.C.; Sanchez-Guerrero, J.; Maciuca, R.; Zhang, D.; Garg, J.P.; Brunetta, P.; et al. Efficacy and Safety of Rituximab in Patients with Active Proliferative Lupus Nephritis: The Lupus Nephritis Assessment with Rituximab Study. Arthritis Rheum. 2012, 64, 1215–1226. [Google Scholar] [CrossRef]
- Merrill, J.T.; Neuwelt, C.M.; Wallace, D.J.; Shanahan, J.C.; Latinis, K.M.; Oates, J.C.; Utset, T.O.; Gordon, C.; Isenberg, D.A.; Hsieh, H.J.; et al. Efficacy and Safety of Rituximab in Moderately-to-Severely Active Systemic Lupus Erythematosus: The Randomized, Double-Blind, Phase II/III Systemic Lupus Erythematosus Evaluation of Rituximab Trial. Arthritis Rheum. 2010, 62, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mysler, E.F.; Spindler, A.J.; Guzman, R.; Bijl, M.; Jayne, D.; Furie, R.A.; Houssiau, F.A.; Drappa, J.; Close, D.; MacIuca, R.; et al. Efficacy and Safety of Ocrelizumab in Active Proliferative Lupus Nephritis: Results from a Randomized, Double-Blind, Phase III Study. Arthritis Rheum. 2013, 65, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, H.; Liu, Z.; Xing, C.; Fu, P.; Ni, Z.; Chen, J.; Lin, H.; Liu, F.; He, Y.; et al. Multitarget Therapy for Induction Treatment of Lupus Nephritis: A Randomized Trial. Ann. Intern. Med. 2015, 162, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Kurasawa, T.; Nishi, E.; Kondo, T.; Okada, Y.; Shibata, A.; Nishimura, K.; Chino, K.; Okuyama, A.; Takei, H.; et al. Efficacy and Safety of Multitarget Therapy with Cyclophosphamide and Tacrolimus for Lupus Nephritis: A Prospective, Single-Arm, Single-Centre, Open Label Pilot Study in Japan. Lupus 2018, 27, 273–282. [Google Scholar] [CrossRef]
- Palmer, S.C.; Tunnicliffe, D.J.; Singh-Grewal, D.; Mavridis, D.; Tonelli, M.; Johnson, D.W.; Craig, J.C.; Tong, A.; Strippoli, G.F.M. Induction and Maintenance Immunosuppression Treatment of Proliferative Lupus Nephritis: A Network Meta-Analysis of Randomized Trials. Am. J. Kidney Dis. 2017, 70, 324–336. [Google Scholar] [CrossRef]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M.; Arriens, C.; Caster, D.J.; Romero-Diaz, J.; Gibson, K.; Kaplan, J.; Lisk, L.; Navarra, S.; et al. Efficacy and Safety of Voclosporin versus Placebo for Lupus Nephritis (AURORA 1): A Double-Blind, Randomised, Multicentre, Placebo-Controlled, Phase 3 Trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef]
- Arriens, C.; Teng, Y.K.O.; Ginzler, E.M.; Parikh, S.V.; Askanase, A.D.; Saxena, A.; Gibson, K.; Caster, D.J.; Atsumi, T.; Lisk, L.; et al. Update on the Efficacy and Safety Profile of Voclosporin: An Integrated Analysis of Clinical Trials in Lupus Nephritis. Arthritis Care Res. 2023, 75, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Ginzler, E.M.; Gibson, K.; Satirapoj, B.; Zuta Santillán, A.E.; Levchenko, O.; Navarra, S.; Atsumi, T.; Yasuda, S.; Chavez-Perez, N.N.; et al. Safety and Efficacy of Long-Term Voclosporin Treatment for Lupus Nephritis in the Phase 3 AURORA 2 Clinical Trial. Arthritis Rheumatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Shupe, J.; Dunn, R.; Kashgarian, M.; Kehry, M.R.; Shlomchik, M.J. Depletion of B Cells in Murine Lupus: Efficacy and Resistance. J. Immunol. 2007, 179, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.M.G.; Cascino, M.D.; Garg, J.; Katsumoto, T.R.; Brakeman, P.; Dall’era, M.; Looney, R.J.; Rovin, B.; Dragone, L.; Brunetta, P. Peripheral Blood B Cell Depletion after Rituximab and Complete Response in Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2018, 13, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Navarra, S.V.; Guzmán, R.M.; Gallacher, A.E.; Hall, S.; Levy, R.A.; Jimenez, R.E.; Li, E.K.M.; Thomas, M.; Kim, H.Y.; León, M.G.; et al. Efficacy and Safety of Belimumab in Patients with Active Systemic Lupus Erythematosus: A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2011, 377, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A Phase III, Randomized, Placebo-Controlled Study of Belimumab, a Monoclonal Antibody That Inhibits B Lymphocyte Stimulator, in Patients with Systemic Lupus Erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef]
- Brunner, H.I.; Abud-Mendoza, C.; Viola, D.O.; Calvo Penades, I.; Levy, D.; Anton, J.; Calderon, J.E.; Chasnyk, V.G.; Ferrandiz, M.A.; Keltsev, V.; et al. Safety and Efficacy of Intravenous Belimumab in Children with Systemic Lupus Erythematosus: Results from a Randomised, Placebo-Controlled Trial. Ann. Rheum. Dis. 2020, 79, 1340–1348. [Google Scholar] [CrossRef]
- Dooley, M.A.; Houssiau, F.; Aranow, C.; D’Cruz, D.P.; Askanase, A.; Roth, D.A.; Zhong, Z.J.; Cooper, S.; Freimuth, W.W.; Ginzler, E.M. Effect of Belimumab Treatment on Renal Outcomes: Results from the Phase 3 Belimumab Clinical Trials in Patients with SLE. Lupus 2013, 22, 63–72. [Google Scholar] [CrossRef]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef]
- Stohl, W.; Kwok, A. Belimumab for the Treatment of Pediatric Patients with Lupus Nephritis. Expert. Opin. Biol. Ther. 2023, 23, 243–251. [Google Scholar] [CrossRef]
- Furie, R.A.; Aroca, G.; Cascino, M.D.; Garg, J.P.; Rovin, B.H.; Alvarez, A.; Fragoso-Loyo, H.; Zuta-Santillan, E.; Schindler, T.; Brunetta, P.; et al. B-Cell Depletion with Obinutuzumab for the Treatment of Proliferative Lupus Nephritis: A Randomised, Double-Blind, Placebo-Controlled Trial. Ann. Rheum. Dis. 2022, 81, 100–107. [Google Scholar] [CrossRef]
- Cinar, O.K.; Marlais, M.; Al Obaidi, M.; Cheng, I.L.; Tullus, K.; Brogan, P.; Moraitis, E. Ofatumumab Use in Juvenile Systemic Lupus Erythematosus: A Single Centre Experience. Lupus 2021, 30, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shen, Q.; Gong, Y.; Li, Y.; Lv, Q.; Liu, H.; Zhao, F.; Yu, H.; Qiu, L.; Li, X.; et al. Safety and Efficacy of Telitacicept in Refractory Childhood-Onset Systemic Lupus Erythematosus: A Self-Controlled before-after Trial. Lupus 2022, 31, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Khamashta, M.; Merrill, J.T.; Werth, V.P.; Furie, R.; Kalunian, K.; Illei, G.G.; Drappa, J.; Wang, L.; Greth, W. Sifalimumab, an Anti-Interferon-α Monoclonal Antibody, in Moderate to Severe Systemic Lupus Erythematosus: A Randomised, Double-Blind, Placebo-Controlled Study. Ann. Rheum. Dis. 2016, 75, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef]
- Jayne, D.; Rovin, B.; Mysler, E.F.; Furie, R.A.; Houssiau, F.A.; Trasieva, T.; Knagenhjelm, J.; Schwetje, E.; Chia, Y.L.; Tummala, R.; et al. Phase II Randomised Trial of Type I Interferon Inhibitor Anifrolumab in Patients with Active Lupus Nephritis. Ann. Rheum. Dis. 2022, 81, 496–506. [Google Scholar] [CrossRef]
- Jayne, D.; Rovin, B.; Mysler, E.; Furie, R.; Houssiau, F.; Trasieva, T.; Knagenhjelm, J.; Schwetje, E.; Tang, W.; Tummala, R.; et al. Anifrolumab in Lupus Nephritis: Results from Second-Year Extension of a Randomised Phase II Trial. Lupus Sci. Med. 2023, 10, e000910. [Google Scholar] [CrossRef]
- Pin, A.; Tesser, A.; Pastore, S.; Moressa, V.; Valencic, E.; Arbo, A.; Maestro, A.; Tommasini, A.; Taddio, A. Biological and Clinical Changes in a Pediatric Series Treated with Off-Label JAK Inhibitors. Int. J. Mol. Sci. 2020, 21, 7767. [Google Scholar] [CrossRef]
- Merrill, J.T.; Werth, V.P.; Furie, R.; van Vollenhoven, R.; Dörner, T.; Petronijevic, M.; Velasco, J.; Majdan, M.; Irazoque-Palazuelos, F.; Weiswasser, M.; et al. Phase 2 Trial of Iberdomide in Systemic Lupus Erythematosus. N. Engl. J. Med. 2022, 386, 1034–1045. [Google Scholar] [CrossRef]
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T Cell Therapy for Refractory Systemic Lupus Erythematosus. Nat. Med. 2022, 28, 2124–2132. [Google Scholar] [CrossRef]
- Lattanzi, B.; Consolaro, A.; Solari, N.; Ruperto, N.; Martini, A.; Ravelli, A. Measures of Disease Activity and Damage in Pediatric Systemic Lupus Erythematosus: British Isles Lupus Assessment Group (BILAG), European Consensus Lupus Activity Measurement (ECLAM), Systemic Lupus Activity Measure (SLAM), Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), Physician’s Global Assessment of Disease Activity (MD Global), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC/ACR DI.; SDI). Arthritis Care Res. 2011, 63 (Suppl. S11), S112–S117. [Google Scholar] [CrossRef]
- Bock, M.; Heijnen, I.; Trendelenburg, M. Anti-C1q Antibodies as a Follow-up Marker in SLE Patients. PLoS ONE 2015, 10, e0123572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, H.; Vanarsa, K.; Gidley, G.; Mok, C.C.; Petri, M.; Saxena, R.; Mohan, C. Association of Urine SCD163 with Proliferative Lupus Nephritis, Fibrinoid Necrosis, Cellular Crescents and Intrarenal M2 Macrophages. Front. Immunol. 2020, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Endo, N.; Tsuboi, N.; Furuhashi, K.; Shi, Y.; Du, Q.; Abe, T.; Hori, M.; Imaizumi, T.; Kim, H.; Katsuno, T.; et al. Urinary Soluble CD163 Level Reflects Glomerular Inflammation in Human Lupus Nephritis. Nephrol. Dial. Transplant. 2016, 31, 2023–2033. [Google Scholar] [CrossRef]
- Qi, S.; Chen, Q.; Xu, D.; Xie, N.; Dai, Y. Clinical Application of Protein Biomarkers in Lupus Erythematosus and Lupus Nephritis. Lupus 2018, 27, 1582–1590. [Google Scholar] [CrossRef]
- Guimarães, J.D.A.R.; Furtado, S. da C.; Lucas, A.C.D.S.; Mori, B.; Barcellos, J.F.M. Diagnostic Test Accuracy of Novel Biomarkers for Lupus Nephritis-An Overview of Systematic Reviews. PLoS ONE 2022, 17, e0275016. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.A.; Haque, A.; Vanarsa, K.; Zhang, T.; Ismail, F.; Lee, K.H.; Pedroza, C.; Greenbaum, L.A.; Mason, S.; Hicks, M.J.; et al. Urine ALCAM, PF4 and VCAM-1 Surpass Conventional Metrics in Identifying Nephritis Disease Activity in Childhood-Onset Systemic Lupus Erythematosus. Front. Immunol. 2022, 13, 885307. [Google Scholar] [CrossRef]
- Yang, G.; Guo, N.; Yin, J.; Wu, J. Elevated Soluble CD163 Predicts Renal Function Deterioration in Lupus Nephritis: A Cohort Study in Eastern China. J. Int. Med. Res. 2021, 49, 03000605211049963. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Zhang, X.L.; Cruz, C.; Cano-Verduzco, M.L.; Shapiro, J.P.; Nagaraja, H.N.; Morales-Buenrostro, L.E.; Rovin, B.H. Urinary Soluble CD163: A Novel Noninvasive Biomarker of Activity for Lupus Nephritis. J. Am. Soc. Nephrol. 2020, 31, 1335–1347. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, A.; Aggarwal, A. Urinary Soluble CD163 Is a Good Biomarker for Renal Disease Activity in Lupus Nephritis. Clin. Rheumatol. 2021, 40, 941–948. [Google Scholar] [CrossRef]
- Inthavong, H.; Vanarsa, K.; Castillo, J.; Hicks, M.J.; Mohan, C.; Wenderfer, S.E. Urinary CD163 Is a Marker of Active Kidney Disease in Childhood-Onset Lupus Nephritis. Rheumatology 2023, 62, 1335–1342. [Google Scholar] [CrossRef]
- Brunner, H.I.; Mueller, M.; Rutherford, C.; Passo, M.H.; Witte, D.; Grom, A.; Mishra, J.; Devarajan, P. Urinary Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Nephritis in Childhood-Onset Systemic Lupus Erythematosus. Arthritis Rheum. 2006, 54, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.; Tullus, K.; Pilkington, C.; Chesters, C.; Marks, S.D.; Newland, P.; Jones, C.A.; Beresford, M.W. Urine Biomarkers for Monitoring Juvenile Lupus Nephritis: A Prospective Longitudinal Study. Pediatr. Nephrol. 2014, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Aljaberi, N.; Wenderfer, S.E.; Mathur, A.; Qiu, T.; Jose, S.; Merritt, A.; Rose, J.; Devarajan, P.; Huang, B.; Brunner, H. Clinical Measurement of Lupus Nephritis Activity Is Inferior to Biomarker-Based Activity Assessment Using the Renal Activity Index for Lupus Nephritis in Childhood-Onset Systemic Lupus Erythematosus. Lupus Sci. Med. 2022, 9, e000631. [Google Scholar] [CrossRef]
- Gutiérrez-Suárez, R.; Ruperto, N.; Gastaldi, R.; Pistorio, A.; Felici, E.; Burgos-Vargas, R.; Martini, A.; Ravelli, A. A Proposal for a Pediatric Version of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index Based on the Analysis of 1,015 Patients with Juvenile-Onset Systemic Lupus Erythematosus. Arthritis Rheum. 2006, 54, 2989–2996. [Google Scholar] [CrossRef]
- Foster, H.E.; Minden, K.; Clemente, D.; Leon, L.; McDonagh, J.E.; Kamphuis, S.; Berggren, K.; Van Pelt, P.; Wouters, C.; Waite-Jones, J.; et al. EULAR/PReS Standards and Recommendations for the Transitional Care of Young People with Juvenile-Onset Rheumatic Diseases. Ann. Rheum. Dis. 2017, 76, 639–646. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, D.K.; Lehman, T.J.; Bernstein, B.; Hanson, V.; King, K.K.; Nadorra, R.; Landing, B.H. Lupus Nephritis: Prognostic Factors in Children. Pediatrics 1992, 89, 240–246. [Google Scholar] [CrossRef]
- Baqi, N.; Moazami, S.; Singh, A.; Ahmad, H.; Balachandra, S.; Tejani, A. Lupus Nephritis in Children: A Longitudinal Study of Prognostic Factors and Therapy. J. Am. Soc. Nephrol. 1996, 7, 924–929. [Google Scholar] [CrossRef]
- Oni, L.; Wright, R.D.; Marks, S.; Beresford, M.W.; Tullus, K. Kidney Outcomes for Children with Lupus Nephritis. Pediatr. Nephrol. 2021, 36, 1377–1385. [Google Scholar] [CrossRef]
- Khandelwal, P.; Govindarajan, S.; Bagga, A. Management and Outcomes in Children with Lupus Nephritis in the Developing Countries. Pediatr. Nephrol. 2023, 38, 987–1000. [Google Scholar] [CrossRef]
- Hiraki, L.T.; Lu, B.; Alexander, S.R.; Shaykevich, T.; Alarcõn, G.S.; Solomon, D.H.; Winkelmayer, W.C.; Costenbader, K.H. End-Stage Renal Disease Due to Lupus Nephritis among Children in the US, 1995–2006. Arthritis Rheum. 2011, 63, 1988–1997. [Google Scholar] [CrossRef]
- Bacchetta, J.; Cochat, P. Primary Disease Recurrence—Effects on Paediatric Renal Transplantation Outcomes. Nat. Rev. Nephrol. 2015, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Wasik, H.; Chadha, V.; Galbiati, S.; Warady, B.; Atkinson, M. Dialysis Outcomes for Children With Lupus Nephritis Compared to Children with Other Forms of Nephritis: A Retrospective Cohort Study. Am. J. Kidney Dis. 2022, 79, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Ugolini-Lopes, M.R.; Seguro, L.P.C.; Castro, M.X.F.; Daffre, D.; Lopes, A.C.; Borba, E.F.; Bonfá, E. Early Proteinuria Response: A Valid Real-Life Situation Predictor of Long-Term Lupus Renal Outcome in an Ethnically Diverse Group with Severe Biopsy-Proven Nephritis? Lupus Sci. Med. 2017, 4, e000213. [Google Scholar] [CrossRef] [PubMed]
- Mackay, M.; Dall’Era, M.; Fishbein, J.; Kalunian, K.; Lesser, M.; Sanchez-Guerrero, J.; Levy, D.M.; Silverman, E.; Petri, M.; Arriens, C.; et al. Establishing Surrogate Kidney End Points for Lupus Nephritis Clinical Trials: Development and Validation of a Novel Approach to Predict Future Kidney Outcomes. Arthritis Rheumatol. 2019, 71, 411–419. [Google Scholar] [CrossRef]
- Sakamoto, A.P.; Silva, C.A.; Islabão, A.G.; Novak, G.V.; Molinari, B.; Nogueira, P.K.; Pereira, R.M.R.; Saad-Magalhães, C.; Clemente, G.; Piotto, D.P.; et al. Chronic Kidney Disease in Patients with Childhood-Onset Systemic Lupus Erythematosus. Pediatr. Nephrol. 2023, 38, 1843–1854. [Google Scholar] [CrossRef]
- Pakchotanon, R.; Gladman, D.D.; Su, J.; Urowitz, M.B. Sustained Complete Renal Remission Is a Predictor of Reduced Mortality, Chronic Kidney Disease and End-Stage Renal Disease in Lupus Nephritis. Lupus 2018, 27, 468–474. [Google Scholar] [CrossRef]
- Mahajan, A.; Amelio, J.; Gairy, K.; Kaur, G.; Levy, R.A.; Roth, D.; Bass, D. Systemic Lupus Erythematosus, Lupus Nephritis and End-Stage Renal Disease: A Pragmatic Review Mapping Disease Severity and Progression. Lupus 2020, 29, 1011–1020. [Google Scholar] [CrossRef]
- Kisaoglu, H.; Baba, O.; Kalyoncu, M. Lupus Low Disease Activity State as a Treatment Target for Pediatric Patients with Lupus Nephritis. Pediatr. Nephrol. 2023, 38, 1167–1175. [Google Scholar] [CrossRef]
- Smith, E.M.D.; Tharmaratnam, K.; Al-Abadi, E.; Armon, K.; Bailey, K.; Brennan, M.; Ciurtin, C.; Gardner-Medwin, J.; Haslam, K.E.; Hawley, D.; et al. Attainment of Low Disease Activity and Remission Targets Reduces the Risk of Severe Flare and New Damage in Childhood Lupus. Rheumatology 2022, 61, 3378–3389. [Google Scholar] [CrossRef]
- Hao, Y.; Oon, S.; Ji, L.; Gao, D.; Fan, Y.; Geng, Y.; Zhang, X.; Li, G.; Morand, E.F.; Nikpour, M.; et al. Determinants and Protective Associations of the Lupus Low Disease Activity State in a Prospective Chinese Cohort. Clin. Rheumatol. 2022, 41, 357–366. [Google Scholar] [CrossRef]
| Activity Index | Definition | Score |
|---|---|---|
| Endocapillary hypercellularity | Endocapillary hypercellularity in <25% (1+), 25–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Neutrophils/karyorrhexis | Neutrophils and/or karyorrhexis in <25% (1+), 25–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Fibrinoid necrosis | Fibrinoid necrosis in <25% (1+), 25–50% (2+), or >50% (3+) of glomeruli | (0–3) × 2 |
| Hyaline deposits | Wire loop lesions and/or hyaline thrombi in <25% (1+), 25–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Cellular/fibrocellular crescents | Cellular and/or fibrocellular crescents in <25% (1+), 25–50% (2+), or >50% (3+) of glomeruli | (0–3) × 2 |
| Interstitial inflammation | Interstitial leukocytes in <25% (1+), 25–50% (2+), or >50% (3+) in the cortex | 0–3 |
| Total | 0–24 | |
| Chronicity Index | Definition | Score |
| Total glomerulosclerosis score | Global and/or segmental sclerosis in <25% (1+), 25–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Fibrous crescents | Fibrous crescents in <25% (1+), 25–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Tubular atrophy | Tubular atrophy in <25% (1+), 25–50% (2+), or >50% (3+) of the cortical tubules | 0–3 |
| Interstitial fibrosis | Interstitial fibrosis in <25% (1+), 25–50% (2+), or >50% (3+) in the cortex | 0–3 |
| Total | 0–12 |
| Renal Domain | Score |
|---|---|
| Proteinuria > 0.5 g/24 h | 4 |
| Renal biopsy class II or V LN | 8 |
| Renal biopsy class III or IV LN | 10 |
| Standard-Dose | Moderate-Dose | Reduced-Dose | |
|---|---|---|---|
| Intravenous methylprednisolone pulses | |||
| Oral prednisone equivalent (daily) | |||
| Week 0–2 | 0.8–1 mg/kg (max 80 mg) | 0.6–0.7 mg/kg (max 50 mg) | 0.5–0.6 mg/kg (max 40 mg) |
| Week 3–4 | 0.6–0.7 mg/kg | 0.5–0.6 mg/kg | 0.3–0.4 mg/kg |
| Week 5–6 | 30 mg | 20 mg | 15 mg |
| Week 7–8 | 25 mg | 15 mg | 10 mg |
| Week 9–10 | 20 mg | 12.5 mg | 7.5 mg |
| Week 11–12 | 15 mg | 10 mg | 5 mg |
| Week 13–14 | 12.5 mg | 7.5 mg | 2.5 mg |
| Week 15–16 | 10 mg | 7.5 mg | 2.5 mg |
| Week 17–18 | 7.5 mg | 5 mg | 2.5 mg |
| Week 19–20 | 7.5 mg | 5 mg | 2.5 mg |
| Week 21–24 | 5 mg | <5 mg | 2.5 mg |
| Week > 25 | <5 mg | <5 mg | <2.5 mg |
| Molecule | Target | Status |
|---|---|---|
| Voclosporin | Calcineurin inhibitor | Approved for adults |
| Obinutuzumab | CD20 | Phase 3 trial |
| Ofatumumab | CD20 | cSLE case series |
| Telitacicept | BLyS, APRIL | Phase 3 trial |
| Ianalumab | BLyS | Phase 3 trial |
| Anifrolumab | IFN-I | Phase 3 trial |
| Sifalimumab | IFN-α | Phase 2 trial (adult SLE) |
| Iberdomide | Ikarios, Aiolos | Phase 2 trial (adult SLE) |
| CAR-T | CD19 | cSLE case series |
| Ravulizumab | C5 complement | Phase 2 trial |
| Vemircopan | Complement factor D | Phase 2 trial |
| Secukinumab | IL-17A | Phase 3 trial |
| Vunakizumab | IL-17A | Phase 2 trial |
| Guselkumab | IL-23 | Phase 2 trial |
| Daratumumab | CD38 | Phase 2 trial |
| Baricitinib | JAK | Phase 3 trial |
| Efgartigimod | FcRn | Phase 2 trial |
| Zetomipzomib | Immunoproteasome | Phase 1/2 trial |
| Zanubrutinib | BTK | Phase 2 trial |
| Iscalimab | CD40 | Phase 2 trial |
| Itolizumab | CD6 | Phase 1 study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennesi, M.; Benvenuto, S. Lupus Nephritis in Children: Novel Perspectives. Medicina 2023, 59, 1841. https://doi.org/10.3390/medicina59101841
Pennesi M, Benvenuto S. Lupus Nephritis in Children: Novel Perspectives. Medicina. 2023; 59(10):1841. https://doi.org/10.3390/medicina59101841
Chicago/Turabian StylePennesi, Marco, and Simone Benvenuto. 2023. "Lupus Nephritis in Children: Novel Perspectives" Medicina 59, no. 10: 1841. https://doi.org/10.3390/medicina59101841
APA StylePennesi, M., & Benvenuto, S. (2023). Lupus Nephritis in Children: Novel Perspectives. Medicina, 59(10), 1841. https://doi.org/10.3390/medicina59101841






