Selecting the Appropriate Radiation Therapy Technique for Extensive Brain Metastases from Tens to Hundreds: Should the Latest Technique Always Be the Best Option?
Abstract
:1. Introduction
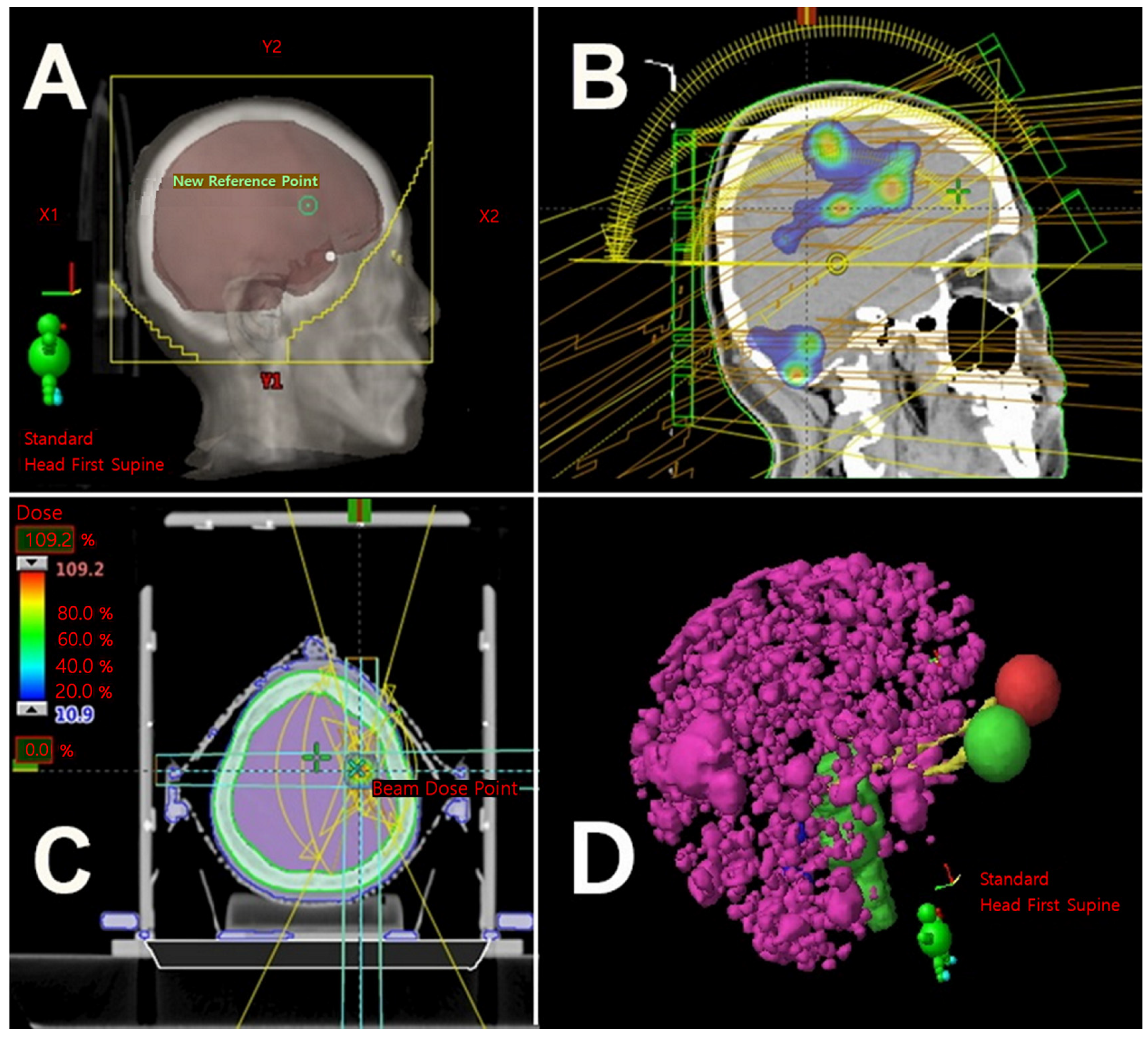
2. Rationale for and Concerns about the Use of LRT in Patients with Extensive Brain Metastases
3. Rationale for and Opinions on Performing WBRT in Patients with Extensive Brain Metastases
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchel, D.K.; Kwon, H.J.; Kubica, P.A.; Huff, W.X.; O’Regan, R.; Dey, M. Brain metastases: An update on the multi-disciplinary approach of clinical management. Neurochirurgie 2022, 6, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5, 5. [Google Scholar] [CrossRef]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef]
- Ryken, T.C.; McDermott, M.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; Kondziolka, D.; et al. The role of steroids in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010, 96, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.M.; Patchell, R.A. Diagnosis and management of brain metastases. Hematol. Oncol. Clin. N. Am. 2001, 15, 1085–1107, vii. [Google Scholar] [CrossRef]
- Soffietti, R.; Cornu, P.; Delattre, J.Y.; Grant, R.; Graus, F.; Grisold, W.; Heimans, J.; Hildebrand, J.; Hoskin, P.; Kalljo, M.; et al. EFNS Guidelines on diagnosis and treatment of brain metastases: Report of an EFNS Task Force. Eur. J. Neurol. 2006, 13, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Shimato, S.; Mitsudomi, T.; Kosaka, T.; Yatabe, Y.; Wakabayashi, T.; Mizuno, M.; Nakahara, N.; Hatano, H.; Natsume, A.; Ishii, D.; et al. EGFR mutations in patients with brain metastases from lung cancer: Association with the efficacy of gefitinib. Neuro Oncol. 2006, 8, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.N.; Rades, D.; Wirth, A.; Lo, S.S.; Danielson, B.L.; Gaspar, L.E.; Sperduto, P.W.; Vogelbaum, M.A.; Radawski, J.D.; Wang, J.Z.; et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. PractRadiat. Oncol. 2012, 2, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.P.; et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- Kondziolka, D.; Patel, A.; Lunsford, L.D.; Kassam, A.; Flickinger, J.C. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular pathways: Radiation-induced cognitive impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance during Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Ewend, M.G.; Morris, D.E.; Carey, L.A.; Ladha, A.M.; Brem, S. Guidelines for the initial management of metastatic brain tumors: Role of surgery, radiosurgery, and radiation therapy. J. Natl. Compr. Cancer Netw. 2008, 6, 505–513, quiz 514. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A. The management of brain metastases. Cancer Treat. Rev. 2003, 29, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Navarria, P.; Minniti, G.; Clerici, E.; Comito, T.; Cozzi, S.; Pinzi, V.; Fariselli, L.; Ciammella, P.; Scoccianti, S.; Borzillo, V.; et al. Brain metastases from primary colorectal cancer: Is radiosurgery an effective treatment approach? Results of a multicenter study of the radiation and clinical oncology Italian association (AIRO). Br. J. Radiol. 2020, 93, 20200951. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Shanley, R.; Luo, X.; Andrews, D.; Werner-Wasik, M.; Valicenti, R.; Bahary, J.P.; Souhami, L.; Won, M.; Mehta, M. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 526–531. [Google Scholar] [CrossRef]
- Raman, S.; Mou, B.; Hsu, F.; Valev, B.; Cheung, A.; Vallieres, I.; Ma, R.; McKenzie, M.; Beaton, L.; Rackley, T.; et al. Whole Brain Radiotherapy Versus Stereotactic Radiosurgery in Poor-Prognosis Patients with One to 10 Brain Metastases: A Randomised Feasibility Study. Clin. Oncol. 2020, 32, 442–451. [Google Scholar] [CrossRef]
- Fritz, C.; Borsky, K.; Stark, L.S.; Tanadini-Lang, S.; Kroeze, S.G.C.; Krayenbuhl, J.; Guckenberger, M.; Andratschke, N. Repeated Courses of Radiosurgery for New Brain Metastases to Defer Whole Brain Radiotherapy: Feasibility and Outcome with Validation of the New Prognostic Metric Brain Metastasis Velocity. Front. Oncol. 2018, 8, 551. [Google Scholar] [CrossRef]
- Lin, B.; Huang, D.; Du, H.; Fan, J.; Zhang, Y.; Feng, G.; Gao, F.; Du, X.B. Whole-Brain Radiation Therapy with Simultaneous Integrated Boost Versus Whole-Brain Radiation Therapy Plus Stereotactic Radiosurgery for the Treatment of Brain Metastasis from Lung Cancer. Front. Oncol. 2021, 11, 631422. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sinclair, G.; Rozati, H.; Hill, L.; Pakzad-Shahabi, L.; Wang, J.; Calvez, K.L.; Paddick, I.; Williams, M. Improving on whole-brain radiotherapy in patients with large brain metastases: A planning study to support the AROMA clinical trial. Radiother. Oncol. 2022, 170, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, D.; Bruynzeel, A.; Eekers, D.; Swinnen, A.; Hurkmans, C.; Wiggenraad, R.; Swaak-Kragten, A.; Dieleman, E.; van der Toorn, P.P.; Oei, B.; et al. A Dutch phase III randomized multicenter trial: Whole brain radiotherapy versus stereotactic radiotherapy for 4–10 brain metastases. Neurooncol. Adv. 2021, 3, vdab021. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, D.; Swinnen, A.; Roberge, D.; Nichol, A.; Zygmanski, P.; Yin, F.F.; Deblois, F.; Hurkmans, C.; Ong, C.L.; Bruynzeel, A.; et al. LINAC based stereotactic radiosurgery for multiple brain metastases: Guidance for clinical implementation. Acta Oncol 2019, 58, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Kim, H.Y.; Chang, J.W.; Park, Y.G.; Chang, J.H. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: Is stereotactic radiosurgery effective for multiple brain metastases? J. Neurosurg. 2010, 113, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Reese, R.A.; Lamba, N.; Catalano, P.J.; Cagney, D.N.; Wen, P.Y.; Aizer, A.A. Incidence and Predictors of Neurologic Death in Patients with Brain Metastases. World Neurosurg. 2022, 162, e401–e415. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Jawahar, A.; Ampil, F.L.; Shi, R.; Nanda, A.; D’Agostino, H. Survival in relation to radiotherapeutic modality for brain metastasis: Whole brain irradiation vs. gamma knife radiosurgery. Am. J. Clin. Oncol. 2004, 27, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Takada, K.; Hasegawa, T.; Yoshida, T.; Murotani, K.; Kobayashi, H.; Sakurai, T.; Yamashita, Y.; Akazawa, N.; Kojima, E. Comparison between stereotactic radiosurgery and whole-brain radiotherapy for 10–20 brain metastases from non-small cell lung cancer. Mol. Clin. Oncol. 2019, 10, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Ahluwalia, M.S.; Khan, O.H.; Asher, A.L.; Wefel, J.S.; Gondi, V. Whole-Brain Radiotherapy for Brain Metastases: Evolution or Revolution? J. Clin. Oncol. 2018, 36, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, D.; Brown, P.; Li, J.; Mehta, M.P. Whole-brain radiotherapy in the management of brain metastasis. J. Clin. Oncol. 2006, 10, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Sze, G.; Mehta, M.; Schultz, C.J.; Schultz, C.; Ford, J.; Roa, W.H.; Leibenhaut, M.; Cinelak, A.J.; Rao, A.; Timmerman, R.; et al. Radiologic response evaluation of brain metastases: Uni-dimensional (ID) W.H.O. recist vs bi-dimensional (2D) or 3-dimensional (3D) criteria. Proceedings of the 3th American Society of Clinical Oncology (ASCO). J. Clini. Oncol. 2001, 20, 59a. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H. Selecting the Appropriate Radiation Therapy Technique for Extensive Brain Metastases from Tens to Hundreds: Should the Latest Technique Always Be the Best Option? Medicina 2023, 59, 1815. https://doi.org/10.3390/medicina59101815
Lee SH. Selecting the Appropriate Radiation Therapy Technique for Extensive Brain Metastases from Tens to Hundreds: Should the Latest Technique Always Be the Best Option? Medicina. 2023; 59(10):1815. https://doi.org/10.3390/medicina59101815
Chicago/Turabian StyleLee, Seok Ho. 2023. "Selecting the Appropriate Radiation Therapy Technique for Extensive Brain Metastases from Tens to Hundreds: Should the Latest Technique Always Be the Best Option?" Medicina 59, no. 10: 1815. https://doi.org/10.3390/medicina59101815




