Effects of Foods of Mesoamerican Origin in Adipose Tissue and Liver-Related Metabolism
Abstract
:1. Introduction
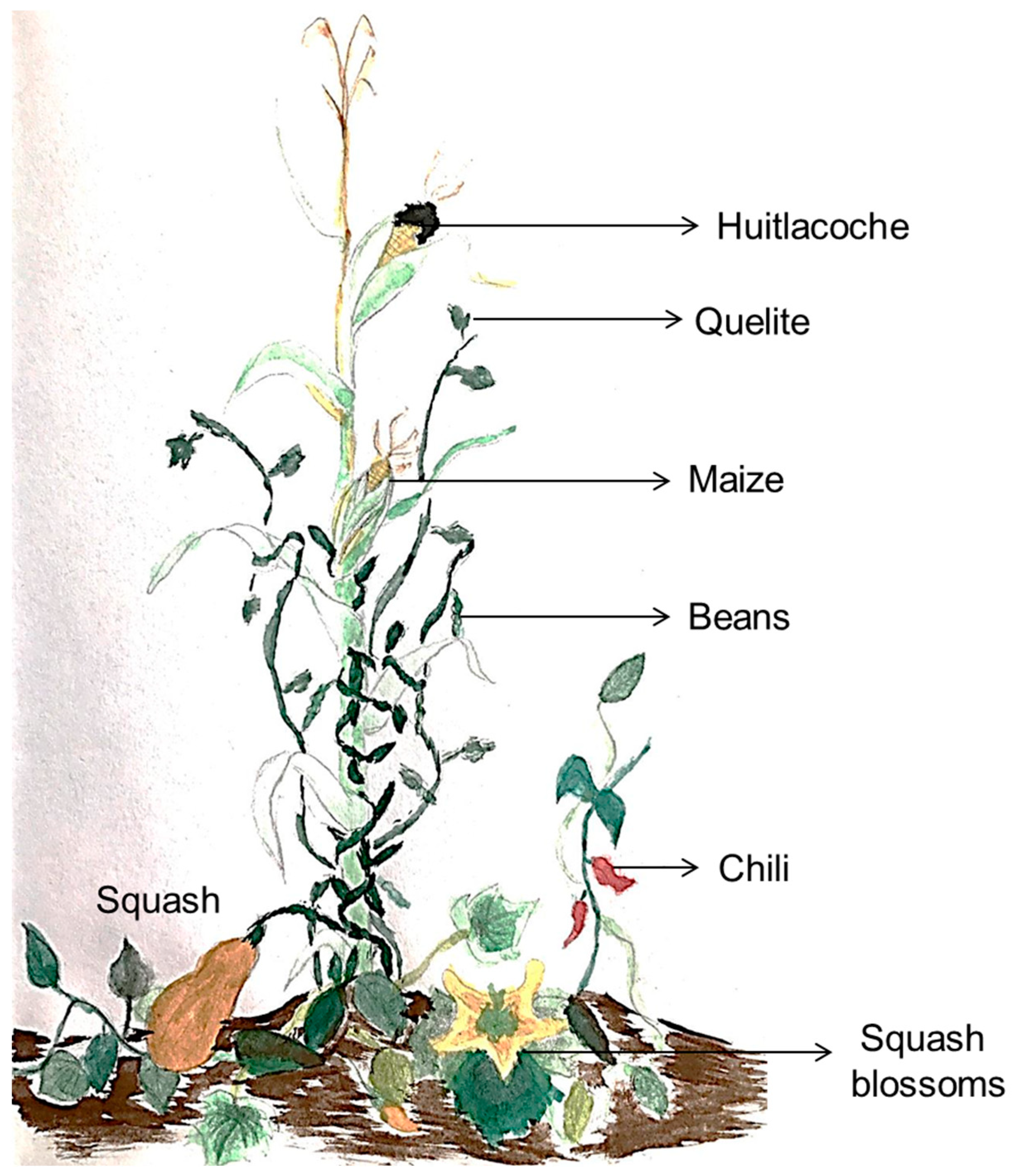
2. Mesoamerican Food and Mexican Milpa Agroecosystem
3. Scientific Evidence of Metabolic Liver Improvement by Mesoamerican Origin Foods and Their Effects in Adipose Tissue and Liver Pathogenic State
3.1. Cacao Bean
3.2. Nopal
3.3. Chili
3.4. Maize
3.5. Black Beans
3.6. Sweet Potato
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Wang, H.; Liang, X.; Roberts, M.S. Hepatic Metabolism in Liver Health and Disease. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Cambridge, UK, 2017; pp. 391–400. [Google Scholar]
- Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Santos, A.; Armendariz-Borunda, J. PPARs as Metabolic Sensors and Therapeutic Targets in Liver Diseases. Int. J. Mol. Sci. 2021, 22, 8298. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Oseini, A.M.; Sanyal, A.J. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017, 37 (Suppl. S1), 97–103. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Tang, W.; Sun, W.; Ma, Y.; Lin, S.; Jing, J.; Jiang, L.; Shi, H.; Song, Z.; et al. Western diet induces severe nonalcoholic steatohepatitis, ductular reaction, and hepatic fibrosis in liver CGI-58 knockout mice. Sci. Rep. 2020, 10, 4701. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Aguilar-López, M.; Pérez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Nmeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Suárez, M.; Boqué, N.; Del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrients 2017, 9, 1052. [Google Scholar] [CrossRef]
- Muñoz Cano, J.M.; Aguilar, A.C.; Hernández, J.C. Lipid-lowering effect of maize-based traditional Mexican food on a metabolic syndrome model in rats. Lipids Health Dis. 2013, 12, 35. [Google Scholar] [CrossRef]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean diet on type 2 diabetes and metabolic Syndrome. J. Nutr. 2015, 146, 920S–927S. [Google Scholar] [CrossRef]
- Schröder, H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J. Nutr. Biochem. 2007, 18, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Burlingame, B.; Dernini, S. Sustainable Diets and Biodiversity: Directions and Solutions for Policy, Research and Action; Food and Agriculture Organization: Québec City, QC, Canada, 2012; 309 p. [Google Scholar]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M. Globalization and food: Implications for the promotion of “Healthy” diets. In Globalization, Diets and Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2002; pp. 1–17. [Google Scholar]
- Montagnini, F. Homegardens of Mesoamerica: Biodiversity, food security, and nutrient management. In Tropical Homegardens; Kumar, B.M., Nair, P.K.R., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 3, pp. 61–84. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Sandoval-Castellanos, E.; Roessler, K.; Gaut, B.S.; Alcaraz, L.D.; Benítez, M.; Escalante, A.E. Seasonal Changes in a Maize-Based Polyculture of Central Mexico Reshape the Co-occurrence Networks of Soil Bacterial Communities. Front. Microbiol. 2017, 8, 2478. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.J.; Wittman, H.; Bacon, C.M.; Ferguson, B.G.; Barrios, L.G.; Barrios, R.G.; Jaffee, D.; Lima, J.; Méndez, V.E.; Morales, H.; et al. Food sovereignty: An alternative paradigm for poverty reduction and biodiversity conservation in Latin America. F1000Research 2013, 2, 235. [Google Scholar] [CrossRef]
- Altieri, M.A.; Funes-Monzote, F.R.; Petersen, P. Agroecologically efficient agricultural systems for smallholder farmers: Contributions to food sovereignty. Agron. Sustain. Dev. 2012, 32, 1–13. [Google Scholar] [CrossRef]
- Crocker-Sagastume, R.; Cosío-González, A.; López-López, M.; Ruiz-Domínguez, L.; Andrade-Ureña, D.; Gutiérrez-Gómez, Y. Interculturalidad alimentario-nutricional en la etnia Wixarika de México. Rev. Esp. Salud Pública 2004, 78, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo Flores, M.E. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients 2019, 11, 751. [Google Scholar] [CrossRef]
- Rosas-Campos, R.; Meza-Rios, A.; Rodriguez-Sanabria, J.S.; la Rosa-Bibiano, R.; Corona-Cervantes, K.; García-Mena, J.; Santos, A.; Sandoval-Rodriguez, A.; Armendariz-Borunda, J. Dietary supplementation with Mexican foods, Opuntia ficus indica, Theobroma cacao, and Acheta domesticus: Improving obesogenic and microbiota features in obese mice. Front. Nutr. 2022, 9, 987222. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Loomba, R. Editorial: Dark chocolate may improve NAFLD and metabolic syndrome by reducing oxidative stress. Aliment. Pharmacol. Ther. 2016, 44, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Martín, M.A.; Goya, L.; Ramos, S. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: Relevance of MAPKs. Mol. Nutr. Food Res. 2015, 59, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, S.; Lambert, J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014, 53, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kang, N.H.; Mukherjee, S.; Yun, J.W. Theobromine, a Methylxanthine in Cocoa Bean, Stimulates Thermogenesis by Inducing White Fat Browning and Activating Brown Adipocytes. Biotechnol. Bioprocess Eng. 2018, 23, 617–626. [Google Scholar] [CrossRef]
- Loffredo, L.; Del Ben, M.; Perri, L.; Carnevale, R.; Nocella, C.; Catasca, E.; Baratta, F.; Ceci, F.; Polimeni, L.; Gozzo, P.; et al. Effects of dark chocolate on NOX-2-generated oxidative stress in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2016, 44, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Cáceres, L.J.; Rabadán-Chávez, G.; Quevedo-Corona, L.; Hernández-Ledesma, B.; Miliar Garcia, A.; Mojica, L.; Lugo-Cervantes, E. Anti-obesity effect of cocoa proteins (Theobroma cacao L.) variety “Criollo” and the expression of genes related to the dysfunction of white adipose tissue in high-fat diet-induced obese rats. J. Funct. Foods 2019, 62, 103519. [Google Scholar] [CrossRef]
- Coronado-Cáceres, L.J.; Hernández-Ledesma, B.; Mojica, L.; Quevedo-Corona, L.; Rabadán-Chávez, G.; Castillo-Herrera, G.A.; Lugo Cervantes, E. Cocoa (Theobroma cacao L.) Seed-Derived Peptides Reduce Blood Pressure by Interacting with the Catalytic Site of the Angiotensin-Converting Enzyme. Foods 2021, 10, 2340. [Google Scholar] [CrossRef] [PubMed]
- Morán-Ramos, S.; Avila-Nava, A.; Tovar, A.R.; Pedraza-Chaverri, J.; López-Romero, P.; Torres, N. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. J. Nutr. 2012, 142, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Mendivil, E.J.; Sandoval-Rodriguez, A.; Meza-Ríos, A.; Zuñiga-Ramos, L.; Dominguez-Rosales, A.; Vazquez-Del Mercado, M.; Sanchez-Orozco, L.; Santos-Garcia, A.; Armendariz-Borunda, J. Capsaicin induces a protective effect on gastric mucosa along with decreased expression of inflammatory molecules in a gastritis model. J. Funct. Foods 2019, 59, 345–351. [Google Scholar] [CrossRef]
- Mendivil, E.J.; Sandoval-Rodríguez, A.; Zuñiga-Ramosa, L.M.; Santos-Garcia, A.; Armendariz-Borunda, J. Capsaicin and sulforaphane prevent experimental liver fibrosis via upregulation of peroxisome proliferator-activated receptor gamma and nuclear factor (erythroid-derived 2)-like 2. J. Funct. Foods 2019, 52, 382–388. [Google Scholar] [CrossRef]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Hepatoprotection of capsaicin in alcoholic and non-alcoholic fatty liver diseases. Arch. Physiol. Biochem. 2021, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Yang, S.M.; Han, I.S. Capsaicin suppresses liver fat accumulation in high-fat diet-induced NAFLD mice. Anim. Cells Syst. 2020, 24, 214–219. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Capsaicin for Weight Control: “Exercise in a Pill” (or Just Another Fad)? Pharmaceuticals 2022, 15, 851. [Google Scholar] [CrossRef]
- Miao, X.; Liu, G.; Xu, X.; Xie, C.; Sun, F.; Yang, Y.; Zhang, T.; Hua, S.; Fan, W.; Li, Q.; et al. High expression of vanilloid receptor-1 is associated with better prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2008, 186, 25–32. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Li, Y.; Liang, X.; Sun, Q.; Yu, H.; Zhong, J.; Ni, Y.; Chen, J.; Zhao, Z.; et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc. Diabetol. 2015, 14, 22. [Google Scholar] [CrossRef]
- Monsereenusorn, Y. Subchronic toxicity studies of capsaicin and capsicum in rats. Res. Commun. Chem. Pathol. Pharmacol. 1983, 41, 95–110. [Google Scholar] [PubMed]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetics and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thail. 2009, 92, 108–113. [Google Scholar]
- Rigamonti, A.E.; Casnici, C.; Marelli, O.; De Col, A.; Tamini, S.; Lucchetti, E.; Tringali, G.; De Micheli, R.; Abbruzzese, L.; Bortolotti, M.; et al. Acute administration of capsaicin increases resting energy expenditure in your obese subjects without affecting energy intake, appetite, and circulating levels of orexigenic/anorexigenic peptides. Nutr. Res. 2018, 52, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zsiborás, C.; Mátics, R.; Hegyi, P.; Balaskó, M.; Pètervári, E.; Szabó, I.; Sarlós, P.; Mikó, A.; Tenk, J.; Rostás, I.; et al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1419–1427. [Google Scholar] [CrossRef]
- Freidel, D.; Reilly, F.K. The Flesh of God: Cosmology, Food, and the Origins of Political Power in Ancient Southeastern Mesoamerica. In Pre-Columbian Foodways; Staller, J., Carrasco, M., Eds.; Springer: New York, NY, USA, 2010; pp. 635–680. [Google Scholar] [CrossRef]
- Buñuelos-Pineda, J.; Gómez-Rodiles, C.C.; Cuéllar- José, R.; Aguirre López, L.O. The Maize Contribution in the Human Health. In Corn—Production and Human Health in Changing Climate; Amanullah, K., Fahad, S., Eds.; InTech: Houston, TX, USA, 2018. [Google Scholar] [CrossRef]
- Magaña-Cerino, J.M.; Tiessen, A.; Soto-Luna, I.C.; Peniche-Pavía, H.A.; Vargas-Guerrero, B.; Domínguez-Rosales, J.A.; García-López, P.M.; Gurrola-Díaz, C.M. Consumption of nixtamal from a new variety of hybrid blue maize ameliorates liver oxidative stress and inflammation in a high-fat diet rat model. J. Funct. Foods 2020, 72, 104075. [Google Scholar] [CrossRef]
- Branca, F.; Lartey, A.; Oenema, S.; Aguayo, V.; Stordalen, G.A.; Richardson, R.; Arvelo, M.; Afshin, A. Transforming the food system to fight non-communicable diseases. BMJ 2019, 364, l296. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Pagliai, G.; Lotti, S.; Corrao, A.; Colombini, B.; Giangrandi, I.; Sofi, F.; Dinu, M. Adherence to the Mediterranean Diet and Ultra-Processed Foods Consumption in a Group of Italian Patients with Celiac Disease. Nutrients 2023, 15, 938. [Google Scholar] [CrossRef]
- Yao, Z.; Song, S.; Li, X.; Wang, W.; Ren, P.; Wang, H.; Xie, Y.; Li, Z. Corn peptides ameliorate nonalcoholic fatty liver disease by suppressing endoplasmic reticulum stress via the AMPKα/Sirt1 pathway in vivo and in vitro. J. Funct. Foods 2022, 93, 105063. [Google Scholar] [CrossRef]
- Wei, Y.; Li, M.; Feng, Z.; Zhang, D.; Sun, M.; Wang, Y.; Chen, X. The Protective Effects of Corn Oligopeptides on Acute Alcoholic Liver Disease by Inhibiting the Activation of Kupffer Cells NF-κB/AMPK Signal Pathway. Nutrients 2022, 14, 4194. [Google Scholar] [CrossRef]
- Li, C.C.; Lee, Y.C.; Lo, H.Y.; Huang, Y.W.; Hsiang, C.Y.; Ho, T.Y. Antihypertensive Effects of Corn Silk Extract and Its Novel Bioactive Constituent in Spontaneously Hypertensive Rats: The Involvement of Angiotensin-Converting Enzyme Inhibition. Molecules 2019, 24, 1886. [Google Scholar] [CrossRef]
- Hernandez-Velazquez, I.; Sanchez-Tapia, M.; Ordaz-Nava, G.; Torres, N.; Tovar, A.R.; Galvez, A. Black bean protein concentrate ameliorates hepatic steatosis by decreasing lipogenesis and increasing fatty acid oxidation in rats fed a high fat-sucrose diet. Food Funct. 2020, 11, 10341–10350. [Google Scholar] [CrossRef]
- Damián-Medina, K.; Milenkovic, D.; Salinas-Moreno, Y.; Corral-Jara, K.F.; Figueroa-Yáñez, L.; Marino-Marmolejo, E.; Lugo-Cervantes, E. Anthocyanin-rich extract from black beans exerts anti-diabetic effects in rats through a multi-genomic mode of action in adipose tissue. Front. Nutr. 2022, 9, 1019259. [Google Scholar] [CrossRef]
- Solverson, P. Anthocyanin Bioactivity in Obesity and Diabetes: The Essential Role of Glucose Transporters in the Gut and Periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Hernández-Velázquez, I.; Pichardo-Ontiveros, E.; Granados-Portillo, O.; Gálvez, A.R.; Tovar, A.; Torres, N. Consumption of Cooked Black Beans Stimulates a Cluster of Some Clostridia Class Bacteria Decreasing Inflammatory Response and Improving Insulin Sensitivity. Nutrients 2020, 12, 1182. [Google Scholar] [CrossRef]
- López-Reyes, A.G.; Arroyo-Curras, N.; Cano, B.G.; Lara-Díaz, V.J.; Guajardo-Salinas, G.E.; Islas, J.F.; Morales-Oyarvide, V.; Morales-Garza, L.A.; Galvez-Gastelum, F.J.; Grijalva, G.; et al. Black bean extract ameliorates liver fibrosis in rats with CCl4-induced injury. Ann. Hepatol. 2008, 7, 130–135. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.; Hua, M.; Miao, X.; Su, Y.; Chi, Y.; Li, Y.; Sun, R.; Niu, H.; Wang, J. Black bean husk and black rice anthocyanin extracts modulated gut microbiota and serum metabolites for improvement in type 2 diabetic rats. Food Funct. 2022, 13, 7377–7391. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, D.; Hua, M.; Miao, X.; Su, Y.; Chi, Y.; Li, Y.; Sun, R.; Niu, H.; Wang, J. Analysis of the alleviating effect of black bean peel anthocyanins on type 2 diabetes based on gut microbiota and serum metabolome. J. Funct. Foods 2023, 102, 105456. [Google Scholar] [CrossRef]
- Kim, H.; Koo, K.A.; Park, W.S.; Kang, D.; Kim, H.S.; Lee, B.Y.; Goo, Y.; Kim, J.; Lee, M.K.; Woo, D.K. Anti-obesity Activity of Anthocyanin and Carotenoid Extracts from Color-fleshed Sweet Potatoes. J. Food Biochem. 2020, 44, e13438. [Google Scholar] [CrossRef] [PubMed]
- Suda, I.; Ishikawa, F.; Hatakeyama, M.; Miyawaki, M.; Kudo, T.; Hirano, K.; Ito, A.; Yamakawa, O.; Horiuchi, S. Intake of purple sweet potato beverage affects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur. J. Clin. Nutr. 2008, 62, 60–67. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, P.; Mu, T. The Differentiation- and Proliferation-Inhibitory Effects of Sporamin from Sweet Potato in 3T3-L1 Preadipocytes. Agric. Sci. China 2009, 8, 1671–2927. [Google Scholar] [CrossRef]
- Nam, S.Y.; Jang, H.H.; Kim, J.B.; Lee, S.H.; Lee, Y.M. Inhibitory effects of anthocyanin-rich fraction from purple sweet potato on high fat diet-induced insulin resistance and hepatic steatosis. J. East Asian Soc. Diet Life 2016, 26, 278–284. [Google Scholar] [CrossRef]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, T.; Rayamajhi, S.; Thapa, A.; Du, W.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; et al. The longitudinal associations between sweet potato intake and the risk of non-alcoholic fatty liver disease: The TCLSIH cohort study. Int. J. Food Sci. Nutr. 2022, 73, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Jung, S.B.; Shin, J.H.; Kim, J.Y.; Kwon, O. Shinzami Korean purple-fleshed sweet potato extract prevents ischaemia-reperfusion-induced liver damage in rats. J. Sci. Food Agric. 2015, 95, 2818–2823. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Wang, Z.; Gao, H.; Su, L.; Xie, J.; Chen, X.; Liang, H.; Wang, C.; Han, Y. Oral hepatoprotective ability evaluation of purple sweet potato anthocyanins on acute and chronic chemical liver injuries. Cell Biochem. Biophys. 2014, 69, 539–548. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Liu, X.; Zhang, M.; Chen, J. Purple sweet potato (Ipomoea batatas L.) anthocyanins: Preventive effect on acute and subacute alcoholic liver damage and dealcoholic effect. J. Agric. Food Chem. 2014, 62, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, Y.; Li, J.; Lian, R.; Qin, L.; Wang, C. Extraction, purification, structural characterization, and hepatoprotective effect of the polysaccharide from purple sweet potato. J. Sci. Food Agric. 2023, 103, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, L.J.; Jiang, S.T.; Mo, Y.W. Protective effects of anthocyanins from purple sweet potato on acute carbon tetrachloride-induced oxidative hepatotoxicity fibrosis in mice. Food Agric. Immnol. 2016, 27, 157–170. [Google Scholar] [CrossRef]
- Salomone, F.; Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Grosso, G.; Godos, J.; Galvano, F.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100069. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lin, X.; Zhang, P.; Liu, Y.; Ling, W.; Guo, H. Upregulated NLRP3 inflammasome activation is attenuated by anthocyanins in patients with nonalcoholic fatty liver disease: A case-control and an intervention study. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101843. [Google Scholar] [CrossRef]
- Hou, W.C.; Lin, Y.H. Dehydroascorbate reductase and monodehydroascorbate reductase activities of trypsin inhibitors, the major sweet potato (Ipomoeas batatas [L.] Lam.) root storage protein. Plant Sci. 1997, 128, 151–158. [Google Scholar] [CrossRef]
- Lee, S.G.; Chae, J.; Kim, D.S.; Lee, J.-B.; Kwon, G.-S.; Kwon, T.K.; Nam, J.-O. Enhancement of the Antiobesity and Antioxidant Effect of Purple Sweet Potato Extracts and Enhancement of the Effects by Fermentation. Antioxidants 2021, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Ree, J.; Park, J.-W.; Choe, H.; Park, Y.I. Anti-Obesity Effects of SPY Fermented with Lactobacillus rhamnosus BST-L.601 via Suppression of Adipogenesis and Lipogenesis in High-Fat Diet-Induced Obese Mice. Foods 2023, 12, 2202. [Google Scholar] [CrossRef]
- Shih, C.K.; Chen, C.M.; Hsiao, T.J.; Liu, C.W.; Li, S.C. White Sweet Potato as Meal Replacement for Overweight White-Collar Workers: A Randomized Controlled Trial. Nutrients 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed]


| Bioactive Molecules Family | Compound Name | Mesoamerican Food | Chemical Structure |
|---|---|---|---|
| Flavanols | (−)-epicatechin C15H14O6 290.27 g/mol | Cacao bean |  |
| (+)-catechin C15H14O6 290.27 g/mol |  | ||
| Procyanidin B2 C30H26O12 578.5 g/mol |  | ||
| Alkaloids | Theobromine C7H8N4O2 180.16 g/mol | Cacao bean |  |
| Capsaicin C18H27NO3 305.40 g/mol | Chili |  | |
| Flavonoids | Quercetin C15H10O7 302.23 g/mol | Nopal, sweet potato |  |
| Myricetin C15H10O8 318.23 g/mol | Sweet potato |  | |
| Luteolin C15H10O6 286.24 g/mol |  | ||
| Apigenin C15H10O5 270.24 g/mol |  | ||
| Kaemferol C15H10O6 286.24 g/mol |  | ||
| Anthocyanins | Cyanidin C15H11O6+ 287.24 g/mol | Blue maize |  |
| Peonidin C16H13O6+ 301.27 g/mol |  | ||
| Delphinidin 3-glucoside C21H21O12+ 465.40 g/mol | Black bean |  | |
| Petunidin 3-glucoside C22H23O12+ 479.40 g/mol |  | ||
| Malvidin 3-glucoside C23H25ClO12 528.90 g/mol |  |
| Type of the Study | Food | Intervention | Principal Findings | Author |
|---|---|---|---|---|
| Pre-clinical | Cacao bean | A total of 8% of Cocoa powder supplementation in HFD-obese mice. | Decrease of inflammation, insulin resistance, and lipid accumulation in the liver. | Gu et al. [26] |
| In vitro administration of the alkaloid theobromine, a cocoa component. | Regulation of the lipolysis process and fat oxidation. | Jang et al. [27] | ||
| Administration of cocoa-derived proteins in a murine model of obesity | Overregulation of PPARγ, PPARα, AMPK, Plin1, SIRT1, and PGC-1alpha; and downregulation of TNFα, Leptin, ACC, and SREBP-1c. Genes related to white adipose tissue dysfunction in obesity. | Coronado-Cáceres et al. [29] | ||
| Pre-clinical | Nopal | Dietary intervention with a mixture of Nopal, Cacao, and cricket powder in obese HFD-fed mice. | Supplementation decreased body weight, amount of fat, serum parameters such as TG, cholesterol, and LDL, and other hormonal parameters such as insulin, leptin, and resistin. Supplementation increased the amount of beneficial intestinal bacteria. | Rosas et al. [23] |
| Oral supplementation of 4% nopal in obese Zucker rats. | The inclusion of nopal in the diet of obese rats decreased the accumulation of hepatic TG, as well as markers of liver damage such as ALT and AST, ROS, and a lower degree of lipid peroxidation. Nopal consumption increased fatty acid oxidation, decreased oxidative stress, and improved insulin signaling. | Morán-Ramos et al. [31] | ||
| Oral supplementation of 5% nopal in obese HFD-rats. | The consumption of nopal modified the composition of the intestinal microbiota, decreased the endotoxemia process, reduced oxidative stress in adipose tissue, and the accumulation of lipids in hepatocytes. | Sánchez-Tapia et al. [7] | ||
| Pre-clinical | Chili | Administration of capsaicin in a murine animal model of liver fibrosis. | Orogastric supplementation of capsaicin showed a hepatoprotective effect. | Mendivil et al. [33] |
| Pre-clinical | Maize | Addition of blue nixtamalized corn in a murine model with high fat diet. | The experimental diet decreased markers of liver damage, ALT and AST, increased antioxidant status, decreased levels of liver tissue inflammation, and consequent damage. | Magaña-Cerino [46] |
| Inclusion of maize products, pozol, and tortilla, in a murine model of metabolic syndrome. | The experimental strategy decreased TG and LDL levels and generated a hepatoprotective effect. | Muñoz-Cano et al. [10] | ||
| Administration of corn-derived peptides in an in vivo murine model. | The main results include decreased oxidative stress and endoplasmic reticulum stress, decreased hepatic lipid accumulation, and protection against hepatic steatosis. | Yao et al. [49] | ||
| Administration of corn-derived peptides to a murine model of liver damage. | Treatment decreased inflammatory cytokines, liver damage enzymes (AST and ALT), TLR4 receptor downregulation, and inhibition of the NF-κB/AMPK signaling pathway in recurrent liver macrophages (Kupffer cells). | Wei et al. [50] | ||
| Pre-clinical | Black bean | Dietary strategy of bean meal and bean protein concentrate in murine model of obesity fed with HFD and high sucrose. | The treatment prevented weight gain and reduced the amount of body fat. | Hernandez-Velazquez et al. [52] |
| Inclusion of black beans in the diet of obese rats fed a high-fat, high-sucrose diet. | The treated group decreased serum glucose and insulin levels, as well as endotoxemia generated by lipopolysaccharides, and increased energy expenditure. | Sánchez-Tapia et al. [55] | ||
| Administration of anthocyanin-rich black bean extract in diabetic murine model. | The administration of the extract impacts the adipogenesis process on several molecules related to this process. The extract was a protective agent against the development of diabetes. | Damián-Medina et al. [53] | ||
| Supplementation with black bean skin extract in murine model of type 2 diabetes. | The treatment decreased glucose and insulin levels. The extract showed anti-inflammatory effect decreasing proinflammatory cytokines, favoring the control of oxidative stress. The supplementation modified the intestinal microbiota, increasing the number of beneficial bacteria. | Sun et al. [57,58] | ||
| Clinical | Sweet potato | Association between the risk of developing MAFLD and sweet potato intake in adult population (follow-up from 2013 to 2019). | Regular consumption of sweet potato is inversely proportional to the development of MAFLD. | Yang et al. [64] |
| As a meal replacement, 132 g of sweet potato per day was included in the diet of overweight workers for 8 weeks. | The treatment decreased parameters associated with overweight such as body weight, BMI, body fat, among others. | Shih et al. [77] | ||
| Pre-clinical | Treatment of 3T3-L1 cells with sporamin (soluble sweet potato protein). | Treatment inhibited preadipocyte differentiation and intracellular lipid accumulation. | Zhi-dong et al. [61] | |
| Oral administration of carotenoid- and anthocyanin-rich extract of sweet potato in a murine model of obesity. | Supplementation decreased body weight, TG levels, and had a hepatoprotective effect. | Kim et al. [59] | ||
| Supplementation with fermented sweet potato extract in a murine model of obesity. | Treated animals showed no abnormal expansion of white fat tissue. | Lee et al. [75] | ||
| Administration of sweet potato extract fermented with Lactobacillus rhamnosus to animals fed a high-fat diet. | The treatment decreased parameters”rela’ed to adipogenesis, such as weight gain, corporal amount of fat, adipocyte size, etc. | Kang et al. [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Rios, A.; López-Villalobos, E.F.; Anguiano-Sevilla, L.A.; Ruiz-Quezada, S.L.; Velazquez-Juarez, G.; López-Roa, R.I.; Marin-Molina, A.L.; Zepeda-Morales, A.S.M. Effects of Foods of Mesoamerican Origin in Adipose Tissue and Liver-Related Metabolism. Medicina 2023, 59, 1907. https://doi.org/10.3390/medicina59111907
Meza-Rios A, López-Villalobos EF, Anguiano-Sevilla LA, Ruiz-Quezada SL, Velazquez-Juarez G, López-Roa RI, Marin-Molina AL, Zepeda-Morales ASM. Effects of Foods of Mesoamerican Origin in Adipose Tissue and Liver-Related Metabolism. Medicina. 2023; 59(11):1907. https://doi.org/10.3390/medicina59111907
Chicago/Turabian StyleMeza-Rios, Alejandra, Erika Fabiola López-Villalobos, Luis Alberto Anguiano-Sevilla, Sandra Luz Ruiz-Quezada, Gilberto Velazquez-Juarez, Rocío Ivette López-Roa, Ana Laura Marin-Molina, and Adelaida Sara Minia Zepeda-Morales. 2023. "Effects of Foods of Mesoamerican Origin in Adipose Tissue and Liver-Related Metabolism" Medicina 59, no. 11: 1907. https://doi.org/10.3390/medicina59111907
APA StyleMeza-Rios, A., López-Villalobos, E. F., Anguiano-Sevilla, L. A., Ruiz-Quezada, S. L., Velazquez-Juarez, G., López-Roa, R. I., Marin-Molina, A. L., & Zepeda-Morales, A. S. M. (2023). Effects of Foods of Mesoamerican Origin in Adipose Tissue and Liver-Related Metabolism. Medicina, 59(11), 1907. https://doi.org/10.3390/medicina59111907






