Neuroimaging Modalities Used for Ischemic Stroke Diagnosis and Monitoring
Abstract
:1. Introduction
2. Methodology
2.1. Study Design
2.2. Search Strategy
2.3. Selection Criteria
2.4. Data Extraction
3. Results
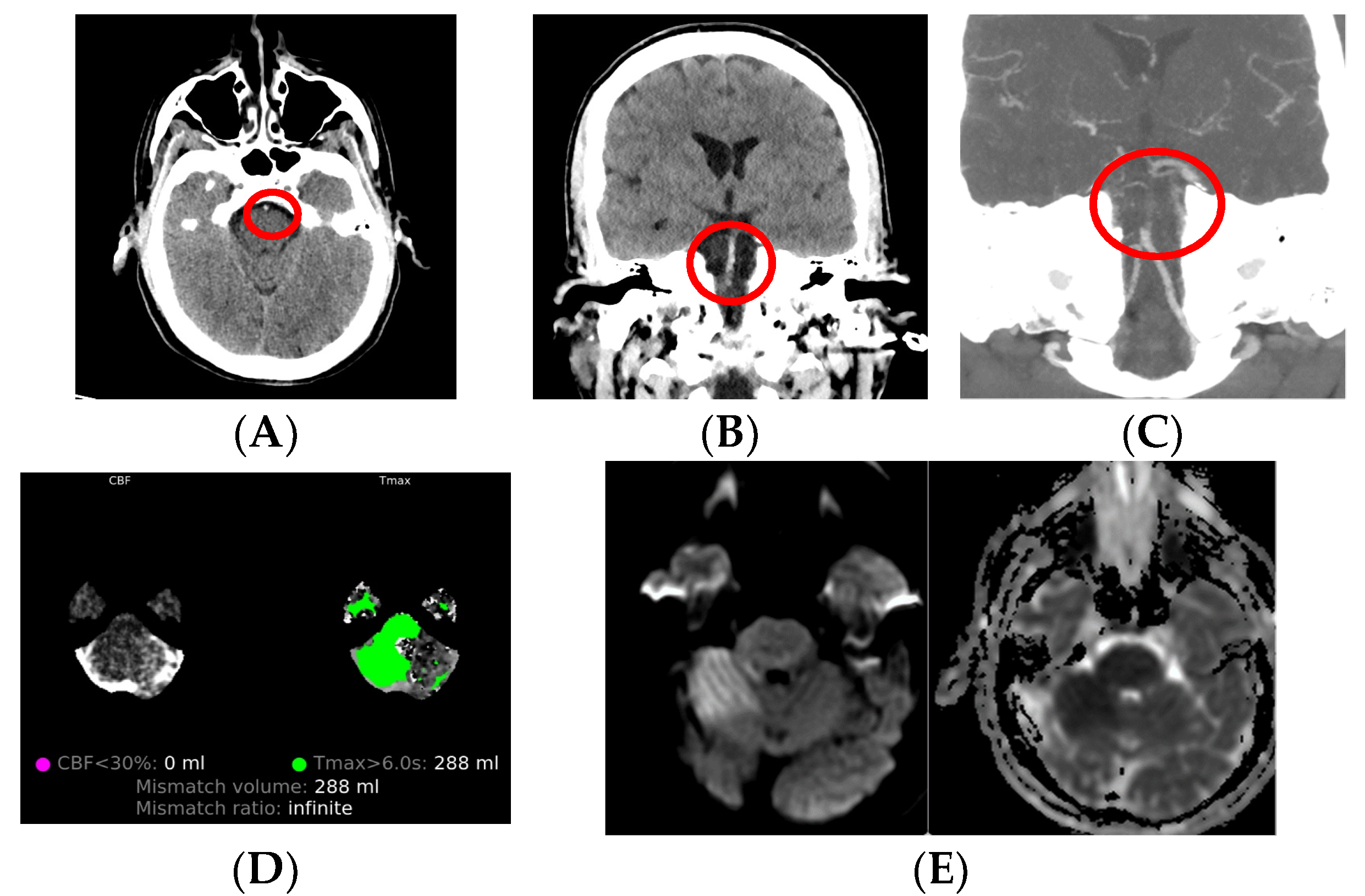
3.1. Non-Contrast CT
3.1.1. Acute Ischemic Stroke
3.1.2. Aspects Score
3.1.3. Subacute Phase
3.1.4. Chronic Phase
3.2. CT Angiography
3.3. CT Perfusion
3.4. MRI in Ischemic Stroke
3.4.1. Early Hyperacute
3.4.2. Late Hyperacute
3.4.3. Acute
3.4.4. Subacute + Chronic Ischemic Strokes
3.5. MR Angiography
3.6. Magnetic Resonance Perfusion Imaging
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Maier, O.; Menze, B.H.; von der Gablentz, J.; Häni, L.; Heinrich, M.P.; Liebrand, M.; Winzeck, S.; Basit, A.; Bentley, P.; Chen, L.; et al. A public evaluation benchmark for ischemic stroke lesion segmentation from multispectral MRI. Med. Image Anal. 2017, 35, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wakhloo, A.K.; Fisher, M. Advances in Acute Ischemic Stroke Therapy. Circ. Res. 2022, 130, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Nael, K.; Sakai, Y.; Khatri, P.; Prestigiacomo, C.J.; Puig, J.; Vagal, A. Imaging-based Selection for Endovascular Treatment in Stroke. Radiographics 2019, 39, 1696–1713. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Fan, T.; Zhao, W.; Abbas, G.; Han, B.; Zhang, K.; Li, N.; Liu, N.; Liang, W.; Huang, H.; et al. Recent advances in the development of nanomedicines for the treatment of ischemic stroke. Bioact. Mater. 2021, 6, 2854–2869. [Google Scholar] [CrossRef]
- Bruch, G.E.; Fernandes, L.F.; Bassi, B.L.; Alves, M.T.R.; Pereira, I.O.; Frézard, F.; Massensini, A.R. Liposomes for drug delivery in stroke. Brain Res. Bull. 2019, 152, 246–256. [Google Scholar] [CrossRef] [PubMed]
- El-Koussy, M.; Schroth, G.; Brekenfeld, C.; Arnold, M. Imaging of acute ischemic stroke. Eur. Neurol. 2014, 72, 309–316. [Google Scholar] [CrossRef] [PubMed]
- You, S.H.; Kim, B.; Kim, B.K.; Park, S.E. Fast MRI in Acute Ischemic Stroke: Applications of MRI Acceleration Techniques for MR-Based Comprehensive Stroke Imaging. Investig. Magn. Reson. Imaging 2021, 25, 81–92. [Google Scholar] [CrossRef]
- Allen, L.M.; Hasso, A.N.; Handwerker, J.; Farid, H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics 2012, 32, 1285–1297, discussion 1297–1299. [Google Scholar] [CrossRef]
- Potter, C.A.; Vagal, A.S.; Goyal, M.; Nunez, D.B.; Leslie-Mazwi, T.M.; Lev, M.H. CT for Treatment Selection in Acute Ischemic Stroke: A Code Stroke Primer. Radiographics 2019, 39, 1717–1738. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Derdeyn, C.P.; Biller, J.; Coffey, C.S.; Hoh, B.L.; Jauch, E.C.; Johnston, K.C.; Johnston, S.C.; Khalessi, A.A.; Kidwell, C.S.; et al. American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 3020–3035. [Google Scholar] [CrossRef] [PubMed]
- Vojinovic, R.; Opancina, V. The Role of Computed Tomography in Evalution of The Acute Ischemic Stroke. Med. Časopis 2016, 50, 139–143. [Google Scholar] [CrossRef]
- Hughes, R.E.; Tadi, P.; Bollu, P.C. TPA Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482376/ (accessed on 23 July 2023).
- Rekik, I.; Allassonnière, S.; Carpenter, T.K.; Wardlaw, J.M. Medical image analysis methods in MR/CT-imaged acute-subacute ischemic stroke lesion: Segmentation, prediction and insights into dynamic evolution simulation models. A critical appraisal. Neuroimage Clin. 2012, 1, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Salinas, C.; Wintermark, M. Imaging of acute ischemic stroke. Neuroimaging Clin. N. Am. 2010, 20, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.P.; Liebeskind, D.S. Imaging of Ischemic Stroke. Continuum 2016, 22, 1399–1423. [Google Scholar] [CrossRef] [PubMed]
- Radhiana, H.; Syazarina, S.O.; Shahizon Azura, M.M.; Hilwati, H.; Sobri, M.A. Non-contrast Computed Tomography in Acute Ischaemic Stroke: A Pictorial Review. Med. J. Malays. 2013, 68, 93–100. [Google Scholar]
- Marks, M.P.; Holmgren, E.B.; Fox, A.J.; Patel, S.; von Kummer, R.; Froehlich, J. Evaluation of early computed tomographic findings in acute ischemic stroke. Stroke 1999, 30, 389–392. [Google Scholar] [CrossRef]
- Scott, J.N.; Buchan, A.M.; Sevick, R.J. Correlation of neurologic dysfunction with CT findings in early acute stroke. Can. J. Neurol. Sci. 1999, 26, 182–189. [Google Scholar] [CrossRef]
- Pop, N.O.; Tit, D.M.; Diaconu, C.C.; Munteanu, M.A.; Babes, E.E.; Stoicescu, M.; Popescu, M.I.; Bungau, S. The Alberta Stroke Program Early CT score (ASPECTS): A predictor of mortality in acute ischemic stroke. Exp. Ther. Med. 2021, 22, 1371. [Google Scholar] [CrossRef]
- Cagnazzo, F.; Derraz, I.; Dargazanli, C.; Lefevre, P.-H.; Gascou, G.; Riquelme, C.; Bonafe, A.; Costalat, V. Mechanical thrombectomy in patients with acute ischemic stroke and ASPECTS ≤ 6: A meta-analysis. J. NeuroInterv. Surg. 2020, 12, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Puetz, V.; Dzialowski, I.; Hill, M.D.; Demchuk, A.M. The Alberta Stroke Program Early CT Score in clinical practice: What have we learned? Int. J. Stroke 2009, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Pexman, J.H.W.; Barber, P.A.; Hill, M.D.; Sevick, R.J.; Demchuk, A.M.; Hudon, M.E.; Hu, W.Y.; Buchan, A.M. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am. J. Neuroradiol. 2001, 22, 1534–1542. [Google Scholar] [PubMed]
- Tei, H.; Uchiyama, S.; Usui, T.; Ohara, K. Posterior circulation ASPECTS on diffusion-weighted MRI can be a powerful marker for predicting functional outcome. J. Neurol. 2010, 257, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Thomalla, G. A Critical Review of Alberta Stroke Program Early CT Score for Evaluation of Acute Stroke Imaging. Front. Neurol. 2017, 7, 245. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Desch, H.; Hacker, H.; Pencz, A. CT fogging effect with ischemic cerebral infarcts. Neuroradiology 1979, 18, 185–192. [Google Scholar] [CrossRef]
- Skriver, E.B.; Olsen, T.S. Transient disappearance of cerebral infarcts on CT scan, the so-called fogging effect. Neuroradiology 1981, 22, 61–65. [Google Scholar] [CrossRef]
- Liu, H.M. Neovasculature and blood-brain barrier in ischemic brain infarct. Acta Neuropathol. 1988, 75, 422–426. [Google Scholar] [CrossRef]
- Bahn, M.M.; Oser, A.B.; Cross, D.T. 3rd. CT and MRI of stroke. J. Magn. Reson. Imaging 1996, 6, 833–845. [Google Scholar] [CrossRef]
- Saver, J.L.; Johnston, K.C.; Homer, D.; Wityk, R.; Koroshetz, W.; Truskowski, L.L.; Haley, E.C. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke 1999, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, T.; Öman, O.; Hokkinen, L.; Wilppu, U.; Salli, E.; Savolainen, S.; Kangasniemi, M. Automatic CT Angiography Lesion Segmentation Compared to CT Perfusion in Ischemic Stroke Detection: A Feasibility Study. J. Digit. Imaging 2022, 35, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Polito, V.; La Piana, R.; Del Pilar Cortes, M.; Tampieri, D. Assessment of clot length with multiphase CT angiography in patients with acute ischemic stroke. Neuroradiol. J. 2017, 30, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Power, S.; McEvoy, S.H.; Cunningham, J.; Ti, J.P.; Looby, S.; O'Hare, A.; Williams, D.; Brennan, P.; Thornton, J. Value of CT angiography in anterior circulation large vessel occlusive stroke: Imaging findings, pearls, and pitfalls. Eur. J. Radiol. 2015, 84, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Ontario Health (Quality). Automated CT Perfusion Imaging to Aid in the Selection of Patients with Acute Ischemic Stroke for Mechanical Thrombectomy: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2020, 20, 1–87. [Google Scholar]
- Ramos, M.M.; Giadas, T.C. Vascular assessment in stroke codes: Role of computed tomography angiography. Radiologia 2015, 57, 156–166. [Google Scholar] [CrossRef]
- Kim, D.-E.; Schellingerhout, D.; Ryu, W.-S.; Lee, S.-K.; Jang, M.U.; Jeong, S.-W.; Na, J.-Y.; Park, J.E.; Lee, E.J.; Cho, K.-H.; et al. Mapping the Supratentorial Cerebral Arterial Territories Using 1160 Large Artery Infarcts. JAMA Neurol. 2019, 76, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kuybu, O.; Tadi, P.; Dossani, R.H. Posterior Cerebral Artery Stroke. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jolugbo, P.; Ariëns, R.A. Thrombus Composition and Efficacy of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. Stroke 2021, 52, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Tahir, R.A.; Haider, S.; Kole, M.; Griffith, B.; Marin, H. Anterior Cerebral Artery: Variant Anatomy and Pathology. J. Vasc. Interv. Neurol. 2019, 10, 16–22. [Google Scholar]
- Medrano-Martorell, S.; Pumar-Pérez, M.; González-Ortiz, S.; Capellades-Font, J. A review of the anatomy of the middle cerebral artery for the era of thrombectomy: A radiologic tool based on CT angiography and perfusion CT. Radiologia 2021, 63, 505–511. [Google Scholar] [CrossRef]
- Salerno, A.; Strambo, D.; Nannoni, S.; Dunet, V.; Michel, P. Patterns of ischemic posterior circulation strokes: A clinical, anatomical, and radiological review. Int. J. Stroke 2022, 17, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.B.; LaMuraglia, G.M.; Lancaster, R.T.; Clouse, W.D.; Kwolek, C.J.; Conrad, M.F.; Cambria, R.P.; Patel, V.I. Severe contralateral carotid stenosis or occlusion does not have an impact on risk of ipsilateral stroke after carotid endarterectomy. J. Vasc. Surg. 2018, 67, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Young, J.Y.; Schaefer, P.W. Acute ischemic stroke imaging: A practical approach for diagnosis and triage. Int. J. Cardiovasc. Imaging 2016, 32, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.H.; Phillips, T.J.; Phatouros, C.C.; Singh, T.P.; Hankey, G.J.; Blacker, D.J.; McAuliffe, W. CT perfusion in acute stroke calls: A pictorial review and differential diagnoses. J. Med. Imaging Radiat. Oncol. 2016, 60, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Václavík, D.; Volný, O.; Cimflová, P.; Švub, K.; Dvorníková, K.; Bar, M. The importance of CT perfusion for diagnosis and treatment of ischemic stroke in anterior circulation. J. Integr. Neurosci. 2022, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Boned, S.; Padroni, M.; Rubiera, M.; Tomasello, A.; Coscojuela, P.; Romero, N.; Muchada, M.; Rodríguez-Luna, D.; Flores, A.; Rodríguez, N.; et al. Admission CT perfusion may overestimate initial infarct core: The ghost infarct core concept. J. Neurointerv. Surg. 2017, 9, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Peerlings, D.; van Ommen, F.; Bennink, E.; Dankbaar, J.W.; Velthuis, B.K.; Emmer, B.J.; Hoving, J.W.; Majoie, C.B.L.M.; Marquering, H.A.; de Jong, H.W.A.M. Probability maps classify ischemic stroke regions more accurately than CT perfusion summary maps. Eur. Radiol. 2022, 32, 6367–6375. [Google Scholar] [CrossRef] [PubMed]
- Feil, K.; Reidler, P.; Kunz, W.G.; Küpper, C.; Heinrich, J.; Laub, C.; Müller, K.; Vöglein, J.; Liebig, T.; Dieterich, M.; et al. Addressing a real-life problem: Treatment with intravenous thrombolysis and mechanical thrombectomy in acute stroke patients with an extended time window beyond 4.5 h based on computed tomography perfusion imaging. Eur. J. Neurol. 2020, 27, 168–174. [Google Scholar] [CrossRef]
- Ghadimi, M.; Sapra, A. Magnetic Resonance Imaging Contraindications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kakkar, P.; Kakkar, T.; Patankar, T.; Saha, S. Current approaches and advances in the imaging of stroke. Dis. Model. Mech. 2021, 14, dmm048785. [Google Scholar] [CrossRef]
- Lee, H.; Yang, Y.; Liu, B.; Castro, S.A.; Shi, T. Patients with Acute Ischemic Stroke Who Receive Brain Magnetic Resonance Imaging Demonstrate Favorable In-Hospital Outcomes. J. Am. Heart Assoc. 2020, 9, e016987. [Google Scholar] [CrossRef]
- Tedyanto, E.H.; Tini, K.; Pramana, N.A.K. Magnetic Resonance Imaging in Acute Ischemic Stroke. Cureus 2022, 14, e27224. [Google Scholar] [CrossRef] [PubMed]
- Wey, H.-Y.; Desai, V.R.; Duong, T.Q. A review of current imaging methods used in stroke research. Neurol. Res. 2013, 35, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Liu, Z.; Liu, G.; Jin, S.; Xia, S. Ability of weakly supervised learning to detect acute ischemic stroke and hemorrhagic infarction lesions with diffusion-weighted imaging. Quant. Imaging Med. Surg. 2022, 12, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Ferrier, M.; Patsoura, S.; Gramada, R.; Meluchova, Z.; Cazzola, V.; Darcourt, J.; Cognard, C.; Viguier, A.; Bonneville, F. Magnetic resonance imaging of arterial stroke mimics: A pictorial review. Insights Imaging 2018, 9, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Meshksar, A.; Villablanca, J.P.; Khan, R.; Carmody, R.; Coull, B.; Nael, K. Role of EPI-FLAIR in patients with acute stroke: A comparative analysis with FLAIR. AJNR Am. J. Neuroradiol. 2014, 35, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Ulu, E.; Ozturk, B.; Atalay, K.; Okumus, I.B.; Erdem, D.; Gul, M.K.; Terzi, O. Diffusion-Weighted Imaging of Brain Metastasis: Correlation of MRI Parameters with Histologic Type. Turk. Neurosurg. 2022, 32, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gu, B.; Zuo, T.; Xu, X.; Chen, Y.-C.; Yin, X.; Feng, G. Predictive value of Alberta stroke program early CT score for perfusion weighted imaging—Diffusion weighted imaging mismatch in stroke with middle cerebral artery occlusion. Medicine 2020, 99, e23490. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.Y.; Tee, Y.K.; Harston, G.; Leigh, R.; Chappell, M.A. Amide proton transfer imaging in stroke. NMR Biomed. 2023, 36, e4734. [Google Scholar] [CrossRef]
- Mandeville, E.T.; Ayata, C.; Zheng, Y.; Mandeville, J.B. Translational MR Neuroimaging of Stroke and Recovery. Transl. Stroke Res. 2017, 8, 22–32. [Google Scholar] [CrossRef]
- Le Bras, A.; Raoult, H.; Ferré, J.-C.; Ronzière, T.; Gauvrit, J.-Y. Optimal MRI sequence for identifying occlusion location in acute stroke: Which value of time-resolved contrast-enhanced MRA? AJNR Am. J. Neuroradiol. 2015, 36, 1081–1088. [Google Scholar] [CrossRef]
- Boujan, T.; Neuberger, U.; Pfaff, J.; Nagel, S.; Herweh, C.; Bendszus, M.; Möhlenbruch, M.A. Value of Contrast-Enhanced MRA versus Time-of-Flight MRA in Acute Ischemic Stroke MRI. AJNR Am. J. Neuroradiol. 2018, 39, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, G.; Sun, Q.; Sun, D.-H. Application of MAGnetic resonance imaging compilation in acute ischemic stroke. World J. Clin. Cases 2021, 9, 10828–10837. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, F.; Yuan, Q.; Chen, Y.; Liu, C.-Y.; Fan, Y. Advances in differential diagnosis of cerebrovascular diseases in magnetic resonance imaging: A narrative review. Quant. Imaging Med. Surg. 2023, 13, 2712–2734. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, F.; Hacking, C.; Sharma, R.; Worsley, C.; Saber, M.; Anan, R.A.; Bell, D.; Murphy, A.; Deng, F.; Baba, Y.; et al. Ischemic Stroke. Available online: Radiopaedia.org (accessed on 13 August 2023).
- Dmytriw, A.A.; Sawlani, V.; Shankar, J. Diffusion-Weighted Imaging of the Brain: Beyond Stroke. Can. Assoc. Radiol. J. 2017, 68, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, F.; Petrovic, A.; Bell, D.; Knipe, H.; Goel, A.; Mudgal, P.; St-Amant, M. T2 Shine through. Available online: Radiopaedia.org (accessed on 14 August 2023).
- Gaillard, F.; Saber, M.; Murphy, A.; Bell, D.; Thurston, M.; Di Muzio, B. Fogging Phenomenon (Cerebral Infarct). Available online: Radiopaedia.org (accessed on 14 August 2023).
- Isla, L.G.; Márquez, I.G.; Naranjo, P.P.; Paniza, M.R.; Ventura, J.M.; Rubia, L.D.; Conesa, M.F. Magnetic resonance imaging of ischemic stroke and its correlation with multimodal computed tomography. In Proceedings of the European Congress of Radiology-ECR 2020, Vienna, Austria, 15–19 July 2020. [Google Scholar] [CrossRef]
- Kraniotis, P.; Solomou, A. Subacute cortical infarct: The value of contrast-enhanced FLAIR images in inconclusive DWI. Radiol. Bras. 2019, 52, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Non Contrast Enhanced MR Angiography. Available online: Radiopaedia.org (accessed on 14 August 2023).
- Kamalian, S.; Lev, M.H. Stroke Imaging. Radiol. Clin. N. Am. 2019, 57, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; González, R.G.; Schaefer, P.W. Conventional MRI and MR Angiography of Stroke. In Acute Ischemic Stroke; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Kurz, K.D.; Ringstad, G.; Odland, A.; Advani, R.; Farbu, E.; Kurz, M.W. Radiological imaging in acute ischaemic stroke. Eur. J. Neurol. 2016, 23 (Suppl. 1), 8–17. [Google Scholar] [CrossRef] [PubMed]
- Monsour, R.; Dutta, M.; Mohamed, A.Z.; Borkowski, A.; Viswanadhan, N.A. Neuroimaging in the Era of Artificial Intelligence: Current Applications. Fed. Pract. 2022, 39 (Suppl. 1), S14–S20. [Google Scholar] [CrossRef] [PubMed]


| Advantages | Disadvantages | |
|---|---|---|
| Non-contrast CT | High availability, cost-effectiveness, and rapid image acquisition; no contrast. | Ionizing radiation. Limited in posterior fossa and small lesions, as well as hyperacute and acute IS. |
| CT angiography | Locates source of thrombi or emboli and the clot dimensions in order to plan the reperfusion treatment. | Ionizing radiation. Contrast contraindications. |
| CT perfusion | Provides an accurate delimitation of the infarct core and penumbra area and thus good selection of patients for reperfusion treatment. | Ionizing radiation. Limited availability. |
| MRI | No radiation; greater sensitivity than CT; better detection of small lesions in comparison to CT. | Slower than CT; higher cost; limited availability. |
| MR angiography | Locate source of thrombi or emboli; no contrast. | Flow-dependent images may be inaccurate, unlike CTA which presents true anatomy of vessel lumen. |
| MR perfusion | Assesses brain tissue perfusion level. | Higher cost; limited availability; use of contrast. |
| Variant | CBF | CBV | MTT | TTP |
|---|---|---|---|---|
| Penumbra | 12–20 mL/100 g/min | >2 mL/100 g | 145% | Increased |
| Infarct core | <10–12 mL/100 g/min | <2 mL/100 g | 145% | Increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nukovic, J.J.; Opancina, V.; Ciceri, E.; Muto, M.; Zdravkovic, N.; Altin, A.; Altaysoy, P.; Kastelic, R.; Velazquez Mendivil, D.M.; Nukovic, J.A.; et al. Neuroimaging Modalities Used for Ischemic Stroke Diagnosis and Monitoring. Medicina 2023, 59, 1908. https://doi.org/10.3390/medicina59111908
Nukovic JJ, Opancina V, Ciceri E, Muto M, Zdravkovic N, Altin A, Altaysoy P, Kastelic R, Velazquez Mendivil DM, Nukovic JA, et al. Neuroimaging Modalities Used for Ischemic Stroke Diagnosis and Monitoring. Medicina. 2023; 59(11):1908. https://doi.org/10.3390/medicina59111908
Chicago/Turabian StyleNukovic, Jasmin J., Valentina Opancina, Elisa Ciceri, Mario Muto, Nebojsa Zdravkovic, Ahmet Altin, Pelin Altaysoy, Rebeka Kastelic, Diana Maria Velazquez Mendivil, Jusuf A. Nukovic, and et al. 2023. "Neuroimaging Modalities Used for Ischemic Stroke Diagnosis and Monitoring" Medicina 59, no. 11: 1908. https://doi.org/10.3390/medicina59111908






