Giant Mandibular Ameloblastoma with Rare Hypercalcemia: A Case Report and Literature Review
Abstract
:1. Introduction
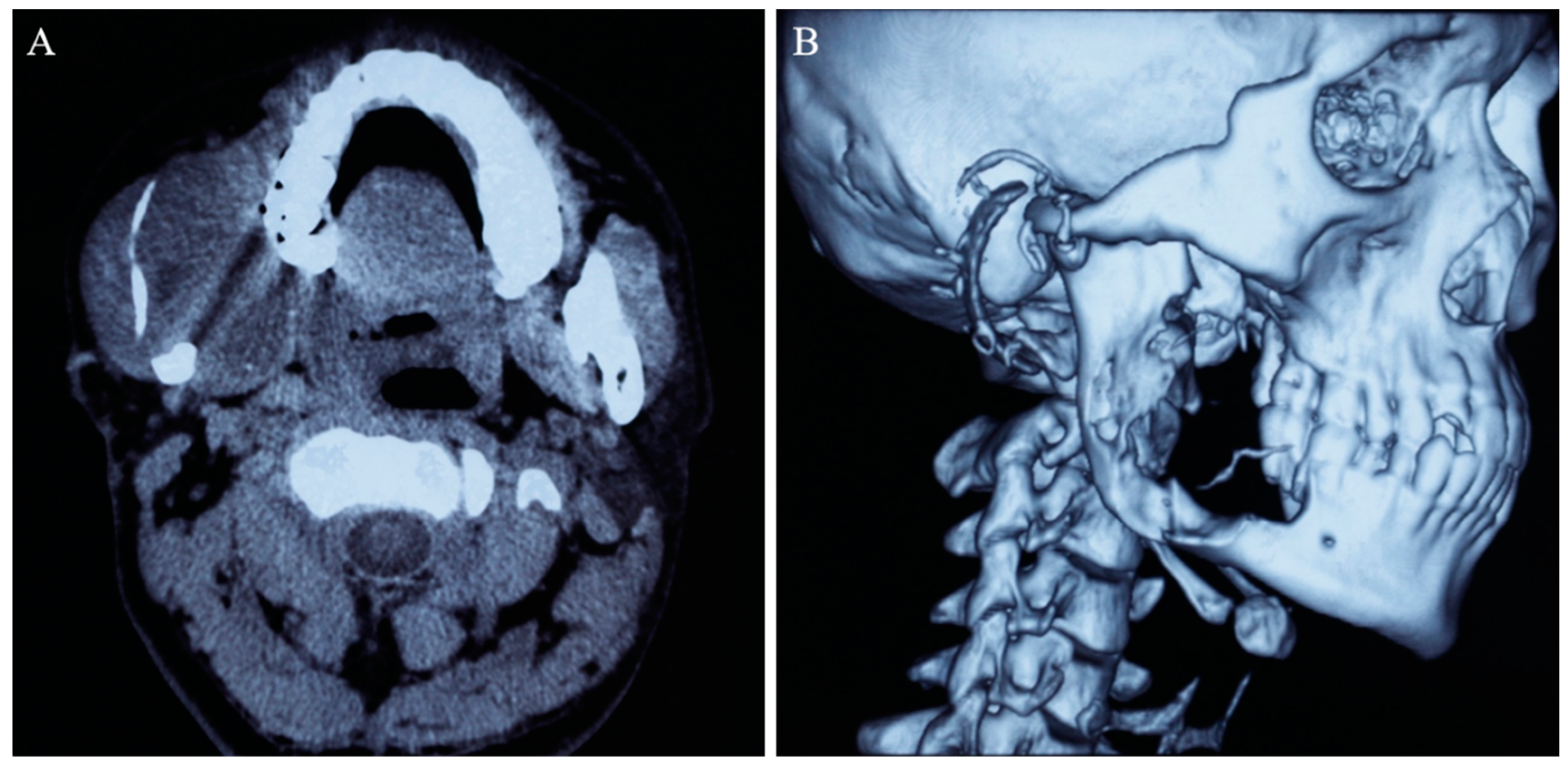
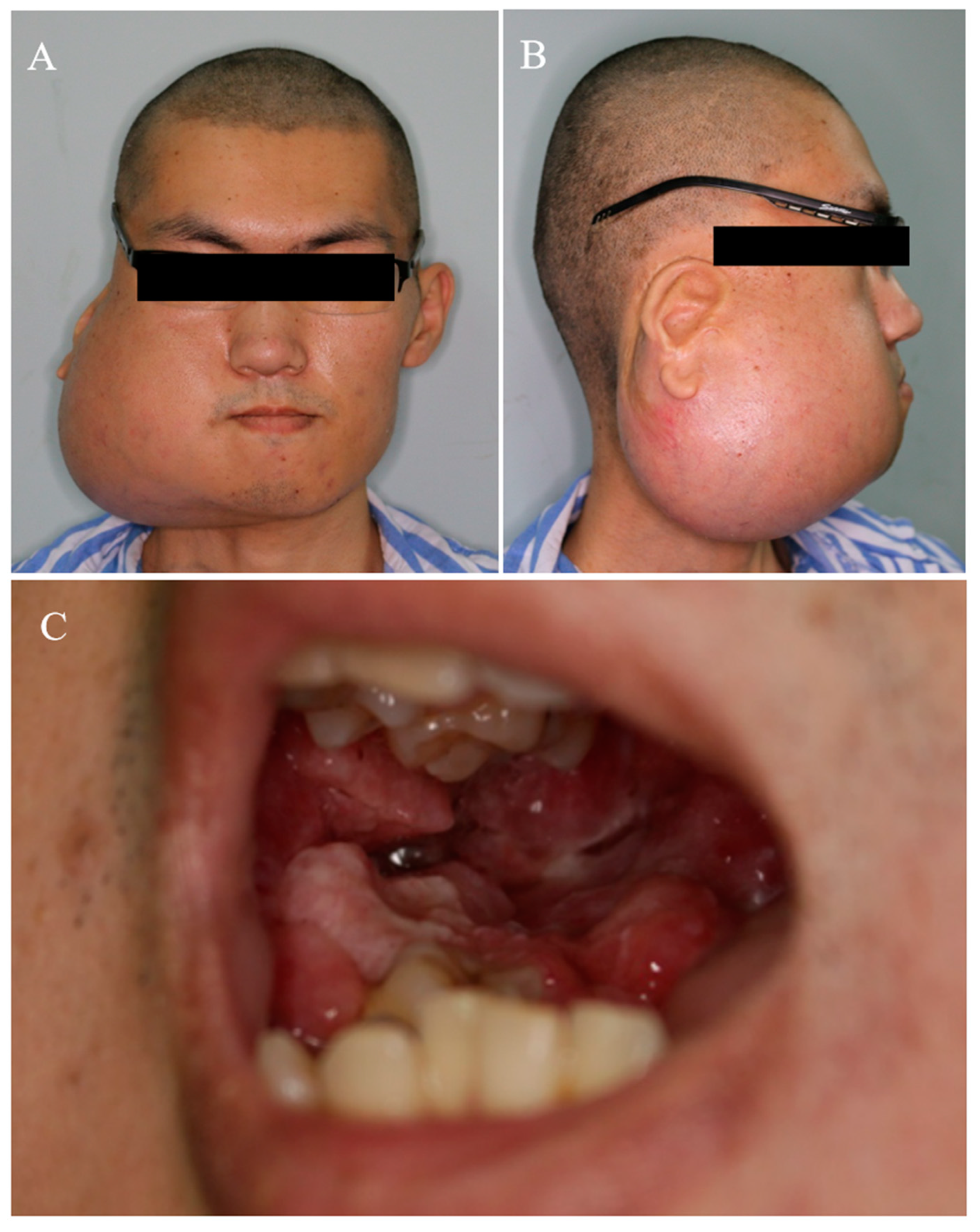
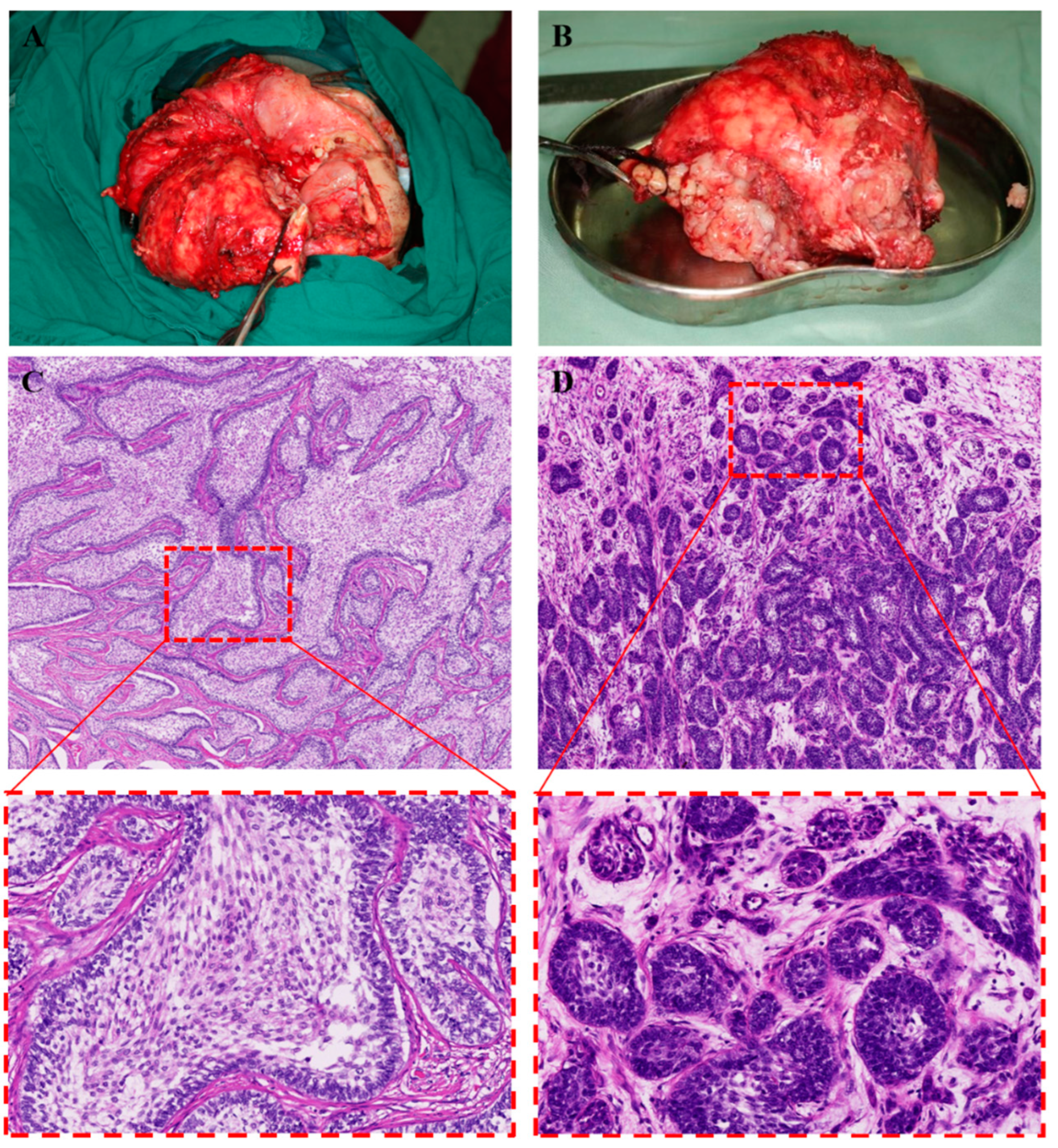
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soluk-Tekkesin, M.; Wright, J.M. The world health organization classification of odontogenic lesions: A summary of the changes of the 2022 (5th) edition. Turk. J. Pathol. 2022, 38, 168–184. [Google Scholar]
- Effiom, O.A.; Ogundana, O.M.; Akinshipo, A.O.; Akintoye, S.O. Ameloblastoma: Current etiopathological concepts and management. Oral Dis. 2018, 24, 307–316. [Google Scholar] [CrossRef]
- Laborde, A.; Nicot, R.; Wojcik, T.; Ferri, J.; Raoul, G. Ameloblastoma of the jaws: Management and recurrence rate. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 7–11. [Google Scholar] [CrossRef]
- Singh, T.; Wiesenfeld, D.; Clement, J.; Chandu, A.; Nastri, A. Ameloblastoma: Demographic data and treatment outcomes from Melbourne, Australia. Aust. Dent. J. 2015, 60, 24–29. [Google Scholar] [CrossRef] [PubMed]
- McClary, A.C.; West, R.B.; Pollack, J.R.; Fischbein, N.J.; Hosinger, C.G.; Sunwoo, J.; Colevas, A.D.; Sirjani, D. Ameloblastoma: A clinical review and trends in management. Eur. Arch. Otorhinolaryngol. 2016, 273, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- Gravvanis, A.; Koumoullis, H.D.; Anterriotis, D.; Tsoutsos, D.; Katsikeris, N. Recurrent giant mandibular ameloblastoma in young adults. Head Neck 2016, 38, E1947–E1954. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, Q.; Yang, L.; Zheng, G.-S.; Zhang, S.-E.; Lao, X.-M.; Liang, Y.-J.; Liao, G.-Q. Marsupialization of mandibular cystic ameloblastoma: Retrospective study of 7 years. Head Neck 2018, 40, 2172–2180. [Google Scholar] [CrossRef]
- Wu, K.; Luo, H.; Yuan, Z.; Wang, Y.; Qin, X.; He, J. Clinical evaluation of fenestration decompression combined with secondary curettage for ameloblastoma of the jaw: Retrospective radiographic analysis. BMC Oral Health 2022, 22, 443. [Google Scholar] [CrossRef]
- Minisola, S.; Pepe, J.; Piemonte, S.; Cipriani, C. The diagnosis and management of hypercalcaemia. BMJ 2015, 350, h2723. [Google Scholar] [CrossRef]
- Zagzag, J.; Hu, M.I.; Fisher, S.B.; Perrier, N.D.; Perrier, F.N.D. Hypercalcemia and cancer: Differential diagnosis and treatment. CA Cancer J. Clin. 2018, 68, 377–386. [Google Scholar] [CrossRef]
- Dickens, L.T.; Derman, B.; Alexander, J.T. Endocrine Society Hypercalcemia of Malignancy Guidelines. JAMA Oncol. 2023, 9, 430–431. [Google Scholar] [CrossRef]
- Tonon, C.R.; Silva, T.A.A.L.; Pereira, F.W.L.; Queiroz, D.A.R.; Junior, E.L.F.; Martins, D.; Azevedo, P.S.; Okoshi, M.P.; Zornoff, L.A.M.; de Paiva, S.A.R.; et al. A Review of Current Clinical Concepts in the Pathophysiology, Etiology, Diagnosis, and Management of Hypercalcemia. Experiment. Med. Sci. Monit. 2022, 28, e935821. [Google Scholar] [CrossRef]
- Asonitis, N.; Angelousi, A.; Zafeiris, C.; Lambrou, I.G.; Dontas, I.; Kassi, E. Diagnosis, Pathophysiology and Management of Hypercalcemia in Malignancy: A Review of the Literature. Horm. Metab. Res. 2019, 51, 770–778. [Google Scholar] [CrossRef]
- Muggia, F. Overview of cancer-related hypercalcemia: Epidemiology and etiology. Semin. Oncol. 1990, 17, 3–9. [Google Scholar] [PubMed]
- Stewart, A.F. Clinical practice. Hypercalcemia associated with cancer. N. Engl. J. Med. 2005, 352, 373–379. [Google Scholar] [CrossRef]
- Stewart, A.F.; Horst, R.; Deftos, L.J.; Cadman, E.C.; Lang, R.; Broadus, A.E. Biochemical evaluation of patients with cancer-associated hypercalcemia: Evidence for humoral and nonhumoral groups. N. Engl. J. Med. 1980, 303, 1377–1383. [Google Scholar] [CrossRef]
- Almuradova, E.; Cicin, I. Cancer-related hypercalcemia and potential treatments. Front. Endocrinol. 2023, 14, 1039490. [Google Scholar] [CrossRef]
- Feldenzer, K.L.; Sarno, J. Hypercalcemia of Malignancy. J. Adv. Pract. Oncol. 2018, 9, 496–504. [Google Scholar] [PubMed]
- Seward, G.R.; Beales, S.J.; Jonson, N.W.; Sita, L.E. A metastasising ameloblastoma associated with renal calculi and hypercalcaemia. Cancer 1975, 36, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Madiedo, G.; Choi, H.; Kleinman, J.G. Ameloblastoma of the Maxilla with Distant Metastases and Hypercalcemia. Am. J. Clin. Pathol. 1981, 75, 585–591. [Google Scholar] [CrossRef]
- McGuirt, W.F.; Scruggs, M.S.; Koufman, J.A. Hypercalcemia Secondary to a Pseudoparathormone-Secreting Ameloblastoma. Arch. Otolaryngol. 1981, 107, 487–490. [Google Scholar] [CrossRef]
- Inoue, N.; Shimojyo, M.; Iwai, H.; Ohtsuki, H.; Yasumizu, R.; Shintaku, M.; Taniguchi, N.; Inada, M.; Arika, T.; Morita, S.; et al. Malignant Ameloblastoma with Pulmonary Metastasis and Hypercalcemia: Report of an Autopsy Case and Review of the Literature. Am. J. Clin. Pathol. 1988, 90, 474–481. [Google Scholar] [CrossRef]
- Harada, K.; Suda, S.; Kayano, T.; Nagura, H.; Enomoto, S. Ameloblastoma with metastasis to the lung and associated hypercalcemia. J. Oral Maxillofac. Surg. 1989, 47, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.P.; Muller, S.; Carlson, G.W.; Murray, D. Ameloblastic carcinoma ex ameloblastoma of the mandible with malignancy-associated hypercalcemia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2000, 90, 716–722. [Google Scholar] [CrossRef]
- Papaioannou, M.; Manika, K.; Tsaoussis, B.; Cheva, A.; Sichletidis, L.; Kioumis, J. Ameloblastoma of the mandible with pulmonary metastases 45 years after initial diagnosis. Respirology 2009, 14, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Aoki, T.; Otsuru, M.; Hirabayashi, K.; Nakamura, N.; Tsukinoki, K. Huge ameloblastoma associated with hypercalcemia, leukocytosis, and elevated tumor markers via production of parathyroid hormone-related protein and granulocyte colony-stimulating factor. J. Oral Maxillofac. Surg. 2012, 70, 1380–1385. [Google Scholar] [CrossRef]
- Ghiam, A.; Al, Z.A.; Feld, R. A case of recurrent metastatic ameloblastoma and hypercalcaemia successfully treated with carboplatin and paclitaxel: Long survival and prolonged stable disease. E Cancer Med. Sci. 2013, 7, 323. [Google Scholar]
- Açıkgöz, Y.; Sendur, M.A.N.; Özdemir, N.Y.; Aksoy, S.; Uncu, D.; Zengin, N. Longest survival of lung metastatic ameloblastoma with a rare cause of malignant hypercalcemia. J. Cranio-Maxillofac. Surg. 2014, 42, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.E.N.; Villafuerte, C.V.; Acampado, L.T. Overwhelming hypercalcaemia in mandibular ameloblastoma. BMJ Case Rep. 2014, 2014, 491. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sasaki, E.; Nakamura, R.; Sawabe, M.; Hagiwara, S.; Hyodo, I.; Hanai, N. Recurrent ameloblastoma with both hypercalcemia and BRAF mutation: A case report. Clin. Case Rep. 2020, 8, 3462–3466. [Google Scholar] [CrossRef]
- Macpherson, D.; Hopper, C.; Meghji, S. Hypercalcaemia and the synthesis of interleukin-1 by an ameloblastoma. Br. J. Oral Maxillofac. Surg. 1991, 29, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Abdelsayed, R.A.; Vartanian, R.K.; Smith, K.K.; Ibrahim, A.N. Parathyroid hormone-related protein (PTHrP) expression in ameloblastoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2004, 97, 208–219. [Google Scholar] [CrossRef]
- Zeballos, R.; Bologna-Molina, R.; Pereira-Prado, V.; Villarroel-Dorrego, M. Expression of parathyroid hormone related protein (PTHRP) in ameloblastomas. J. Clin. Exp. Dent. 2018, 10, e172–e176. [Google Scholar]
- Kumamoto, H.; Ooya, K. Expression of parathyroid hormone-related protein (PTHrP), osteoclast differentiation factor (ODF)/receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoclastogenesis inhibitory factor (OCIF)/osteoprotegerin (OPG) in ameloblastomas. J. Oral Pathol. Med. 2004, 33, 46–52. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Chewcharat, A.; Mao, M.A.; Bathini, T.; Vallabhajosyula, S.; Thirunavukkarasu, S.; Kashani, K.B. Impact of admission serum ionized calcium levels on risk of acute kidney injury in hospitalized patients. Sci. Rep. 2020, 10, 12316. [Google Scholar] [CrossRef]
- De Francesco, D.E.; Martiniano, L.V.M.; Lima, L.L.L.; Leite Filho, N.C.V.; de Oliveira Souz, L.E.; Duarte Fernandes, P.H.P.; da Silva, S.L.; da Silva, G.B.J. Acute kidney injury due to excessive and prolonged intramuscular injection of veterinary supplements containing vitamins A, D and E: A series of 16 cases. Nefrologia 2017, 37, 61–67. [Google Scholar] [CrossRef]
- Kruger, J.M.; Osborne, C.A.; Nachreiner, R.F.; Refsal, K.R. Hypercalcemia and renal failure. Etiology, pathophysiology, diagnosis, and treatment. Vet. Clin. North Am. Small Anim. Pract. 1996, 26, 1417–1445. [Google Scholar] [CrossRef]
- Berretta, L.; Melo, G.; Mello, F.; Lizio, G.; Rivero, E. Effectiveness of marsupialisation and decompression on the reduction of cystic jaw lesions: A systematic review. Br. J. Oral Maxillofac. Surg. 2021, 59, E17–E42. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Samman, N. Recurrence related to treatment modalities of unicystic ameloblastoma: A systematic review. Int. J. Oral Maxillofac. Surg. 2006, 35, 681–690. [Google Scholar] [CrossRef]
- Nakamura, N.; Higuchi, Y.; Mitsuyasu, T.; Sandra, F.; Ohishi, M. Comparison of long-term results between different approaches to ameloblastoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 93, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Higuchi, Y.; Tashiro, H.; Ohishi, M. Marsupialization of cystic ameloblastoma: A clinical and histopathologic study of the growth characteristics before and after marsupialization. J. Oral Maxillofac. Surg. 1995, 53, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G. A pathologist’s approach to the treatment of ameloblastoma. J. Oral Maxillofac. Surg. 1984, 42, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, S.P.; Raubenheimer, E.J.; Noffke, C.E. Internal morphology of ameloblastomas: A study of 24 resected specimens. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 754–762. [Google Scholar] [CrossRef]
- Yokobayashi, Y.; Yokobayashi, T.; Nakajima, T.; Oyama, T.; Fukushima, M.; Ishiki, T. Marsupialization as a possible diagnostic aid in cystic ameloblastoma. Case report. J. Maxillofac. Surg. 1983, 11, 137–141. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.A.; Baldwin, D.R. Multidisciplinary team management in thoracic oncology: More than just a concept? Eur. Respir. J. 2014, 43, 1776–1786. [Google Scholar] [CrossRef]
- Guillem, P.; Bolla, M.; Courby, S.; Descotes, J.-L.; Laramas, M.; Sibilot, D.-M. Multidisciplinary team meetings in cancerology: Setting priorities for improvement. Bull. Cancer 2011, 98, 989–998. [Google Scholar] [CrossRef]
- Nikolovski, Z.; Watters, D.; Stupart, D.; Guest, G.D. Colorectal multidisciplinary meetings: How do they affect the timeliness of treatment? ANZ J. Surg. 2017, 87, E112–E115. [Google Scholar]





| Department | Professional Recommendations |
|---|---|
| Nephrology | 1. Chronic nephropathy may be associated with hypercalcemia. 2. Attention should be paid to maintaining water-electrolyte balance and acid–base balance in perioperative period. 3. If the patient’s renal function remains abnormal after surgery, further renal investigation should be considered. |
| Endocrinology | 1. Hypercalcemia caused by hyperparathyroidism is ruled out by low parathyroid hormone levels and normal parathyroid imaging. 2. Lowering blood calcium levels to the normal range before surgery. |
| Radiology | CTA is recommended when necessary to clarify the relationship between the tumor and neck vessels. |
| Neurosurgery | If the tumor has a rich blood supply from right external carotid artery, preoperative external carotid artery embolization can be considered to prevent intraoperative bleeding. |
| Anesthesiology | 1. The long duration of surgery, massive hemorrhage, and hemodynamic instability implies high risks of anesthesia. 2. Taking aggressive measures to optimize preoperative nutritional status. 3. Adequate supply of compatible blood and blood products should be prepared. 4. Establishing peripheral venous access before surgery. |
| Hematology | Low hemoglobin, low ferritin levels, and microcytic hypochromic anaemia—iron supplementation is recommended. |
| Biochemical Parameter | Result | Normal Values | Interpretation |
|---|---|---|---|
| Hemoglobin | 91 g/L | 131~172 g/L | Recurrent oral bleeding and inadequate nutrient intake may lead to anemia. |
| Hematokrit | 28.6% | 38.0~50.8% | |
| Total serum calcium | 3.64 mmol/L | 2.03~2.54 mmol/L | Hypercalcemia is likely related to the giant ameloblastoma. |
| Serum potassium | 3.07 mmol/L | 3.50~5.20 mmol/L | Deficiency of oral intake. |
| Serum phosphorus | 0.71 mmol/L | 0.87~1.45 mmol/L | Deficiency of oral intake. |
| Creatinine | 131 umol/L | 59~104 umol/L | Hypercalcemia may results in damaged renal function. |
| Parathyroid hormone (PTH) | 6.7 pg/mL | 15.0~65.0 pg/mL | Hypercalcemia may lead to secondary reduction in PTH levels. |
| 25-hydroxyvitamin D | 7.5 nmol/L | 12.3~107.0 nmol/L | Hypercalcemia feedback inhibited vitamin D synthesis; insufficient intake of vitamin D. |
| Author | Publish Year | Age of Ameloblastoma | Age of Hypercalcemia | Sex | Primary Site | Tumor Size | Infection | Number of Operations | Pathological Type | Metastasis | Metastasis Site | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seward [19] | 1975 | 13 | 27 | M | Mandible | - | - | 3 | Metastasizing | Yes | Lungs Liver Spine | Yes |
| Madiedo [20] | 1981 | 49 | 54 | M | Maxilla | - | - | 3 | Metastasizing | Yes | Lungs Small intestine Peritoneal | Yes |
| Mcguirt [21] | 1981 | 53 | 55 | F | Mandible | - | - | 2 | Conventional | No | No | Yes |
| Inoue [22] | 1988 | 51 | 67 | F | Maxilla | - | - | 2 | Metastasizing | Yes | Lungs | Yes |
| Harada [23] | 1989 | 33 | 51 | M | Mandible | - | - | 4 | Metastasizing | Yes | Lungs | Yes |
| Cox [24] | 2000 | 25 | 43 | M | Mandible | 17 × 16 × 13 cm | Yes | 5 | Conventional | No | No | Yes |
| Papaioannou [25] | 2009 | 7 | 52 | F | Mandible | - | - | 6 | Metastasizing | Yes | Lungs | Yes |
| Ota Y. [26] | 2012 | 32 | 32 | F | Mandible | 272 × 203 × 151 mm | - | 2 | Conventional | No | No | No |
| Ghiam [27] | 2013 | 26 | 47 | M | Mandible | - | - | 2 | Metastasizing | Yes | Lungs | Yes |
| Acikgoz [28] | 2014 | 48 | 52 | M | Mandible | - | - | 2 | Metastasizing | Yes | Lungs | Yes |
| Lo T.E. [29] | 2014 | 20 | 20 | F | Mandible | 12 × 12 × 8 cm | Yes | 1 | Conventional | No | No | No |
| Suzuki [30] | 2020 | 49 | 49 | F | Mandible | 110 mm | Yes | 1 | Conventional | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Xu, C.; Wang, P.; Chen, J.; Yu, D.; Zhu, H. Giant Mandibular Ameloblastoma with Rare Hypercalcemia: A Case Report and Literature Review. Medicina 2023, 59, 1956. https://doi.org/10.3390/medicina59111956
Shen W, Xu C, Wang P, Chen J, Yu D, Zhu H. Giant Mandibular Ameloblastoma with Rare Hypercalcemia: A Case Report and Literature Review. Medicina. 2023; 59(11):1956. https://doi.org/10.3390/medicina59111956
Chicago/Turabian StyleShen, Wenyi, Chenlu Xu, Pan Wang, Junpeng Chen, Dan Yu, and Huiyong Zhu. 2023. "Giant Mandibular Ameloblastoma with Rare Hypercalcemia: A Case Report and Literature Review" Medicina 59, no. 11: 1956. https://doi.org/10.3390/medicina59111956
APA StyleShen, W., Xu, C., Wang, P., Chen, J., Yu, D., & Zhu, H. (2023). Giant Mandibular Ameloblastoma with Rare Hypercalcemia: A Case Report and Literature Review. Medicina, 59(11), 1956. https://doi.org/10.3390/medicina59111956






