Lung Ultrasound Is Useful for Evaluating Lung Damage in COVID-19 Patients Treated with Bamlanivimab and Etesevimab: A Single-Center Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Lung Ultrasound
2.4. Statistics
3. Results
3.1. Population Differences
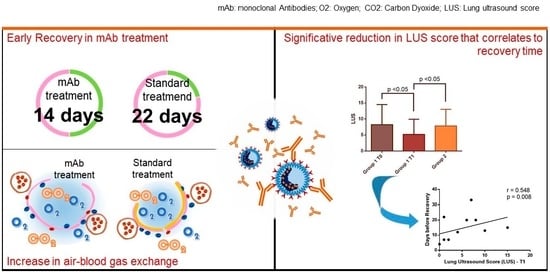
3.2. mAb role in COVID-19 Recovery
3.3. Clinical Outcome
3.4. Lung Ultrasound Score Evaluation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cates, J.; Lucero-Obusan, C.; Dahl, R.M.; Schirmer, P.; Garg, S.; Oda, G.; Hall, A.J.; Langley, G.; Havers, F.P.; Holodniy, M.; et al. Risk for In-Hospital Complications Associated with COVID-19 and Influenza—Veterans Health Administration, United States, October 1, 2018–May 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, V.; Tun, N.L.; Haque, W.Z.; Gilbert Majella, M.; Kumar Sivakumar, R.; Kumar, A.; Hsu, A.T.W.; Ishak, I.A.; Nur, A.A.; Ayeh, S.K.; et al. Factors associated with disease severity and mortality among patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241541. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Seibel, A.; Heinz, W.; Greim, C.; Weber, S. Lungensonographie bei COVID-19. Wien. Klin. Mag. 2021, 24, 164–172. [Google Scholar] [CrossRef]
- Cicco, S.; Vacca, A.; Cariddi, C.; Carella, R.; Altamura, G.; Solimando, A.G.; Lauletta, G.; Pappagallo, F.; Cirulli, A.; Stragapede, A.; et al. Imaging Evaluation of Pulmonary and Non-Ischaemic Cardiovascular Manifestations of COVID-19. Diagnostics 2021, 11, 1271. [Google Scholar] [CrossRef]
- Bavaro, D.; Diella, L.; Solimando, A.; Cicco, S.; Buonamico, E.; Stasi, C.; Ciannarella, M.; Marrone, M.; Carpagnano, F.; Resta, O.; et al. Bamlanivimab and Etesevimab administered in an outpatient setting for SARS-CoV-2 infection. Pathog. Glob. Health 2022, 116, 297–304. [Google Scholar] [CrossRef]
- Elsaghir, H.; Adnan, G. Best practices for administering monoclonal antibody therapy for coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2021, 325, 632–644. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco. Definizione delle Modalita’ e delle Condizioni di Impiego Dell’anticorpo Monoclonale Bamlanivimab-Etesevimab. (Determina n. DG/318/2021); Gazzetta Ufficiale: Rome, Italy, 2021; No. 66.
- Xue, H.; Li, C.; Cui, L.; Tian, C.; Li, S.; Wang, Z.; Liu, C.; Ge, Q. M-BLUE protocol for coronavirus disease-19 (COVID-19) patients: Interobserver variability and correlation with disease severity. Clin. Radiol. 2021, 76, 379–383. [Google Scholar] [CrossRef]
- Solimando, A.G.; Susca, N.; Borrelli, P.; Prete, M.; Lauletta, G.; Pappagallo, F.; Buono, R.; Inglese, G.; Forina, B.M.; Bochicchio, D.; et al. Short-Term Variations in Neutrophil-to-Lymphocyte and Urea-to-Creatinine Ratios Anticipate Intensive Care Unit Admission of COVID-19 Patients in the Emergency Department. Front. Med. 2021, 7, 625176. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Mudd, P.A.; West, C.P.; Wilber, E.; Wilber, S.T. Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Examinations, Laboratory Tests, Imaging Accuracy, and Biases. Acad. Emerg. Med. 2020, 27, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.W.; Wu, X.X.; Jiang, X.G.; Xu, K.J.; Ying, L.J.; Ma, C.L.; Li, S.B.; Wang, H.Y.; Zhang, S.; Gao, H.N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef]
- McCreary, E.K.; Pogue, J.M. COVID-19 Treatment: A Review of Early and Emerging Options. Open Forum Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Ferré, E.M.N.; Schmitt, M.M.; Ochoa, S.; Rosen, L.B.; Shaw, E.R.; Burbelo, P.D.; Stoddard, J.L.; Rampertaap, S.; DiMaggio, T.; Bergerson, J.R.E.; et al. SARS-CoV-2 Spike Protein-Directed Monoclonal Antibodies May Ameliorate COVID-19 Complications in APECED Patients. Front. Immunol. 2021, 12, 720205. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cicco, S.; Mozzini, C.; Marozzi, M.; De Fazio, G.; Carella, R.; Vacca, A.; Cariddi, C.; Pappagallo, F.; Solimando, A.G.; Ria, R. Cardiovascular risk score may be useful in stratify death risk in hospitalized covid19 patients. J. Hypertens. 2022, 40, e172. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Başaran, S.; Şimşek-Yavuz, S.; Meşe, S.; Çağatay, A.; Medetalibeyoğlu, A.; Öncül, O.; Özsüt, H.; Ağaçfidan, A.; Gül, A.; Eraksoy, H. The effect of tocilizumab, anakinra and prednisolone on antibody response to SARS-CoV-2 in patients with COVID-19: A prospective cohort study with multivariate analysis of factors affecting the antibody response. Int. J. Infect. Dis. 2021, 105, 756–762. [Google Scholar] [CrossRef]
- Masci, G.M.; Iafrate, F.; Ciccarelli, F.; Pambianchi, G.; Panebianco, V.; Pasculli, P.; Ciardi, M.R.; Mastroianni, C.M.; Ricci, P.; Catalano, C.; et al. Tocilizumab effects in COVID-19 pneumonia: Role of CT texture analysis in quantitative assessment of response to therapy. Radiol. Medica 2021, 126, 1170–1180. [Google Scholar] [CrossRef]
- Sava, M.; Sommer, G.; Daikeler, T.; Woischnig, A.K.; Martinez, A.E.; Leuzinger, K.; Hirsch, H.; Erlanger, T.; Wiencierz, A.; Bassetti, S.; et al. Ninety-day outcome of patients with severe COVID-19 treated with tocilizumab—A single centre cohort study. Swiss Med. Wkly. 2021, 151, w20550. [Google Scholar] [CrossRef]
- Guigon, A.; Faure, E.; Lemaire, C.; Chopin, M.C.; Tinez, C.; Assaf, A.; Lazrek, M.; Hober, D.; Bocket, L.; Engelmann, I.; et al. Emergence of Q493R mutation in SARS-CoV-2 spike protein during Bamlanivimab/Etesevimab treatment and resistance to viral clearance. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Lee, W.; Hur, J.; Park, D. Chest Computed Tomography Findings in Asymptomatic Patients with COVID-19. Respiration 2020, 99, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Fujikawa, A.; Jitsu, M.; Kunishima, N.; Watanabe, S.; Suzuki, Y.; Umeda, S.; Uwabe, Y. Chest ct findings in cases from the cruise ship diamond princess with coronavirus disease (Covid-19). Radiol. Cardiothorac. Imaging 2020, 2, e200110. [Google Scholar] [CrossRef]
- So, M.; Kabata, H.; Fukunaga, K.; Takagi, H.; Kuno, T. Radiological and functional lung sequelae of COVID-19: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef]
- Munker, D.; Veit, T.; Barton, J.; Mertsch, P.; Mümmler, C.; Osterman, A.; Khatamzas, E.; Barnikel, M.; Hellmuth, J.C.; Münchhoff, M.; et al. Pulmonary function impairment of asymptomatic and persistently symptomatic patients 4 months after COVID-19 according to disease severity. Infection 2022, 50, 157–168. [Google Scholar] [CrossRef]
- Smith, M.J.; Hayward, S.A.; Innes, S.M.; Miller, A.S.C. Point-of-care lung ultrasound in patients with COVID-19—A narrative review. Anaesthesia 2020, 75, 1096–1104. [Google Scholar] [CrossRef]
- Cicco, S.; Cicco, G.; Racanelli, V.; Vacca, A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediat. Inflamm. 2020, 2020, 7527953. [Google Scholar] [CrossRef]
- Cicco, S.; Vacca, A.; Cittadini, A.; Marra, A.M. Long-Term Follow-Up May be Useful in Coronavirus Disease 2019 Survivors to Prevent Chronic Complications. Infect. Chemother. 2020, 52, 407. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Finelli, C. Obesity, COVID-19 and immunotherapy: The complex relationship! Immunotherapy 2020, 12, 1105–1109. [Google Scholar] [CrossRef]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef] [PubMed]



| mAb | No mAb | p-Value | |
|---|---|---|---|
| Age | 64.50 ± 7.26 | 59.71 ± 11.68 | Ns |
| Sex (M/F) | 8/6 | 16/12 | Ns |
| Body mass index (kg/m2) | 34.70 ± 3.07 | 26.48 ± 1.57 | 0.0001 |
| Comorbidities number (IQR) | 2 (–2.5) | 1 (1.2) | Ns |
| Diabetes | 2 | 2 | Ns |
| Arterial hypertension | 10 | 15 | Ns |
| Cardiovascular disease | 7 | 4 | Ns |
| Chronic obstructive pulmonary disease | 4 | 3 | Ns |
| Other lung diseases (Asthma, Obstructive sleep apnea) | 1 | 2 | Ns |
| Chronic kidney disease | 1 | 0 | Ns |
| Chronic liver disease | 1 | 0 | Ns |
| Autoimmune disease | 1 | 4 | Ns |
| Cancer | 2 | 3 | Ns |
| Current Smoker | 2 | 4 | Ns |
| mAb | No mAb | ||
|---|---|---|---|
| Time of recovery (first negative swab) | 13.85 ± 7.91 | 21.65 ± 7.08 | 0.0007 |
| 14-day recovery (%) | 10 (71.43) | 2 (7.14) | <0.0001 |
| 28-day recovery (%) | 12 (85.71) | 21 (75.00) | Ns |
| Home-treated number (steroids, NSAIDs, paracetamol, prophylactic antibiotic) | 9 | 18 | Ns |
| Need of Oxygen (%) | 2 (14.29) | 1 (3.57) | Ns |
| Steroid (Prednisone) | 3 | 9 | Ns |
| Antibiotics | 6 | 10 | Ns |
| Low molecular weight heparin | 3 | 1 | Ns |
| COVID Symptoms median number (IQR) | 3 (2–4) | 4 (3–7) | 0.05 |
| Fever | 7 | 22 | Ns |
| Max Temperature | 37.06 ± 0.98 | 38.66 ± 0.52 | 0.0001 |
| Cough | 12 | 26 | Ns |
| Dyspnea | 2 | 12 | Ns |
| Tachypnea (>22 arpm) | 0 | 6 | Ns |
| Fatigue | 5 | 13 | Ns |
| Hypo/anosmia | 3 | 14 | Ns |
| Dysgeusia | 4 | 14 | Ns |
| Sore Throat | 4 | 7 | Ns |
| Nausea/vomiting | 0 | 3 | Ns |
| Diarrhea | 2 | 7 | Ns |
| Myalgia/arthralgia | 7 | 9 | Ns |
| Confusion | 0 | 1 | Ns |
| Headache | 2 | 9 | Ns |
| Conjunctivitis | 0 | 2 | Ns |
| mAb | No mAb | p-Value | |||
|---|---|---|---|---|---|
| T0 | T1 | p-Value vs T0 | p-Value vs T1 | ||
| Vital Signs | |||||
| Systolic blood pressure (mmHg) | 146.90 ± 15.18 | 128.00 ± 15.67 | 0.04 | 127.50 ± 7.58 | Ns |
| Diastolic blood pressure (mmHg) | 81.30 ± 7.62 | 71.00 ± 7.75 | 0.03 | 81.67 ± 9.31 | 0.03 |
| Heart rate (bpm) | 74.57 ± 13.67 | 86.00 ± 13.70 | Ns | 78.86 ± 8.21 | Ns |
| Respiration rate (apm) | 19.33 ± 2.45 | 18.67 ±2.00 | Ns | 16.00 ± 2.83 | Ns |
| Temperature (°C) | 37.06 ± 0.98 | 35.87 ± 0.42 | 0.001 | - | |
| Blood Gas Analysis | |||||
| pH | 7.45 ± 0.03 | 7.42 ± 0.03 | 0.01 | 7.46 ± 0.05 | 0.04 |
| pCO2 (mmHg) | 37.91 ± 4.11 | 37.27 ± 2.97 | ns | 40.50 ± 1.29 | 0.03 |
| SO2 (mmHg) | 76.09 ± 11.84 | 88.27 ± 10.25 | 0.008 | 75.00 ± 9.83 | 0.04 |
| HCO3− (mEq/L) | 26.89 ± 2.34 | 24.81 ± 1.76 | 0.02 | 28.38 ± 3.13 | 0.01 |
| SO2 (%) | 96.69 ± 1.84 | 98.08 ± 0.76 | 0.02 | 95.25 ± 2.36 | 0.004 |
| A-aDO2 (mmHg) | 26.52 ± 13.76 | 15.14 ± 9.74 | 0.02 | 24.38 ± 10.84 | 0.02 |
| P/F | 362.40 ± 56.22 | 420.30 ± 48.76 | 0.005 | 357.00 ± 46.72 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicco, S.; Marozzi, M.S.; Palumbo, C.A.; Sturdà, E.; Fusillo, A.; Scarilli, F.; Albanese, F.; Morelli, C.; Bavaro, D.F.; Diella, L.; et al. Lung Ultrasound Is Useful for Evaluating Lung Damage in COVID-19 Patients Treated with Bamlanivimab and Etesevimab: A Single-Center Pilot Study. Medicina 2023, 59, 203. https://doi.org/10.3390/medicina59020203
Cicco S, Marozzi MS, Palumbo CA, Sturdà E, Fusillo A, Scarilli F, Albanese F, Morelli C, Bavaro DF, Diella L, et al. Lung Ultrasound Is Useful for Evaluating Lung Damage in COVID-19 Patients Treated with Bamlanivimab and Etesevimab: A Single-Center Pilot Study. Medicina. 2023; 59(2):203. https://doi.org/10.3390/medicina59020203
Chicago/Turabian StyleCicco, Sebastiano, Marialuisa Sveva Marozzi, Carmen Alessandra Palumbo, Elisabetta Sturdà, Antonio Fusillo, Flavio Scarilli, Federica Albanese, Claudia Morelli, Davide Fiore Bavaro, Lucia Diella, and et al. 2023. "Lung Ultrasound Is Useful for Evaluating Lung Damage in COVID-19 Patients Treated with Bamlanivimab and Etesevimab: A Single-Center Pilot Study" Medicina 59, no. 2: 203. https://doi.org/10.3390/medicina59020203
APA StyleCicco, S., Marozzi, M. S., Palumbo, C. A., Sturdà, E., Fusillo, A., Scarilli, F., Albanese, F., Morelli, C., Bavaro, D. F., Diella, L., Saracino, A., Pappagallo, F., Solimando, A. G., Lauletta, G., Ria, R., & Vacca, A. (2023). Lung Ultrasound Is Useful for Evaluating Lung Damage in COVID-19 Patients Treated with Bamlanivimab and Etesevimab: A Single-Center Pilot Study. Medicina, 59(2), 203. https://doi.org/10.3390/medicina59020203