Trends in Long COVID Symptoms in Japanese Teenage Patients
Abstract
:1. Introduction
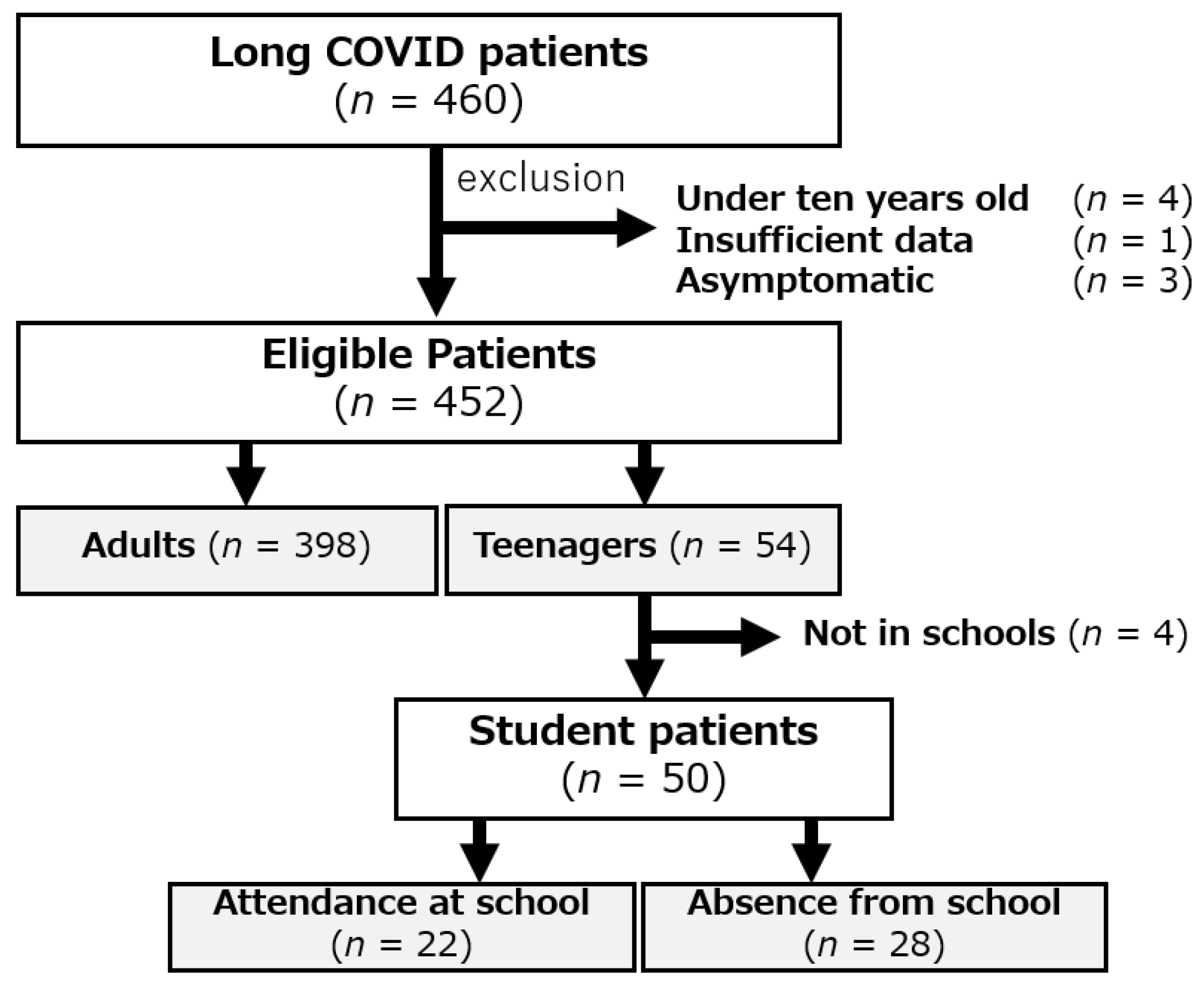
2. Patients and Methods
2.1. Study Design and Patients’ Characteristics
2.2. Definitions of the Delta Variant and Omicron Variant Periods
2.3. Statistical Analysis
2.4. Ethical Approval
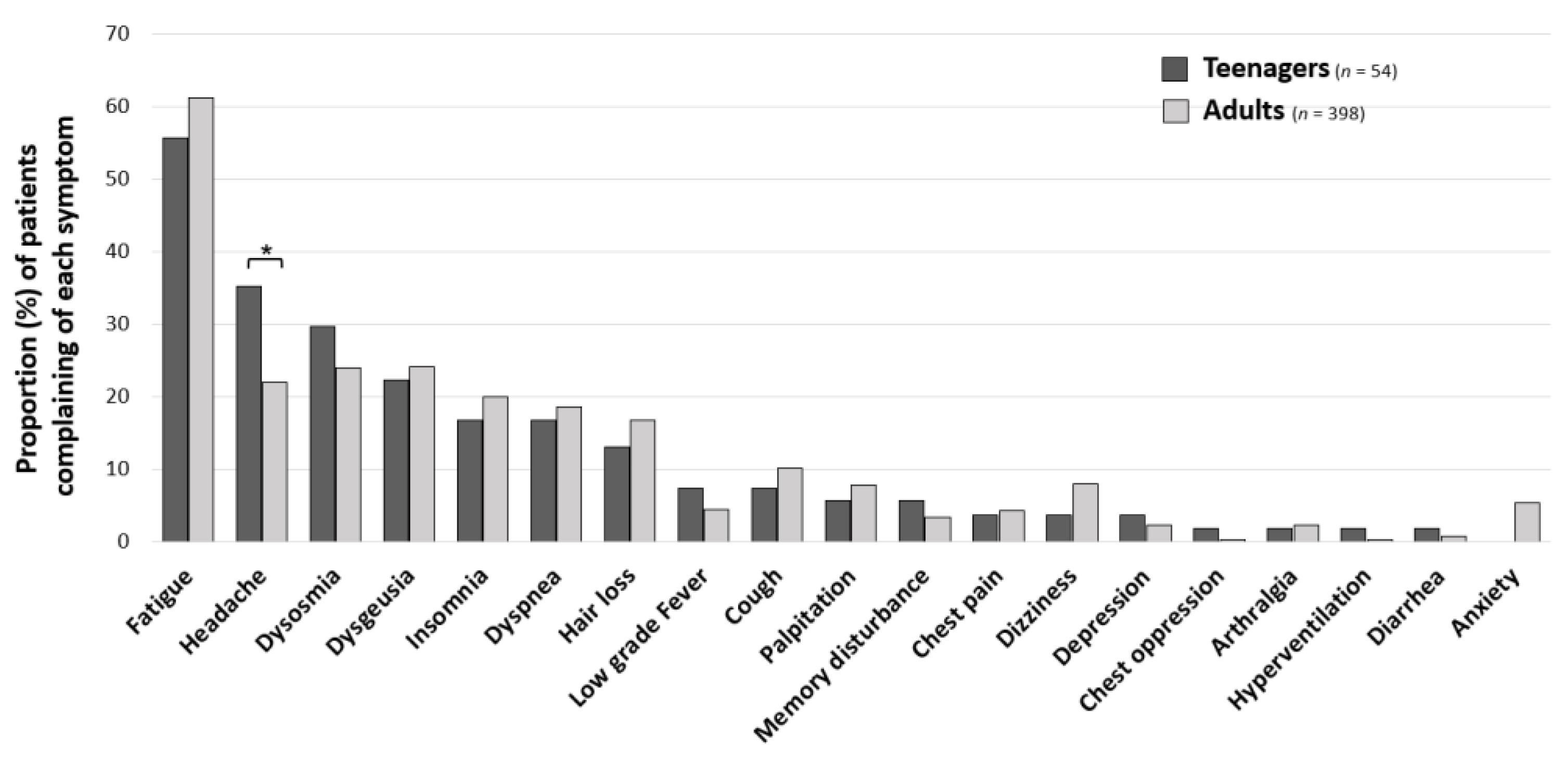
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Pittet, L.F.; Curtis, N. How Common is Long COVID in Children and Adolescents? Pediatr. Infect. Dis. J. 2021, 40, e482–e487. [Google Scholar] [CrossRef]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef] [PubMed]
- Fainardi, V.; Meoli, A.; Chiopris, G.; Motta, M.; Skenderaj, K.; Grandinetti, R.; Bergomi, A.; Antodaro, F.; Zona, S.; Esposito, S. Long COVID in Children and Adolescents. Life 2022, 12, 285. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, N.; Esang, M. Long COVID in Children and Adolescents. Prim. Care Companion CNS Disord. 2022, 24, 40720. [Google Scholar] [CrossRef]
- Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Honda, H.; Sakurada, Y.; Sunada, N.; Omura, D.; Hasegawa, K.; Hagiya, H.; Obika, M.; et al. Clinical Characteristics of Japanese Patients Who Visited a COVID-19 Aftercare Clinic for Post-Acute Sequelae of COVID-19/Long COVID. Cureus 2021, 13, e18568. [Google Scholar] [CrossRef]
- Sakurada, Y.; Sunada, N.; Honda, H.; Tokumasu, K.; Otsuka, Y.; Nakano, Y.; Hanayama, Y.; Furukawa, M.; Hagiya, H.; Otsuka, F. Serial Changes of Long COVID Symptoms and Clinical Utility of Serum Antibody Titers for Evaluation of Long COVID. J. Clin. Med. 2022, 11, 1309. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. Case Management of COVID-19 (Secondary Version). JMA J. 2021, 4, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Otsuka, Y.; Honda, H.; Sunada, N.; Tokumasu, K.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; Ochi, K.; Hagiya, H.; et al. Transitional Changes in Fatigue-Related Symptoms Due to Long COVID: A Single-Center Retrospective Observational Study in Japan. Medicina 2022, 58, 1393. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Chiappini, E.; Licari, A.; Galli, L.; Marseglia, G.L. Prevalence and clinical presentation of long COVID in children: A systematic review. Eur. J. Pediatr. 2022, 181, 3995–4009. [Google Scholar] [CrossRef]
- Roge, I.; Smane, L.; Kivite-Urtane, A.; Pucuka, Z.; Racko, I.; Klavina, L.; Pavare, J. Comparison of Persistent Symptoms after COVID-19 and Other Non-SARS-CoV-2 Infections in Children. Front. Pediatr. 2021, 9, 752385. [Google Scholar] [CrossRef]
- Radtke, T.; Ulyte, A.; Puhan, M.A.; Kriemler, S. Long-term Symptoms after SARS-CoV-2 Infection in Children and Adolescents. JAMA 2021, 326, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. Eur. J. Pediatr. 2022, 181, 1597–1607. [Google Scholar] [CrossRef]
- Shoji, K.; Akiyama, T.; Tsuzuki, S.; Matsunaga, N.; Asai, Y.; Suzuki, S.; Iwamoto, N.; Funaki, T.; Ohmagari, N. Clinical characteristics of COVID-19 in hospitalized children during the Omicron variant predominant period. J. Infect. Chemother. 2022, 28, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef]
- Parslow, R.M.; Harris, S.; Broughton, J.; Alattas, A.; Crawley, E.; Haywood, K.; Shaw, A. Children’s experiences of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review and meta-ethnography of qualitative studies. BMJ Open 2017, 7, e012633. [Google Scholar] [CrossRef]
- Morrow, A.K.; Ng, R.; Vargas, G.; Jashar, D.T.; Henning, E.; Stinson, N.; Malone, L.A. Postacute/Long COVID in Pediatrics: Development of a Multidisciplinary Rehabilitation Clinic and Preliminary Case Series. Am. J. Phys. Med. Rehabil. 2021, 100, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Faedda, N.; Cerutti, R.; Verdecchia, P.; Migliorini, D.; Arruda, M.; Guidetti, V. Behavioral management of headache in children and adolescents. J. Headache Pain 2016, 17, 80. [Google Scholar] [CrossRef] [Green Version]
- Tokumasu, K.; Honda, H.; Sunada, N.; Sakurada, Y.; Matsuda, Y.; Yamamoto, K.; Nakano, Y.; Hasegawa, T.; Yamamoto, Y.; Otsuka, Y.; et al. Clinical Characteristics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Diagnosed in Patients with Long COVID. Medicina 2022, 58, 850. [Google Scholar] [CrossRef]
- Kikkenborg Berg, S.; Dam Nielsen, S.; Nygaard, U.; Bundgaard, H.; Palm, P.; Rotvig, C.; Vinggaard Christensen, A. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Meherali, S.; Punjani, N.; Louie-Poon, S.; Abdul Rahim, K.; Das, J.K.; Salam, R.A.; Lassi, Z.S. Mental Health of Children and Adolescents Amidst COVID-19 and Past Pandemics: A Rapid Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3432. [Google Scholar] [CrossRef] [PubMed]
- Tso, W.W.Y.; Wong, R.S.; Tung, K.T.S.; Rao, N.; Fu, K.W.; Yam, J.C.S.; Chua, G.T.; Chen, E.Y.H.; Lee, T.M.C.; Chan, S.K.W.; et al. Vulnerability and resilience in children during the COVID-19 pandemic. Eur. Child Adolesc. Psychiatry 2022, 31, 161–176. [Google Scholar] [CrossRef]
- Allen, C.W.; Diamond-Myrsten, S.; Rollins, L.K. School Absenteeism in Children and Adolescents. Am. Fam. Physician 2018, 98, 738–744. [Google Scholar]
- Norris, T.; Collin, S.M.; Tilling, K.; Nuevo, R.; Stansfeld, S.A.; Sterne, J.A.; Heron, J.; Crawley, E. Natural course of chronic fatigue syndrome/myalgic encephalomyelitis in adolescents. Arch. Dis. Child. 2017, 102, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Sunada, N.; Honda, H.; Nakano, Y.; Yamamoto, K.; Tokumasu, K.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; Otsuka, Y.; Obika, M.; et al. Hormonal trends in patients suffering from long COVID symptoms. Endocr. J. 2022, 69, 1173–1181. [Google Scholar] [CrossRef]
- Soejima, Y.; Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Harada, K.; Nakamoto, K.; Sunada, N.; Sakurada, Y.; Hasegawa, K.; Hagiya, H.; et al. Late-Onset Hypogonadism in a Male Patient with Long COVID Diagnosed by Exclusion of ME/CFS. Medicina 2022, 58, 536. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Otsuka, Y.; Sunada, N.; Tokumasu, K.; Nakano, Y.; Honda, H.; Sakurada, Y.; Hagiya, H.; Hanayama, Y.; Otsuka, F. Detection of Male Hypogonadism in Patients with Post COVID-19 Condition. J. Clin. Med. 2022, 11, 1955. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Otsuka, F. Possibility of endocrine dysfunction in post coronavirus disease 2019 (COVID-19) condition. Endocr. J. 2022, 69, 1357. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Chew-Graham, C.A.; Briggs, T.A.; Kane, B. Long COVID in children and young people: Uncertainty and contradictions. Br. J. Gen. Pract. 2022, 72, 253–254. [Google Scholar] [CrossRef]


| Teenagers (n = 54) | Adults (n = 398) | p-Value | |
|---|---|---|---|
| Age years, mean (SD) | |||
| 15.3 (1.8) | 42.9 (13.7) | <0.001 (a) ** | |
| Gender, n (%) | |||
| Male | 23 (42.6) | 183 (46) | 0.639 (b) |
| Female | 31 (57.4) | 215 (54.1) | |
| Symptom onset phase, n (%) | |||
| Preceding phase | 9 (16.7) | 102 (25.6) | 0.253 (b) |
| Delta variant phase | 15 (27.8) | 117 (29.4) | |
| Omicron variant phase | 30 (55.6) | 179 (45) | |
| Period from the onset until the first visit, n (%) | |||
| <60 days | 14 (25.9) | 129 (32.4) | 0.289 (b) |
| 60~90 days | 19 (35.2) | 101 (25.4) | |
| >90 | 21 (38.9) | 168 (42.2) | |
| Severity of COVID-19 in acute phase, n (%) | |||
| Mild | 54 (100) | 321 (80.7) | <0.001 (b) ** |
| Moderate–Severe | 0 (0) | 77 (19.4) | |
| Attendance at School (n = 22) | Absence from School (n = 28) | p-Value | |
|---|---|---|---|
| Age years, mean (SD) | |||
| 14.7 (2) | 15.4 (1.4) | 0.556 (a) | |
| Gender, n (%) | |||
| Male | 11 (50) | 10 (35.7) | 0.310 (b) |
| Female | 11 (50) | 18 (64.3) | |
| Symptom onset phase, n (%) | |||
| Preceding phase | 2 (9.1) | 6 (21.4) | 0.214 (b) |
| Delta variant phase | 9 (40.9) | 6 (21.4) | |
| Omicron variant phase | 11 (50) | 16 (57.1) | |
| Period from the onset until the first visit, n (%) | |||
| <60 days | 4 (18.2) | 10 (35.7) | 0.159 (b) |
| <60 days | 4 (18.2) | 10 (35.7) | 0.159 (b) |
| 60~90 days | 7 (31.8) | 11 (39.3) | |
| >90 | 11 (50) | 7 (25) | |
| Vaccination, n (%) | |||
| <2 times | 11 (50) | 12 (42.9) | 0.696 (b) |
| 2 times | 10 (45.5) | 13 (46.4) | |
| >3 times | 1 (4.6) | 3 (10.7) | |
| School, n (%) | |||
| Elementary school | 3 (13.6) | 1 (3.6) | 0.294 (b) |
| Junior high school | 8 (36.4) | 8 (28.6) | |
| High school | 11 (50) | 19 (67.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurada, Y.; Otsuka, Y.; Tokumasu, K.; Sunada, N.; Honda, H.; Nakano, Y.; Matsuda, Y.; Hasegawa, T.; Ochi, K.; Hagiya, H.; et al. Trends in Long COVID Symptoms in Japanese Teenage Patients. Medicina 2023, 59, 261. https://doi.org/10.3390/medicina59020261
Sakurada Y, Otsuka Y, Tokumasu K, Sunada N, Honda H, Nakano Y, Matsuda Y, Hasegawa T, Ochi K, Hagiya H, et al. Trends in Long COVID Symptoms in Japanese Teenage Patients. Medicina. 2023; 59(2):261. https://doi.org/10.3390/medicina59020261
Chicago/Turabian StyleSakurada, Yasue, Yuki Otsuka, Kazuki Tokumasu, Naruhiko Sunada, Hiroyuki Honda, Yasuhiro Nakano, Yui Matsuda, Toru Hasegawa, Kanako Ochi, Hideharu Hagiya, and et al. 2023. "Trends in Long COVID Symptoms in Japanese Teenage Patients" Medicina 59, no. 2: 261. https://doi.org/10.3390/medicina59020261





