Culprit versus Complete Revascularization during the Initial Intervention in Patients with Acute Coronary Syndrome Using a Virtual Treatment Planning Tool: Results of a Single-Center Pilot Study
Abstract
:1. Introduction
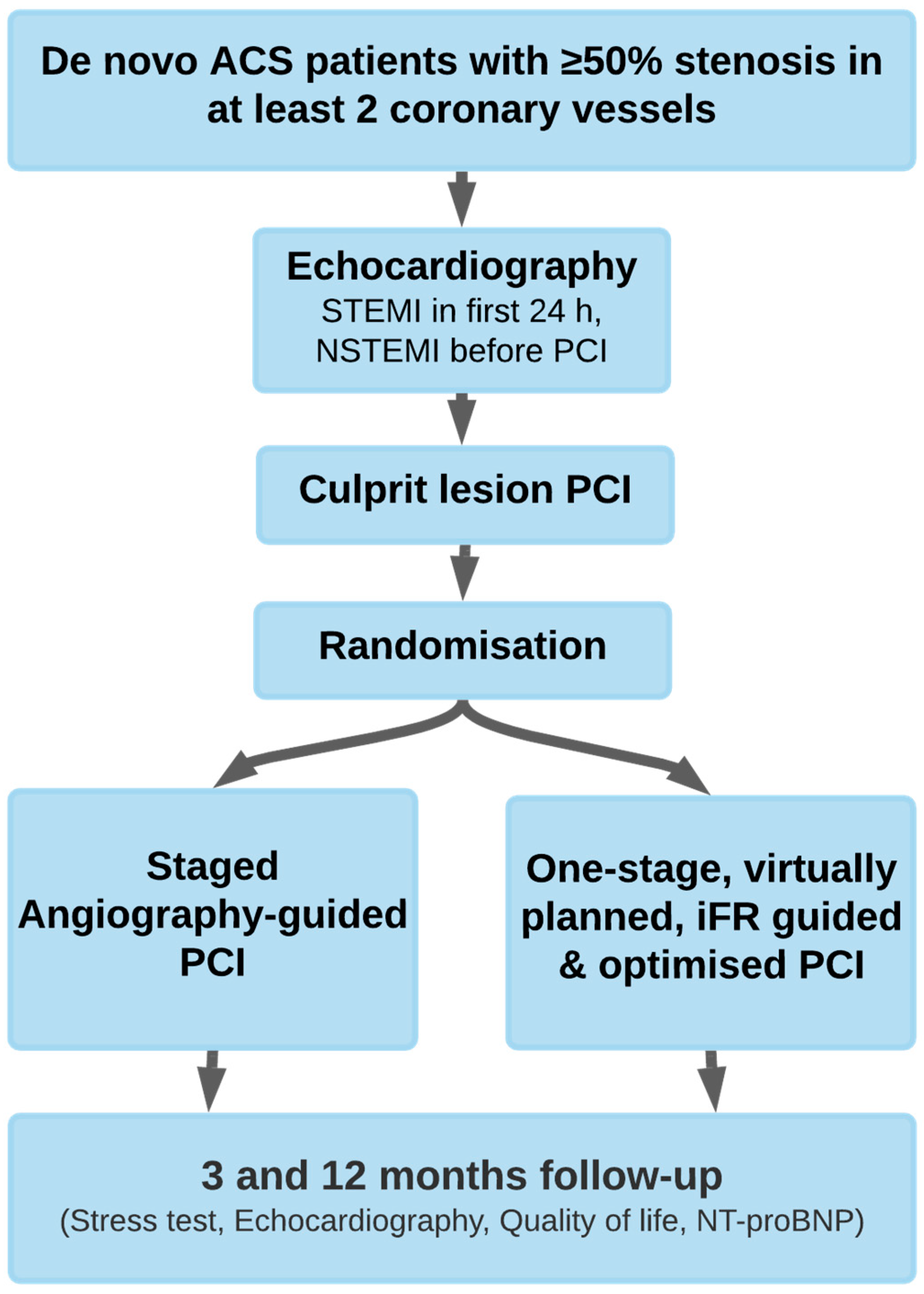
2. Materials and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Instantaneous Wave-Free Ratio Measurements
2.4. Primary Safety Endpoints
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, K.; Lyu, S.; Song, X.; Liu, H.; Yuan, F.; Xu, F.; Zhang, M.; Wang, W.; Zhang, M.; Zhang, D.; et al. Long-Term safety and efficacy of staged percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction and multivessel coronary disease. Am. J. Cardiol. 2019, 124, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Sharma, P.K.; Cohen, D.J.; Fonarow, G.C.; Kaltenbach, L.A.; Effron, M.B.; Zettler, M.E.; Peterson, E.D.; Wang, T.Y. Multivessel versus culprit vessel-only percutaneous coronary intervention among patients with acute myocardial infarction: Insights from the TRANSLATE-ACS observational study. J. Am. Heart Assoc. 2017, 6, e006343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.W.; Clare, R.M.; Schulte, P.J.; Pieper, K.S.; Shaw, L.K.; Califf, R.M.; Magnus Ohman, E.; Van De Werf, F.; Hirji, S.; Harrington, R.A.; et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA 2014, 312, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.A.W.; Mishra, A.; Worthley, M.I.; Nelson, A.J.; Psaltis, P.J. Management of multivessel coronary artery disease in patients with non-ST-elevation myocardial infarction: A complex path to precision medicine. Ther. Adv. Chronic Dis. 2020, 11, 2040622320938527. [Google Scholar] [CrossRef]
- Atti, V.; Gwon, Y.; Narayanan, M.A.; Garcia, S.; Sandoval, Y.; Brilakis, E.S.; Basir, M.B.; Turagam, M.K.; Khandelwal, A.; Mena-Hurtado, C.; et al. Multivessel versus culprit-only revascularization in STEMI and multivessel coronary artery disease: Meta-analysis of randomized trials. JACC Cardiovasc. Interv. 2020, 13, 1571–1582. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Bauersachs, J.; Dendale, P.; Edvardsen, T.; Gale, C.P.; Jobs, A.; Lambrinou, E.; Mehilli, J.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.C.; Hyun, J.Y.; Ahn, Y.; Bae, S.; Hyun, D.Y.; Cho, K.H.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; Jeong, M.H.; et al. Optimal revascularization strategy in non–ST-segment–elevation myocardial infarction with multivessel coronary artery disease: Culprit-only versus one-stage versus multistage revascularization. J. Am. Heart Assoc. 2020, 9, e016575. [Google Scholar] [CrossRef]
- Khan, M.S.; Khan, A.R.; Khan, A.I.; Seo, M.; Yasmin, F.; Usman, M.S.; Moustafa, A.; Schmid, C.H.; Kalra, A.; Ikram, S. Comparison of revascularization strategies in patients with acute coronary syndrome and multivessel coronary disease: A systematic review and network meta-analysis. Catheter. Cardiovasc. Interv. 2020, 96, E447–E454. [Google Scholar] [CrossRef]
- Rai, D.; Tahir, M.W.; Bandyopadhyay, D.; Chowdhury, M.; Kharsa, A.; Pendala, V.S.; Ali, H.; Naidu, S.S.; Baibhav, B. Meta-analysis and trial sequential analysis of randomized controlled trials for multivessel PCI versus culprit artery only PCI in STEMI without cardiogenic shock. Curr. Probl. Cardiol. 2021, 46, 100646. [Google Scholar] [CrossRef] [PubMed]
- Bryer, E.; Stein, E.; Goldberg, S. Multivessel coronary artery disease: The limitations of a “one-size-fits-all” approach. Mayo Clin Proc. Innov. Qual. Outcomes 2020, 4, 638–641. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Xu, Q.; Chen, X. Staged versus one-time complete revascularization with percutaneous coronary intervention in STEMI patients with multivessel disease: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169406. [Google Scholar] [CrossRef] [Green Version]
- Pimor, A.; Auffret, V.; Didier, R.; Delaunay, R.; Filippi, E.; Hacot, J.P.; Saouli, D.; Rouault, G.; Druelles, P.; Bot, E.; et al. Immediate complete revascularization in patients with ST-segment elevation myocardial infarction and multivessel disease treated by primary percutaneous coronary intervention: Insights from the ORBI registry. Arch. Cardiovasc. Dis. 2018, 111, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kobayashi, Y. Percutaneous coronary intervention strategies in patients with acute myocardial infarction and multivessel disease: Completeness, timing, lesion assessment, and patient status. J. Cardiol. 2019, 74, 95–101. [Google Scholar] [CrossRef]
- Ong, P.; Sechtem, U. Controversies in the treatment of patients with STEMI and multivessel disease: Is it time for PCI of all lesions? Clin. Res. Cardiol. 2016, 105, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.R.; Bossard, M. Acute coronary syndromes and multivessel disease: Completing the dvidence. JACC Cardiovasc. Interv. 2020, 13, 1568–1570. [Google Scholar] [CrossRef]
- Michail, M.; Thakur, U.; Mehta, O.; Ramzy, J.M.; Comella, A.; Ihdayhid, A.R.; Cameron, J.D.; Nicholls, S.J.; Hoole, S.P.; Brown, A.J. Non-hyperaemic pressure ratios to guide percutaneous coronary intervention. Open Heart 2020, 7, e001308. [Google Scholar] [CrossRef]
- Baumann, S.; Chandra, L.; Skarga, E.; Renker, M.; Borggrefe, M.; Akin, I.; Lossnitzer, D. Instantaneous wave-free ratio (iFR®) to determine hemodynamically significant coronary stenosis: A comprehensive review. World J. Cardiol. 2018, 10, 267–277. [Google Scholar] [CrossRef]
- Younus, M.; Seto, A.H. Clinical outcomes data for instantaneous wave-free ratio-guided percutaneous coronary intervention. Interv. Cardiol. Clin. 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Indolfi, C.; Mongiardo, A.; Spaccarotella, C.; Torella, D.; Caiazzo, G.; Polimeni, A.; Sorrentino, S.; Micieli, M.; Sabatino, J.; Curcio, A.; et al. The instantaneous wave-free ratio (iFR) for evaluation of non-culprit lesions in patients with acute coronary syndrome and multivessel disease. Int. J. Cardiol. 2015, 178, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Meucci, M.C.; Niccoli, G. The management of non-culprit coronary lesions in patients with acute coronary syndrome. Eur. Heart J. 2021, 22, L170–L175. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Jeremias, A.; Maehara, A.; Matsumura, M.; Zhang, Z.; Schneider, J.; Tang, K.; Talwar, S.; Marques, K.; Shammas, N.W.; et al. 1-Year Outcomes of Blinded Physiological Assessment of Residual Ischemia After Successful PCI. J. Am. Coll. Cardiol. Interv. 2022, 15, 52–61. [Google Scholar] [CrossRef]
- Tonino, P.A.L.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U.; Ikeno, F.; Vant Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [Green Version]
- AL-Obaidi, F.R.; Fearon, W.F.; Yong, A.S.C. Invasive physiological indices to determine the functional significance of coronary stenosis. IJC Heart Vasc. 2018, 18, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Lee, J.M.; Kim, H.K.; Kim, J.; Park, J.; Hwang, D.; Rhee, T.M.; Park, T.K.; Yang, J.H.; Song YBin Shin, E.S.; et al. Fractional flow reserve and instantaneous wave-free ratio for nonculprit stenosis in patients with acute myocardial infarction. JACC Cardiovasc. Interv. 2018, 11, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Shah, R. Accuracy of fractional flow reserve during acute myocardial infarction. Eur. Heart J. 2020, 41, 2597. [Google Scholar] [CrossRef]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.-J.; Boxma-de Klerk, B.M.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. Fractional flow reserve–guided multivessel angioplasty in myocardial infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; Clemmensen, P.; et al. Complete revascularization versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): An open-label, randomised controlled trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Layland, J.; Oldroyd, K.G.; Curzen, N.; Sood, A.; Balachandran, K.; Das, R.; Junejo, S.; Ahmed, N.; Lee, M.M.Y.; Shaukat, A.; et al. Fractional flow reserve vs. Angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: The British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur. Heart J. 2015, 36, 100–111. [Google Scholar] [CrossRef]
- Puymirat, E.; Cayla, G.; Simon, T.; Steg, P.G.; Montalescot, G.; Durand-Zaleski, I.; Le Bras, A.; Gallet, R.; Khalife, K.; Morelle, J.-F.; et al. Multivessel PCI guided by FFR or angiography for myocardial infarction. N. Engl. J. Med. 2021, 385, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Musto, C.; De Felice, F.; Rigattieri, S.; Chin, D.; Marra, A.; Nazzaro, M.S.; Cifarelli, A.; Violini, R. Instantaneous wave-free ratio and fractional flow reserve for the assessment of nonculprit lesions during the index procedure in patients with ST-segment elevation myocardial infarction: The WAVE study. Am. Heart J. 2017, 193, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Götberg, M.; Christiansen, E.H.; Gudmundsdottir, I.; Sandhall, L.; Omerovic, E.; James, S.K.; Erlinge, D.; Fröbert, O. Instantaneous wave-free ratio versus fractional flow reserve guided intervention (iFR-SWEDEHEART): Rationale and design of a multicenter, prospective, registry-based randomized clinical trial. Am. Heart J. 2015, 170, 945–950. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Garcia-Garcia, H.M.; Scarsini, R.; Hideo-Kajita, A.; Gonzalo López, N.; Leone, A.M.; Sarno, G.; Daemen, J.; Shlofmitz, E.; Jeremias, A.; et al. Novel indices of coronary physiology: Do we need alternatives to fractional flow reserve? Circ. Cardiovasc. Interv. 2020, 13, e008487. [Google Scholar] [CrossRef] [PubMed]
- Götberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.-E.; Öhagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; Tarkin, J.; Petraco, R.; Broyd, C.; Jabbour, R.; et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: Results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef] [Green Version]
- Nijjer, S.S.; Sen, S.; Petraco, R.; Mayet, J.; Francis, D.P.; Davies, J.E.R. The Instantaneous wave-Free Ratio (iFR) pullback: A novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovasc. Revascularization Med. 2015, 16, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Jeremias, A.; Davies, J.E.; Maehara, A.; Matsumura, M.; Schneider, J.; Tang, K.; Talwar, S.; Marques, K.; Shammas, N.W.; Gruberg, L.; et al. Blinded physiological assessment of residual ischaemia after successful angiographic percutaneous coronary intervention: The DEFINE PCI Study. JACC Cardiovasc. Interv. 2019, 12, 1991–2001. [Google Scholar] [CrossRef]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Pasquale GDi López-Sendón, J.; Faxon, D.P.; Mauri, L.; et al. Complete revascularization with multivessel PCI for myocardial infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef] [Green Version]
- Wald, D.S.; Morris, J.K.; Wald, N.J.; Chase, A.J.; Edwards, R.J.; Hughes, L.O.; Berry, C.; Oldroyd, K.G. Randomized trial of preventive angioplasty in myocardial infarction. N. Engl. J. Med. 2013, 369, 1115–1123. [Google Scholar] [CrossRef]
- Rathod, K.S.; Koganti, S.; Jain, A.K.; Astroulakis, Z.; Lim, P.; Rakhit, R.; Kalra, S.S.; Dalby, M.C.; O’Mahony, C.; Malik, I.S.; et al. Complete versus culprit-only lesion intervention in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2018, 72, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Mahmoud, A.N.; Kumbhani, D.J.; Bhatt, D.L.; Bavry, A.A. Complete or culprit-only revascularization for patients With multivessel coronary artery disease undergoing percutaneous coronary intervention: A pairwise and network meta-analysis of randomized trials. JACC Cardiovasc. Interv. 2017, 10, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gaffar, R.; Habib, B.; Filion, K.B.; Reynier, P.; Eisenberg, M.J. Optimal timing of complete revascularization in acute coronary syndrome: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e005381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardella, G.; Lucisano, L.; Garbo, R.; Pennacchi, M.; Cavallo, E.; Stio, R.E.; Calcagno, S.; Ugo, F.; Boccuzzi, G.; Fedele, F.; et al. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients: The SMILE trial. J. Am. Coll. Cardiol. 2016, 67, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershlick, A.H.; Khan, J.N.; Kelly, D.J.; Greenwood, J.P.; Sasikaran, T.; Curzen, N.; Blackman, D.J.; Dalby, M.; Fairbrother, K.L.; Banya, W.; et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for stemi and multivessel disease: The CvLPRIT trial. J. Am. Coll. Cardiol. 2015, 65, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Vlaar, P.J.; Mahmoud, K.D.; Holmes, D.R.; Van Valkenhoef, G.; Hillege, H.L.; Van der Horst, I.C.; Zijlstra, F.; De Smet, B.J.G.L. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: A pairwise and network meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 692–703. [Google Scholar] [CrossRef]

| Baseline Variable | iFR-Guided Group (n = 9) | Angiography-Guided Group (n = 8) | p-Value |
|---|---|---|---|
| NSTEMI + UA | 5 (55.6%) | 7 (87.5%) | 0.294 |
| STEMI | 4 (44.4%) | 1 (12.5%) | 0.294 |
| Female | 2 (22.2%) | 5 (62.5%) | 0.153 |
| Male | 7 (77.8%) | 3 (37.5%) | 0.153 |
| Age, mean years | 62.7 ± 4.5 | 69.6 ± 8.2 | 0.046 |
| BMI, mean (kg/m2) | 26.1 ± 4.8 | 29.3 ± 4.1 | 0.236 |
| Smokers | 5 (55.6%) | 4 (50.0%) | 0.637 |
| Pack-years, mean | 25.4 ± 19.7 | 20.6 ± 23.1 | 0.727 |
| History of hypertension | 5 (55.6%) | 7 (87.5%) | 0.294 |
| History of diabetes mellitus | 0 | 3 (37.5%) | 0.082 |
| History of congestive heart failure | 6 (66.7%) | 3 (37.5%) | 0.347 |
| NT-proBNP, mean (pg/mL) | 1270 ± 800.8 | 2054 ± 1721.6 | 0.386 |
| Creatinine, mean (µmol/L) | 73.7 ± 22.3 | 86 ± 26.6 | 0.148 |
| Hgb, mean (g/dL) | 13 ± 1.1 | 12 ± 2.4 | 0.643 |
| HbA1c, mean (%) | 5.86 ± 0.5 | 7 ± 1.8 | 0.051 |
| Peak index hs-troponin I (pg/mL) | 2227 ± 1486.8 | 3886 ± 3816.9 | 0.685 |
| LDL cholesterol, mean (mmol/L) | 4.13 ± 1.3 | 3.42 ± 1.5 | 0.290 |
| Total cholesterol, mean (mmol/L) | 5.28 ± 1.3 | 5.55 ± 0.7 | 0.558 |
| HDL cholesterol, mean (mmol/L) | 1.05 ± 0.3 | 1.14 ± 0.3 | 0.773 |
| Index EF, mean (%) | 51.9 ± 19.9 | 57.4 ± 11.4 | 0.314 |
| Procedural Outcomes | Angiography-Guided Group (n = 8) | iFR-Guided Group (n = 9) | p-Value |
|---|---|---|---|
| Index PCI stent length used (mm) | 39.9 ± 19 | 55.2 ± 31 | 0.335 |
| Index PCI Number of stents used | 1.5 ± 0.5 | 1.89 ± 1.1 | 0.526 |
| Index PCI volume of contrast media (mL) | 192.5 ± 52.8 | 360 ± 97.9 | 0.003 |
| Index PCI duration (minutes) | 47 ± 15.5 | 119.4 ± 40.7 | 0.004 |
| Index hospital stay (days) | 5 ± 1.6 | 4.8 ± 1.1 | 0.878 |
| Index PCI related MACE | Median = 0.0 IQR = 0.0–0.0 | Median = 0.0 IQR = 0.0–0.5 | 0.168 |
| Total stent length used (mm) | 67.6 ± 42.7 | 55.2 ± 31 | 0.441 |
| Total number of stents used for complete revascularization | 2.8 ± 1.3 | 1.9 ± 1.1 | 0.134 |
| Total volume of contrast media (mL) | 313.8 ± 164.4 | 360 ± 97.9 | 0.563 |
| Total PCI duration (minutes) | 101 ± 55 | 119 ± 40 | 0.563 |
| Total hospital stay (days) | 6.5 ± 2.3 | 4.8 ± 1.1 | 0.058 |
| Total PCI number till complete revascularization achieved | 1.8 ± 0.7 | 1.0 ± 0.0 | 0.007 |
| Total PCI related MACE | Median = 0.0 IQR = 0.0–0.0 | Median = 0.0 IQR = 0.0–0.5 | 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiljevs, D.; Kakurina, N.; Pontaga, N.; Kokina, B.; Osipovs, V.; Sorokins, N.; Pikta, S.; Trusinskis, K.; Lejnieks, A. Culprit versus Complete Revascularization during the Initial Intervention in Patients with Acute Coronary Syndrome Using a Virtual Treatment Planning Tool: Results of a Single-Center Pilot Study. Medicina 2023, 59, 270. https://doi.org/10.3390/medicina59020270
Vasiljevs D, Kakurina N, Pontaga N, Kokina B, Osipovs V, Sorokins N, Pikta S, Trusinskis K, Lejnieks A. Culprit versus Complete Revascularization during the Initial Intervention in Patients with Acute Coronary Syndrome Using a Virtual Treatment Planning Tool: Results of a Single-Center Pilot Study. Medicina. 2023; 59(2):270. https://doi.org/10.3390/medicina59020270
Chicago/Turabian StyleVasiljevs, Deniss, Natalja Kakurina, Natalja Pontaga, Baiba Kokina, Vladimirs Osipovs, Nikolajs Sorokins, Sergejs Pikta, Karlis Trusinskis, and Aivars Lejnieks. 2023. "Culprit versus Complete Revascularization during the Initial Intervention in Patients with Acute Coronary Syndrome Using a Virtual Treatment Planning Tool: Results of a Single-Center Pilot Study" Medicina 59, no. 2: 270. https://doi.org/10.3390/medicina59020270







