Achieving Precise Cup Positioning in Direct Anterior Total Hip Arthroplasty: A Narrative Review
Abstract
1. Introduction
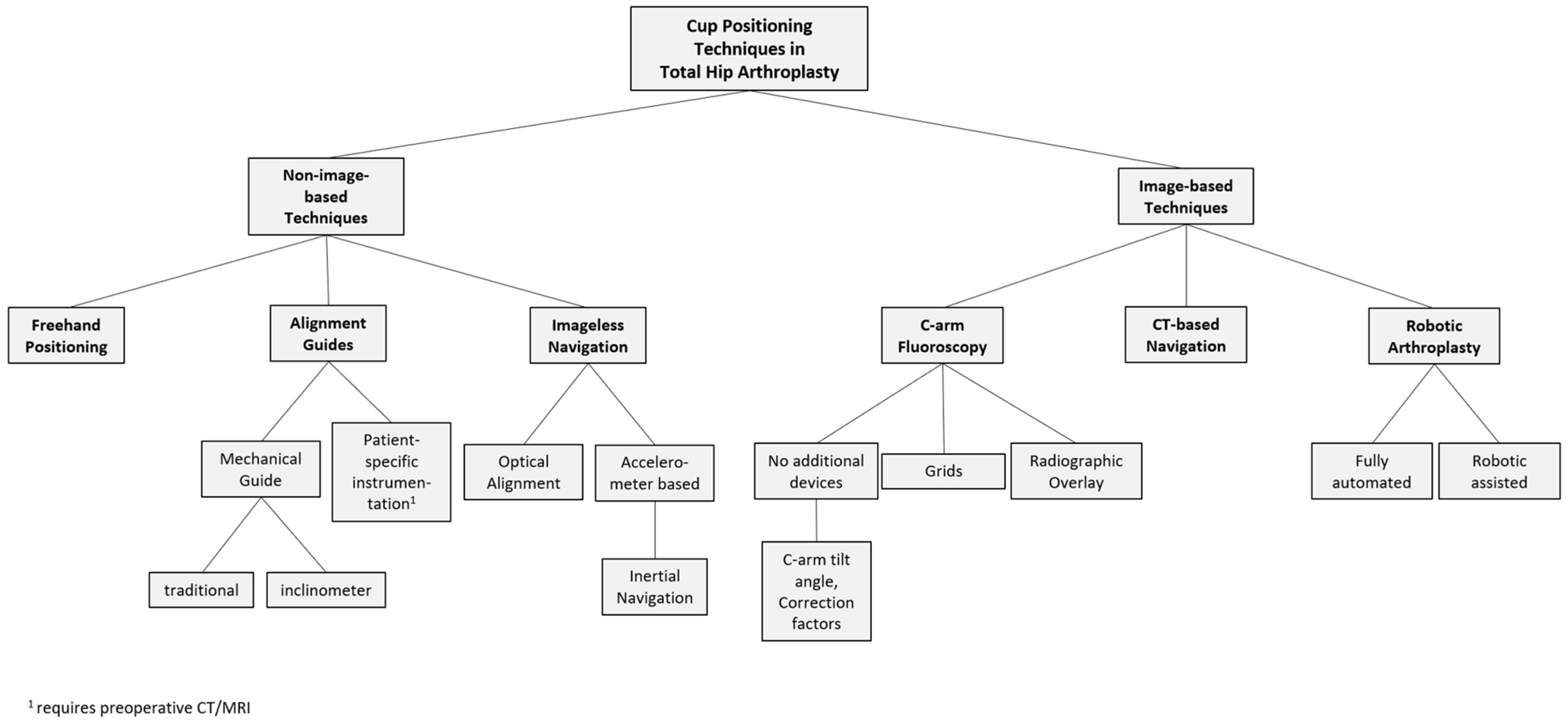
2. Cup Positioning Techniques
2.1. Freehand Positioning Based on Anatomic Landmarks
2.2. Positioning with Intraoperative C-Arm Imaging
2.2.1. C-Arm Imaging without Additional Devices
2.2.2. iPad App
2.2.3. Grids
2.2.4. Radiographic Overlay Technique
2.3. Mechanical Alignment Guides
2.4. Patient-Specific Instrumentation
2.5. Navigation Systems without Imaging
2.5.1. Optical Alignment
2.5.2. Accelerometer Based Alignment
2.5.3. Inertial Navigation Systems
2.6. CT-Based Navigation
2.7. Robotic Total Hip Arthroplasty
3. Discussion
3.1. Freehand Positioning and Mechanical Alignment Guides
3.2. Challenges in Intraoperative Fluoroscopy and How to Address Them
3.2.1. Grids
3.2.2. Radiographic Overlay Technique
3.2.3. Fluoroscopy without Additional Applications
3.3. Imageless Navigation
3.4. Robotic-Assisted Hip Replacements
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, M.; Craiovan, B.; Woerner, M.L.; Schwarz, T.; Grifka, J.; Renkawitz, T.F. Predictors of Outcome After Primary Total Joint Replacement. J. Arthroplast. 2018, 33, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Bota, N.C.; Nistor, D.-V.; Caterev, S.; Todor, A. Historical overview of hip arthroplasty: From humble beginnings to a high-tech future. Orthop. Rev. 2021, 13, 8773. [Google Scholar] [CrossRef] [PubMed]
- Daines, B.K.; Dennis, D.A. The importance of acetabular component position in total hip arthroplasty. Orthop. Clin. North Am. 2012, 43, e23–e34. [Google Scholar] [CrossRef] [PubMed]
- Dora, C.; Houweling, M.; Koch, P.; Sierra, R.J. Iliopsoas impingement after total hip replacement: The results of non-operative management, tenotomy or acetabular revision. J. Bone Jt. Surg. Br. 2007, 89, 1031–1035. [Google Scholar] [CrossRef]
- Patil, S.; Bergula, A.; Chen, P.C.; Colwell, C.W., Jr.; D’Lima, D.D. Polyethylene wear and acetabular component orientation. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. S4), 56–63. [Google Scholar] [CrossRef]
- Weber, M.; Woerner, M.; Craiovan, B.; Voellner, F.; Worlicek, M.; Springorum, H.-R.; Grifka, J.; Renkawitz, T. Current standard rules of combined anteversion prevent prosthetic impingement but ignore osseous contact in total hip arthroplasty. Int. Orthop. 2016, 40, 2495–2504. [Google Scholar] [CrossRef]
- Lewinnek, G.E.; Lewis, J.L.; Tarr, R.; Compere, C.L.; Zimmerman, J.R. Dislocations after total hip-replacement arthroplasties. J. Bone Jt. Surg. Am. 1978, 60, 217–220. [Google Scholar] [CrossRef]
- Leichtle, U.G.; Leichtle, C.I.; Taslaci, F.; Reize, P.; Wünschel, M. Dislocation after total hip arthroplasty: Risk factors and treatment options. Acta Orthop. Traumatol. Turc. 2013, 47, 96–103. [Google Scholar] [CrossRef]
- McLawhorn, A.S.; Sculco, P.K.; Weeks, K.D.; Nam, D.; Mayman, D.J. Targeting a New Safe Zone: A Step in the Development of Patient-Specific Component Positioning for Total Hip Arthroplasty. Am. J. Orthop. 2015, 44, 270–276. [Google Scholar]
- Biedermann, R.; Tonin, A.; Krismer, M.; Rachbauer, F.; Eibl, G.; Stöckl, B. Reducing the risk of dislocation after total hip arthroplasty: The effect of orientation of the acetabular component. J. Bone Jt. Surg. Br. 2005, 87, 762–769. [Google Scholar] [CrossRef]
- Danoff, J.R.; Bobman, J.T.; Cunn, G.; Murtaugh, T.; Gorroochurn, P.; Geller, J.A.; Macaulay, W. Redefining the Acetabular Component Safe Zone for Posterior Approach Total Hip Arthroplasty. J. Arthroplast. 2016, 31, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Opperer, M.; Lee, Y.; Nally, F.; Blanes Perez, A.; Goudarz-Mehdikhani, K.; Della Gonzalez Valle, A. A critical analysis of radiographic factors in patients who develop dislocation after elective primary total hip arthroplasty. Int. Orthop. 2016, 40, 703–708. [Google Scholar] [CrossRef]
- Seagrave, K.G.; Troelsen, A.; Malchau, H.; Husted, H.; Gromov, K. Acetabular cup position and risk of dislocation in primary total hip arthroplasty. Acta Orthop. 2017, 88, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; von Kunow, F.; Innmann, M.; Meyer, M.; Thieme, M.; Jerabek, S.; Renkawitz, T. Which Safe Zone Is Safe in Total Hip Arthroplasty? The Effect of Bony Impingement. J. Pers. Med. 2022, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.-H.; Zurfluh, B. Compliant positioning of total hip components for optimal range of motion. J. Orthop. Res. 2004, 22, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Habor, J.; Fischer, M.C.M.; Tokunaga, K.; Okamoto, M.; Radermacher, K. The Patient-Specific Combined Target Zone for Morpho-Functional Planning of Total Hip Arthroplasty. J. Pers. Med. 2021, 11, 817. [Google Scholar] [CrossRef]
- Danaei, B.; McPhee, J. Model-Based Acetabular Cup Orientation Optimization Based on Minimizing the Risk of Edge-Loading and Implant Impingement Following Total Hip Arthroplasty. J. Biomech. Eng. 2022, 144, 111008. [Google Scholar] [CrossRef]
- Waldstein, W.; Merle, C.; Schmidt-Braekling, T.; Boettner, F. Does stem design influence component positioning in total hip arthroplasty using a minimal invasive posterolateral approach? Int. Orthop. 2014, 38, 1347–1352. [Google Scholar] [CrossRef]
- Beverland, D.E.; O’Neill, C.K.J.; Rutherford, M.; Molloy, D.; Hill, J.C. Placement of the acetabular component. Bone Jt. J. 2016, 98, 37–43. [Google Scholar] [CrossRef]
- Hill, J.C.; Gibson, D.P.; Pagoti, R.; Beverland, D.E. Photographic measurement of the inclination of the acetabular component in total hip replacement using the posterior approach. J. Bone Jt. Surg. Br. 2010, 92, 1209–1214. [Google Scholar] [CrossRef]
- Barrack, R.L.; Krempec, J.A.; Clohisy, J.C.; McDonald, D.J.; Ricci, W.M.; Ruh, E.L.; Nunley, R.M. Accuracy of acetabular component position in hip arthroplasty. J. Bone Jt. Surg. Am. 2013, 95, 1760–1768. [Google Scholar] [CrossRef]
- Crawford, D.A.; Adams, J.B.; Hobbs, G.R.; Lombardi, A.J.V., Jr.; Berend, K.R. Surgical Approach and Hip Laterality Affect Accuracy of Acetabular Component Placement in Primary Total Hip Arthroplasty. Surg. Technol. Int. 2019, 35, 377–385. [Google Scholar]
- Martin, J.R.; Masonis, J.L.; Mason, J.B. Anatomic Total Hip Component Position Is More Reproducible with the Direct Anterior Approach Using Intraoperative Fluoroscopy. Arthroplast. Today 2020, 6, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Rocca, A.; Gallinari, G.; Piscitello, S.; Klumpp, R. Targeting the safe zones for cup position without fluoroscopic guidance in total hip arthroplasty: Does the surgical approach affect the outcomes? Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1471–1476. [Google Scholar] [CrossRef]
- Jungwirth-Weinberger, A.; Schmidt-Braekling, T.; Rueckl, K.; Springer, B.; Boettner, F. Anterior hip replacement: Lower dislocation rates despite less restrictions? Arch. Orthop. Trauma Surg. 2022, 142, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Goyal, T.; Choudhury, A.K.; Paul, S.; Gupta, T.; Das, L. Acetabular and Femoral Component Positioning Using Direct Anterior Approach Versus Posterior Approach in Total Hip Arthroplasty. Indian J. Orthop. 2021, 55, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Brun, O.-C.L.; Sund, H.N.; Nordsletten, L.; Röhrl, S.M.; Mjaaland, K.E. Component Placement in Direct Lateral vs Minimally Invasive Anterior Approach in Total Hip Arthroplasty: Radiographic Outcomes from a Prospective Randomized Controlled Trial. J. Arthroplast. 2019, 34, 1718–1722. [Google Scholar] [CrossRef]
- Ha, Y.-C.; Yoo, J.J.; Lee, Y.-K.; Kim, J.Y.; Koo, K.-H. Acetabular component positioning using anatomic landmarks of the acetabulum. Clin. Orthop. Relat. Res. 2012, 470, 3515–3523. [Google Scholar] [CrossRef]
- Molho, D.A.; Kuether, J.P.; Rubin, L.E. Anatomic Navigation Using the Transverse Acetabular Ligament for Acetabular Component Positioning in Total Hip Arthroplasty Through the Direct Anterior Approach. J. Am. Acad. Orthop. Surg. 2022, 30, 100–103. [Google Scholar] [CrossRef]
- Sotereanos, N.G.; Miller, M.C.; Smith, B.; Hube, R.; Sewecke, J.J.; Wohlrab, D. Using intraoperative pelvic landmarks for acetabular component placement in total hip arthroplasty. J. Arthroplast. 2006, 21, 832–840. [Google Scholar] [CrossRef]
- Archbold, H.A.P.; Mockford, B.; Molloy, D.; McConway, J.; Ogonda, L.; Beverland, D. The transverse acetabular ligament: An aid to orientation of the acetabular component during primary total hip replacement: A preliminary study of 1000 cases investigating postoperative stability. J. Bone Jt. Surg. Br. 2006, 88, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Meermans, G.; Grammatopoulos, G.; Innmann, M.; Beverland, D. Cup placement in primary total hip arthroplasty: How to get it right without navigation or robotics. EFORT Open Rev. 2022, 7, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, M.E.; Elibrahimi, A.; Shimi, M.; Elmrini, A. Acetabular component orientation in total hip arthroplasty: The role of acetabular transverse ligament. Acta Ortop. Bras. 2016, 24, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Ding, Z.; Yuan, M.; Zhou, K.; Zhou, Z. The influence of sagittal pelvic malrotation on transverse acetabular ligament guided cup orientation: A retrospective cohort study. BMC Musculoskelet. Disord. 2021, 22, 495. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; DiGioia, A.M., 3rd; Mor, A.B.; Jaramaz, B. A kinematic model for calculating cup alignment error during total hip arthroplasty. J. Biomech. 2005, 38, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.W. The definition and measurement of acetabular orientation. J. Bone Jt. Surg. Br. 1993, 75, 228–232. [Google Scholar] [CrossRef]
- Frandsen, J.J.; Kahn, T.L.; Anderson, L.A.; Pelt, C.E.; Peters, C.L.; Gililland, J.M. Managing Hip-Spine Concepts in the Direct Anterior Approach with Use of Fluoroscopy. J. Arthroplast. 2021, 36, S104–S110. [Google Scholar] [CrossRef]
- Rueckl, K.; Alcaide, D.J.; Springer, B.; Rueckl, S.; Kasparek, M.F.; Boettner, F. Intraoperative measurement of cup inclination using fluoroscopy requires a correction factor. Arch. Orthop. Trauma Surg. 2019, 139, 1511–1517. [Google Scholar] [CrossRef]
- Tannast, M.; Murphy, S.B.; Langlotz, F.; Anderson, S.E.; Siebenrock, K.A. Estimation of pelvic tilt on anteroposterior X-rays--a comparison of six parameters. Skelet. Radiol. 2006, 35, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ackland, M.K.; Bourne, W.B.; Uhthoff, H.K. Anteversion of the acetabular cup. Measurement of angle after total hip replacement. J. Bone Jt. Surg. Br. 1986, 68, 409–413. [Google Scholar] [CrossRef]
- Hassan, D.M.; Johnston, G.H.; Dust, W.N.; Watson, L.G.; Cassidy, D. Radiographic calculation of anteversion in acetabular prostheses. J. Arthroplast. 1995, 10, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-K.; Hou, S.-M.; Yang, R.-S.; Wu, T.-Y.; Fuh, C.-S. A new tool for measuring cup orientation in total hip arthroplasties from plain radiographs. Clin. Orthop. Relat. Res. 2006, 451, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.-H. A simplified method to determine acetabular cup anteversion from plain radiographs. J. Arthroplast. 2004, 19, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Nho, J.-H.; Lee, Y.-K.; Kim, H.J.; Ha, Y.-C.; Suh, Y.-S.; Koo, K.-H. Reliability and validity of measuring version of the acetabular component. J. Bone Jt. Surg. Br. 2012, 94, 32–36. [Google Scholar] [CrossRef]
- Zingg, M.; Boudabbous, S.; Hannouche, D.; Montet, X.; Boettner, F. Standardized fluoroscopy-based technique to measure intraoperative cup anteversion. J. Orthop. Res. 2017, 35, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Boettner, F.; Zingg, M.; Emara, A.K.; Waldstein, W.; Faschingbauer, M.; Kasparek, M.F. The Accuracy of Acetabular Component Position Using a Novel Method to Determine Anteversion. J. Arthroplast. 2017, 32, 1180–1185. [Google Scholar] [CrossRef]
- Bechler, U.; Springer, B.; Rueckl, K.; Rolvien, T.; Boettner, F. Can a simple iPad app improve C-arm based component position in anterior THA? Arch. Orthop. Trauma Surg. 2021, 141, 1401–1409. [Google Scholar] [CrossRef]
- DeJesus, J., IV; Nishioka, S.; Andrews, S.N.; Mathews, K.; Nakasone, C.K. Improved hip symmetry with an adjustable fluoroscopic grid during total hip arthroplasty. Hip Int. 2022, 11207000221089274, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Thorne, T.J.; Nishioka, S.T.; Andrews, S.N.; Mathews, K.A.; Nakasone, C.K. Comparison of Component Placement Accuracy Using Two Intraoperative Fluoroscopic Grid Technologies During Direct Anterior Total Hip Arthroplasty. J. Arthroplast. 2020, 35, 3601–3606. [Google Scholar] [CrossRef]
- Thorne, T.; Nishioka, S.; Andrews, S.; Mathews, K.; Nakasone, C. Component placement accuracy of two digital intraoperative fluoroscopy supplementation systems in direct anterior total hip arthroplasty. Arch. Orthop. Trauma Surg. 2022, 142, 1283–1288. [Google Scholar] [CrossRef]
- Goodell, P.; Ellis, S.; Kokobun, B.; Wilson, H.; Kollmorgen, R.C. Computer Navigation vs. Conventional Overlay Methods in Direct Anterior Total Hip Arthroplasty: A Single Surgeon Experience. Cureus 2022, 14, e29907. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.G.; Parks, N.L.; McDonald, J.F., 3rd; Pfefferle, K.J. A Prospective, Randomized Study of Surgical Positioning Software Shows Improved Cup Placement in Total Hip Arthroplasty. Orthopedics 2019, 42, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Siebenmorgen, J.P.; Stronach, B.M.; Mears, S.C.; Stambough, J.B. The Use of Intraoperative Digital Radiography Alignment Software to Assess Implant Placement in Total Hip Arthroplasty. Curr. Rev. Musculoskelet. Med. 2021, 14, 369–377. [Google Scholar] [CrossRef]
- Bruce-Brand, R.; Magill, P.; O’Neill, C.; Karayiannis, P.; Hill, J.; Beverland, D. Mechanical and Anatomical Alignment Guide Techniques Are Superior to Freehand in Achieving Target Orientation of an Acetabular Component. Arthroplast. Today 2021, 11, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, D.S.; Costello, J.P., 2nd; Dalling, A.D.; Wagner, J.D.; Al-Hardan, W.; Carvajal, J.A. The efficacy of patient specific instrumentation (PSI) in total hip arthroplasty (THA): A systematic review and meta-analysis. J. Orthop. 2022, 34, 404–413. [Google Scholar] [CrossRef]
- Parvizi, J.; Benson, J.R.; Muir, J.M. A new mini-navigation tool allows accurate component placement during anterior total hip arthroplasty. Med. Devices 2018, 11, 95–104. [Google Scholar] [CrossRef]
- Muir, J.M.; Foley, K.A.; Fiaes, K.; Wagler, J.B.; Galaszewicz, M.; Benson, J.R.; Bradley, M.P. Validation of a Novel Software Measurement Tool for Total Hip Arthroplasty. Cureus 2021, 13, e15544. [Google Scholar] [CrossRef]
- Bradley, M.P.; Benson, J.R.; Muir, J.M. Accuracy of Acetabular Component Positioning Using Computer-assisted Navigation in Direct Anterior Total Hip Arthroplasty. Cureus 2019, 11, e4478. [Google Scholar] [CrossRef]
- Tanino, H.; Nishida, Y.; Mitsutake, R.; Ito, H. Portable Accelerometer-Based Navigation System for Cup Placement of Total Hip Arthroplasty: A Prospective, Randomized, Controlled Study. J. Arthroplast. 2020, 35, 172–177. [Google Scholar] [CrossRef]
- Walter, W.L.; Baker, N.A.; Marsden-Jones, D.; Sadeghpour, A. Novel Measure of Acetabular Cup Inclination and Anteversion During Total Hip Arthroplasty. Med. Devices 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Shatrov, J.; Marsden-Jones, D.; Lyons, M.; Walter, W.L. Improving Acetabular Component Positioning in Total Hip Arthroplasty: A Cadaveric Study of an Inertial Navigation Tool and a Novel Registration Method. HSS J. 2022, 18, 358–367. [Google Scholar] [CrossRef]
- Widmer, K.-H.; Grützner, P.A. Joint replacement-total hip replacement with CT-based navigation. Injury 2004, 35 (Suppl. S1), 84–89. [Google Scholar] [CrossRef] [PubMed]
- Perets, I.; Mu, B.H.; Mont, M.A.; Rivkin, G.; Kandel, L.; Domb, B.G. Current topics in robotic-assisted total hip arthroplasty: A review. Hip Int. 2020, 30, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Konan, S.; Ayuob, A.; Ayyad, S.; Haddad, F.S. The current role of robotics in total hip arthroplasty. EFORT Open Rev. 2019, 4, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, P.; Mansour, J.; Assi, C.; Caton, J.; Lustig, S.; Coulomb, R. Current concepts in robotic total hip arthroplasty. SICOT J. 2020, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Hanreich, C.; Streck, L.E.; Boettner, F. Robotik in der Endoprothetik—Neue OP-Unterstützungssysteme. OP-JOURNAL 2022, 38, 96–103. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, T.P.; Berry, D.J. The epidemiology of revision total hip arthroplasty in the United States. J. Bone Jt. Surg. Am. 2009, 91, 128–133. [Google Scholar] [CrossRef]
- Callanan, M.C.; Jarrett, B.; Bragdon, C.R.; Zurakowski, D.; Rubash, H.E.; Freiberg, A.A.; Malchau, H. The John Charnley Award: Risk factors for cup malpositioning: Quality improvement through a joint registry at a tertiary hospital. Clin. Orthop. Relat. Res. 2011, 469, 319–329. [Google Scholar] [CrossRef]
- Suhardi, V.J.; Chiu, Y.-F.; Sculco, P.K.; Della Gonzalez Valle, A. Accuracy of acetabular cup placement positively correlates with level of training. Int. Orthop. 2021, 45, 2797–2804. [Google Scholar] [CrossRef]
- Sariali, E.; Boukhelifa, N.; Catonne, Y.; Pascal Moussellard, H. Comparison of Three-Dimensional Planning-Assisted and Conventional Acetabular Cup Positioning in Total Hip Arthroplasty: A Randomized Controlled Trial. J. Bone Jt. Surg. Am. 2016, 98, 108–116. [Google Scholar] [CrossRef]
- Van Duren, B.H.; Royeca, J.M.; Cunningham, C.M.; Lamb, J.N.; Brew, C.J.; Pandit, H. Can the use of an inclinometer improve acetabular cup inclination in total hip arthroplasty? A review of the literature. Hip Int. 2021, 31, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; van Gaalen, S.M.; De Gast, A. Precision and accuracy of imageless navigation versus freehand implantation of total hip arthroplasty: A systematic review and meta-analysis. Int. J. Med. Robot. 2017, 13, e1843. [Google Scholar] [CrossRef] [PubMed]
- Minoda, Y.; Ohzono, K.; Aihara, M.; Umeda, N.; Tomita, M.; Hayakawa, K. Are acetabular component alignment guides for total hip arthroplasty accurate? J. Arthroplast. 2010, 25, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A.; Iannotti, F.; Proietti, L.; Massafra, C.; Speranza, A.; Laghi, A.; Iorio, R. The Accuracy of Patient-Specific Instrumentation with Laser Guidance in a Dynamic Total Hip Arthroplasty: A Radiological Evaluation. Sensors 2021, 21, 4232. [Google Scholar] [CrossRef] [PubMed]
- Fontalis, A.; Epinette, J.-A.; Thaler, M.; Zagra, L.; Khanduja, V.; Haddad, F.S. Advances and innovations in total hip arthroplasty. SICOT J. 2021, 7, 26. [Google Scholar] [CrossRef]
- McNabb, D.C.; Jennings, J.M.; Levy, D.L.; Miner, T.M.; Yang, C.C.; Kim, R.H. Direct Anterior Hip Replacement Does Not Pose Undue Radiation Exposure Risk to the Patient or Surgeon. J. Bone Jt. Surg. Am. 2017, 99, 2020–2025. [Google Scholar] [CrossRef]
- Curtin, B.M.; Armstrong, L.C.; Bucker, B.T.; Odum, S.M.; Jiranek, W.A. Patient Radiation Exposure During Fluoro-Assisted Direct Anterior Approach Total Hip Arthroplasty. J. Arthroplast. 2016, 31, 1218–1221. [Google Scholar] [CrossRef]
- Schwarz, T.; Weber, M.; Wörner, M.; Renkawitz, T.; Grifka, J.; Craiovan, B. Central X-ray beam correction of radiographic acetabular cup measurement after THA: An experimental study. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 829–837. [Google Scholar] [CrossRef]
- Ross, J.R.; Tannenbaum, E.P.; Nepple, J.J.; Kelly, B.T.; Larson, C.M.; Bedi, A. Functional acetabular orientation varies between supine and standing radiographs: Implications for treatment of femoroacetabular impingement. Clin. Orthop. Relat. Res. 2015, 473, 1267–1273. [Google Scholar] [CrossRef]
- Lembeck, B.; Mueller, O.; Reize, P.; Wuelker, N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop. 2005, 76, 517–523. [Google Scholar] [CrossRef]
- Gililland, J.M.; Anderson, L.A.; Boffeli, S.L.; Pelt, C.E.; Peters, C.L.; Kubiak, E.N. A fluoroscopic grid in supine total hip arthroplasty: Improving cup position, limb length, and hip offset. J. Arthroplast. 2012, 27, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hambright, D.; Hellman, M.; Barrack, R. Intra-operative digital imaging: Assuring the alignment of components when undertaking total hip arthroplasty. Bone Joint J. 2018, 100, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Penenberg, B.L.; Samagh, S.P.; Rajaee, S.S.; Woehnl, A.; Brien, W.W. Digital Radiography in Total Hip Arthroplasty: Technique and Radiographic Results. J. Bone Jt. Surg. Am. 2018, 100, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Vigdorchik, J.M.; Windsor, E.W.; Schwarzkopf, R.; Mayman, D.J.; Sculco, P.K. Abnormal spinopelvic mobility as a risk factor for acetabular placement error in total hip arthroplasty using optical computer-assisted surgical navigation system. Bone Jt. Open 2022, 3, 475–484. [Google Scholar] [CrossRef]
- Migliorini, F.; Cuozzo, F.; Oliva, F.; Eschweiler, J.; Hildebrand, F.; Maffulli, N. Imageless navigation for primary total hip arthroplasty: A meta-analysis study. J. Orthop. Traumatol. 2022, 23, 21. [Google Scholar] [CrossRef]
- Brown, M.L.; Reed, J.D.; Drinkwater, C.J. Imageless computer-assisted versus conventional total hip arthroplasty: One surgeon’s initial experience. J. Arthroplast. 2014, 29, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Kim, B.K.; Jo, M.I.; Ahn, B.M. Which one is more affected by navigation-assisted cup positioning in total hip arthroplasty: Anteversion or inclination? A retrospective matched-pair cohort study in Asian physique. J. Orthop. Surg. 2018, 26, 2309499018780755. [Google Scholar] [CrossRef]
- Ong, C.B.; Chiu, Y.-F.; Premkumar, A.; Della Gonzalez Valle, A. Use of a novel imageless navigation system reduced fluoroscopy exposure and improved acetabular positioning in anterior approach total hip arthroplasty: A case-control study. Arch. Orthop. Trauma Surg. 2022, epub ahead of print, 1–7. [Google Scholar] [CrossRef]
- Hohmann, E.; Bryant, A.; Tetsworth, K. Accuracy of acetabular cup positioning using imageless navigation. J. Orthop. Surg. Res. 2011, 6, 40. [Google Scholar] [CrossRef]
- Zurmühle, C.A.; Zickmantel, B.; Christen, M.; Christen, B.; Zheng, G.; Schwab, J.M.; Tannast, M.; Steppacher, S.D. Image-Less THA Cup Navigation in Clinical Routine Setup: Individual Adjustments, Accuracy, Precision, and Robustness. Medicina 2022, 58, 832. [Google Scholar] [CrossRef]
- Clement, N.D.; Gaston, P.; Bell, A.; Simpson, P.; Macpherson, G.; Hamilton, D.F.; Patton, J.T. Robotic arm-assisted versus manual total hip arthroplasty. Bone Jt. Res. 2021, 10, 22–30. [Google Scholar] [CrossRef]
- Ng, N.; Gaston, P.; Simpson, P.M.; Macpherson, G.J.; Patton, J.T.; Clement, N.D. Robotic arm-assisted versus manual total hip arthroplasty: A systematic review and meta-analysis. Bone Jt. J. 2021, 103, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-H.; Li, X.-M.; Ma, S.-Q.; Zhao, Y.-C.; Qi, C.; Xue, Y. Total Hip Arthroplasty with Robotic Arm Assistance for Precise Cup Positioning: A Case-Control Study. Orthop. Surg. 2022, 14, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Remily, E.A.; Nabet, A.; Sax, O.C.; Douglas, S.J.; Pervaiz, S.S.; Delanois, R.E. Impact of Robotic Assisted Surgery on Outcomes in Total Hip Arthroplasty. Arthroplast. Today 2021, 9, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Needham, K.; Adams, C.; Coppolecchia, A.; Lavernia, C. Robotic-assisted total hip arthroplasty: An economic analysis. J. Comp. Eff. Res. 2021, 10, 1225–1234. [Google Scholar] [CrossRef]
- Khalifa, A.A.; Abdelnasser, M.K.; Ahmed, A.M.; Shetty, G.M.; Abdelaal, A.M. Smartphone Application Helps Improve the Accuracy of Cup Placement by Young, Less-Experienced Surgeons during Primary Total Hip Arthroplasty. Arch. Bone Jt. Surg. 2022, 10, 278–285. [Google Scholar] [CrossRef]
- Arulampalam, J.; Bucknill, A.; Gupta, S.; Li, Q.; McMahon, S.J. Validation of a Prototype Augmented Reality Navigation System for Total Hip Replacement. Surg. Technol. Int. 2022, 41, sti41–1615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streck, L.E.; Boettner, F. Achieving Precise Cup Positioning in Direct Anterior Total Hip Arthroplasty: A Narrative Review. Medicina 2023, 59, 271. https://doi.org/10.3390/medicina59020271
Streck LE, Boettner F. Achieving Precise Cup Positioning in Direct Anterior Total Hip Arthroplasty: A Narrative Review. Medicina. 2023; 59(2):271. https://doi.org/10.3390/medicina59020271
Chicago/Turabian StyleStreck, Laura Elisa, and Friedrich Boettner. 2023. "Achieving Precise Cup Positioning in Direct Anterior Total Hip Arthroplasty: A Narrative Review" Medicina 59, no. 2: 271. https://doi.org/10.3390/medicina59020271
APA StyleStreck, L. E., & Boettner, F. (2023). Achieving Precise Cup Positioning in Direct Anterior Total Hip Arthroplasty: A Narrative Review. Medicina, 59(2), 271. https://doi.org/10.3390/medicina59020271



