Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts
Abstract
1. Introduction
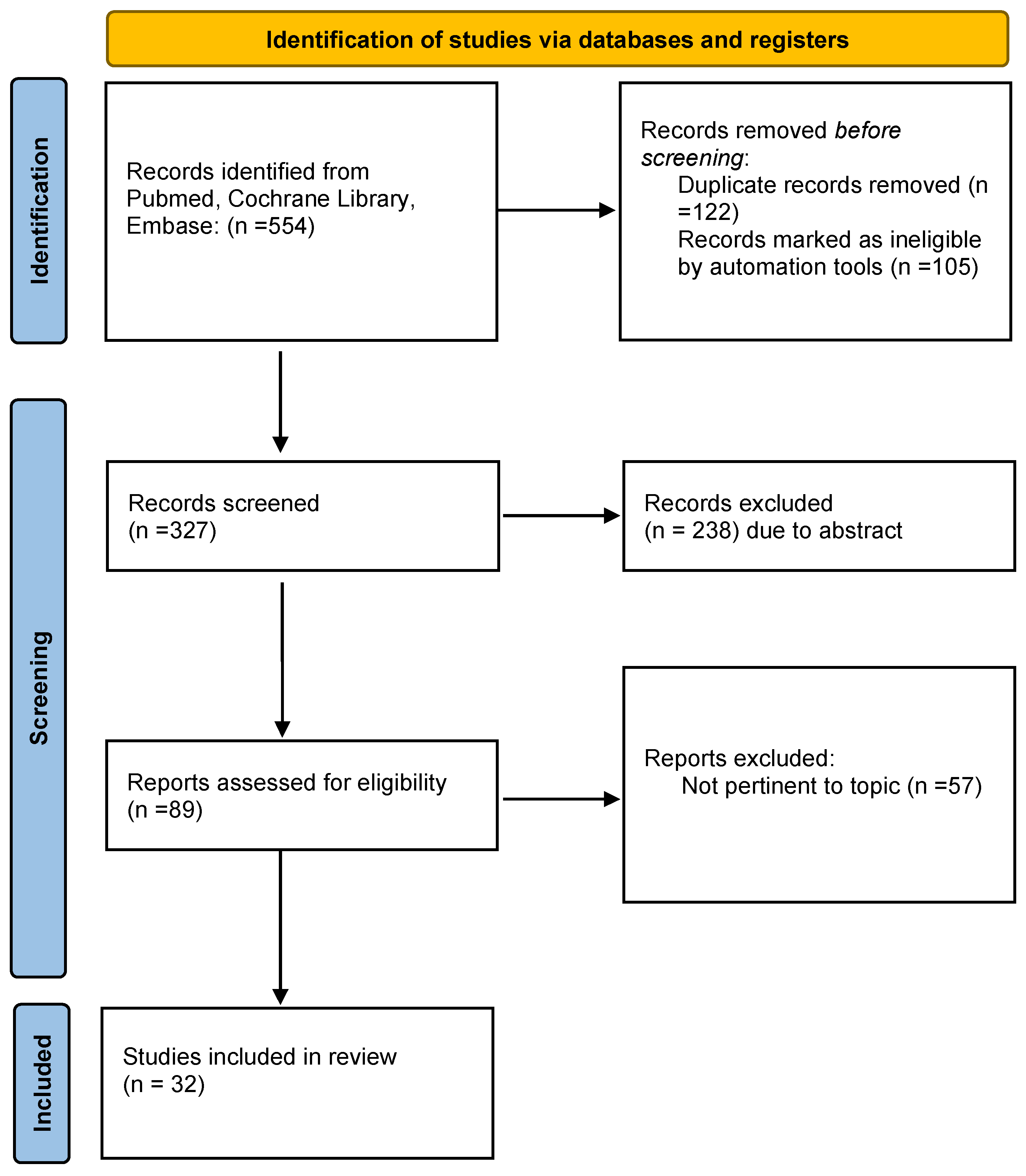
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction and Analysis
3. Results
3.1. Animal Study
3.1.1. Enzymatically Derived ADSC-SVF in Tendinopathy and Tendon Injury
| Authors | Animal Model | Injury | ADSC-SVF Provenience | Treatment | Investigation | Outcomesx |
|---|---|---|---|---|---|---|
| Lee et al. [34] | Sprague Dawley rats-in vivo | Full thickness Achilles tendon defect | Human subcutaneous fat tissue | Group 1: fibrin glue-ADSC injection Group 2: fibrin glue Group 3: control group | Histological Immunohistochemical, Biomechanical, Proteomic evaluation | Better gross morphological and biomechanical recovery in ADSC group. Increased expression of HST-C1 and T-1. |
| Oshita et al. [21] | 16 F344/NSIc rats | Collagenase iatrogenic Achilles tendon injury | inguinal fat pads of two F344/NSlc rats | Group 1: ADSC injection Group 2: saline injection | Histological Immunohistochemical | ADSC group decreased levels of disrupted collagen fibers, cellularity, and hypervascularity. Increased expression COL1, decreased expression COL3. |
| De Aro et al. [35] | Lewis rat | Transected Achilles tendon | Inguinal region of 10 male Lewis rats | Group 1: untreated Group 2: ADSC Group 3: GDF-5 Group 4: ADSC + GDF-5 | Biomechanical, RT-PCR | The ADSC increased expression of Lox, Dcn, and Tgfb1. Lower deformation at higher stress. |
| Chen et al. [36] | Sprague-dawley rats | Collagenase induced rotator cuff injury | Human subcutaneous fat tissue | Group 1: untreated Group 2: ADSC | Histological, RT-PCR, Biomechanical | Improvement in collagen fibres alignment, increased COL1, TNC expression No biomechanical differences at final endpoint |
| Valencia Mora et al. [37] | Sprague-Dawley rats | Detachment and repair of the supraspinatus tendon | Rat subcutaneous fat tissue | Group 1: collagen carrier Group 2: collagen carrier + ADSC Group 3: untreated | Biomechanical Histological | ADSC group showed less acute inflammation signs. No significative differences in biomechanical analysis. |
| Shen et. al. [38] | Mongrel Dogs | Second and fifth flexor digitorum profundus (FDP) zone II tendon transection and repair | Autologous subcutaneous fat tissue+ collagen sheet | Group 1: ADSC + collagen sheet wraps around the tendon Group 2: HA Group 3: suture only | Gene expression Immunostaining Histological | ADSC modulated the inflammatory phase of tendon healing, promoted a regenerative/anti-inflammatory M2 macrophage phenotype and influenced tendon extracellular matrix remodeling, angiogenesis, and cell survival. |
| Canapp et al. [39] | Dogs | Supraspinatus tendinopathy | Autologous subcutaneous fat tissue+PRP | Group 1: ADSC + PRP peritendinous injection by US guidance Group 2: contralateral non-affetcted | MRI, US, XR evaluation; Gait analysis | TPI significant increase in Treated limb |
| Vieira et al. [40] | New Zeland Rabbits | Achilles tendon cut injury | Autologous subcutaneous adipose tissue | Group 1: tendon cut Group 2: tendon suture Group 3: tendon suture + ADSC | Histological Analysis | Increase of vascular score and collagen fibres structural organization in Group 2 and 3 |
| Chen et al. [41] | New Zeland Rabbits | Achilles tendon cut injury | Autologous subcutaneous adipose tissue | Group 1: tendon cut and sutured Group 2: tendon cut and sutured + ADSC | Cell viability, histological, proteomic analysis Biomechanical analysis | Increased tenocyte viability in ADSC group, mechanical strength and increased expression of TM BG Dcn |
| Uysal et al. [42] | Japanese rabbits | Achilles tendon cut injury | Autologous subcutaneous adipose tissue | Group 1: PRP gel Group 2: PRP + ADSC | Histological immunohistochemical, biomechanical analysis | COL1 FGF VEGF higher in ADSC treated group. Tgfb lower. Higher tendon tensile strength in ADSC treated group. |
| Lu et al. [43] | New zeland rabbits | Supraspinatus tendon incision and suture | Autologous subcutaneous adipose tissue | Group 1: ADSC-FG Group 2: FG | Histological, immunohistochemical, biomechanical analysis, MRI analysis | Higher expression of COL1, and ratio of COL3/COL1. Higher biomechanical value in ADSC-FG group, MRI better results after 12 weels |
| Behfar et al. [28] | New zeland rabbits | Deep digital flexor tendon transection and sutured. | Autologous subcutaneous adipose tissue | Group 1: tendon suture Group 2: tendon suture + ADSC | Histological, immunohistochemical, biomechanical analysis | Higher orientation of collagen bundles and fibrillar continuity. Increased expression of COL1. Higher tensile strength parameters. |
| Behfar et al. [44] | New zeland rabbits | Deep digital flexor tendon transection and sutured. | Autologous subcutaneous adipose tissue | Group 1: tendon suture Group 2: tendon suture + ADSC | biomechanical analysis | Significant increases in ultimate and yield load, energy absorption, and stress were noted at both time points when treatment groups were compared to their matched controls |
| Geburek et al. [45] | Warmbloods and trotter horses | Superficial digital flexor tendon surgical core lesion | Autologous subcutaneous adipose tissue | Group 1: inactivated autologous serum Group 2: ADSC injection | Histological, immunohistochemical biomechanical, clinical, US examination | No differences in histological findings, no difference in GAG, biomechanical, US results. |
| Carvalho et al. [46] | Mixed breed horses | Superficial digital flexor tendon collagenase iatrogenic injury | Autologous subcutaneous adipose tissue | Group 1: control Group 2: ADSC injection | Flow cytometric peripheral blood fluorescent microscopy | Cell presence, nanocrystals valuable in vivo markers |
| Conze et al. [47] | Warmblood and standardbreed horses | superficial digital flexor tendon surgical core lesion | Autologous subcutaneous adipose tissue | Group 1: control Group 2: ADSC injection | Histological, immunohistochemical, US analysis | Histology staining significantly higher for new vessels and FVIII. More vascularization in us doppler control. |
| Polly et al. [48] | Mixed breed horses | Superficial digital flexor tendonitis | Autologous subcutaneous adipose tissue | Group1: control Group2: ADSC co-cultured | Genic expression analysis | Significantly higher expression of IGF1, Tgfb1, SDF1. Lower expression of COMP. |
3.1.2. Mechanically Derived ADSC-SVF in Tendinopathy and Tendon Injury
3.2. Human Study
3.2.1. Enzymatically Derived ADSC-SVF in Tendinopathy and Tendon Injury
| Authors | Injury | ADSC-SVF Provenience | ADSC-SVF Delivery | Investigation | Outcomes |
|---|---|---|---|---|---|
| Jo et al. [49] | Partial thickness Rotator cuff tear | Autologous from abdominal area | Intralesional-injection | Clinical (SPADI score, CONSTANT score, VAS) Radiological (MRI), Arthroscopic | Significantly alleviated shoulder pain with more than 70% reduction from the baseline in the high-dose group. At MRI the bursal-side defect significantly decreased by 90% in the high-dose group . Arthroscopic examination volume of the articular- and bursal- side defects decreased by 83% and 90%. |
| Kim et al. [50] | Partial thickness Rotator cuff tear | Autologous, derived from buttocks | ADSC loaded in fibrin glue was injected compared to standard suture | Clinical (VAS, ROM, CONSTANT score, UCLA score) Radiological (MRI) | No clinical differences. At MRI, improved structural outcomes, low retear rate |
| Hurd et al. [51] | Partial thickness Rotator cuff tear | Autologous, derived from either the periumbilical abdominal area, bilateral flanks, or medial thigh fat | Intralesional-injection | Clinical (ASES score, SF-36) | Significantly higher ASES score |
| Freitag et al. [52] | Common extensor tendon chronic tendinopathy | Autologous abdominal fat | Intralesional injection enriched with PRP | Clinical (NPRT, PRTE) Radiological (US, MRI) | Significant improvements of clinical score and tendon structure |
| Khouri et al. [53] | Chronic lateral elbow tendinopathy | Autologous, derived from periumbilical zone | Percutaneous injection to the affected elbow | Clinical (VAS, qDASH) Radiological (MRI) | Improved VAS scores for maximum pain score, QuickDASH -Compulsory score, QuickDASH-Sport score, MRI score for tendinopathy. |
| Lee et al. [54] | Lateral epicondylosis | Allogenic | Intratendinous injection with fibrin glue | Clinical (VAS, MEPI score) Radiological (US) | Safe and improved elbow pain VAS, performance MEPI score, and structural defects |
| Khouri et al. [55] | Patellar tendinopathy | Autologous, derived from periumbilical zone | Percutaneous injection to the affected tendon | Clinical (VAS, Visa-P) Radiological (MRI) | VAS and Visa-P score improved significantly. MRI revealed significantive improvement for tendon thickness, and tear length, width and thickness compared to baseline. |
3.2.2. Mechanically Derived ADSC-SVF in Tendinopathy and Tendon Injury
3.3. Biomaterials Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Torrillas, M.; Rubio, M.; Damia, E.; Cuervo, B.; Del Romero, A.; Peláez, P.; Chicharro, D.; Miguel, L.; Sopena, J.J. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. Int. J. Mol. Sci. 2019, 25, 3105. [Google Scholar] [CrossRef] [PubMed]
- Magnan, B.; Bondi, M.; Pierantoni, S.; Samaila, E. The pathogenesis of Achilles tendinopathy: A systematic review. Foot Ankle Surg. 2014, 20, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Jomaa, G.; Kwan, C.K.; Fu, S.C.; Ling, S.K.K.; Chan, K.M.; Yung, P.S.H.; Rolf, C. A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet. Disord. 2020, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Wardale, J.; Brooks, R.; Henson, F.; Noorani, A.; Rushton, N. Exploring the Application of Stem Cells in Tendon Repair and Regeneration. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1018–1029. [Google Scholar] [CrossRef]
- Schneider, M.; Angele, P.; Jarvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef]
- Gaspar, D.; Spanoudes, K.; Holladay, C.; Pandit, A.; Zeugolis, D. Progress in cell-based therapies for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 240–256. [Google Scholar] [CrossRef]
- Lomas, A.J.; Ryan, C.N.M.; Sorushanova, A.; Shologu, N.; Sideri, A.I.; Tsioli, V.; Fthenakis, G.C.; Tzora, A.; Skoufos, I.; Quinlan, L.R.; et al. The past, present and future in scaffold-based tendon treatments. Adv. Drug Deliv. Rev. 2015, 84, 257–277. [Google Scholar] [CrossRef]
- Childress, M.A.; Beutler, A. Management of chronic tendon injuries. Am. Fam. Physician 2013, 87, 486–490. [Google Scholar]
- Gerdesmeyer, L.; Mittermayr, R.; Fuerst, M.; Al Muderis, M.; Thiele, R.; Saxena, A.; Gollwitzer, H. Current evidence of extracorporeal shock wave therapy in chronic Achilles tendinopathy. Int. J. Surg. 2015, 24, 154–159. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Flavin, N.E.; Vaysbrot, E.; Harvey, W.; McAlindon, T. High-energy extracorporeal shock-wave therapy for treating chronic calcific tendinitis of the shoulder: A systematic review. Ann. Intern. Med. 2014, 160, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Couppé, C.; Svensson, R.B.; Silbernagel, K.G.; Langberg, H.; Magnusson, S.P. Eccentric or concentric exercises for the treatment of tendinopathies? J. Orthop. Sports Phys. Ther. 2015, 45, 853–863. [Google Scholar] [CrossRef]
- Barker-Davies, R.M.; Nicol, A.; McCurdie, I.; Watson, J.; Baker, P.; Wheeler, P.; Fong, D.; Lewis, M.; Bennett, A.N. Study protocol: A double blind randomised control trial of high volume image guided injections in Achilles and patellar tendinopathy in a young active population. BMC Musculoskelet. Disord. 2017, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A. Effectiveness of dry needling and high-volume imageguided injection in the management of chronic mid-portion Achilles tendinopathy in adult population: A literature review. Eur. J. Orthop Surg. Traumatol. 2017, 27, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, M.; Diehl, N.; Schmitt, C.; Kohn, D.M.; Lorbach, O. Results of surgical treatment of chronic patellar tendinosis (Jumper’s knee): A systematic review of the literature. Arthroscopy 2015, 31, 2424–2429.e3. [Google Scholar] [CrossRef]
- Ahmad, Z.; Siddiqui, N.; Malik, S.S.; Abdus-Samee, M.; Tytherleigh-Strong, G.; Rushton, N. Lateral epicondylitis: A review of pathology and management. Bone Jt. J. 2013, 95, 1158–1164. [Google Scholar] [CrossRef]
- Baltes, T.P.A.; Zwiers, R.; Wiegerinck, J.I. Surgical treatment for midportion Achilles tendinopathy: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1817–1838. [Google Scholar] [CrossRef]
- Shapiro, E.; Grande, D.; Drakos, M. Biologics in Achilles tendon healing and repair: A review. Curr. Rev. Musculoskelet. Med. 2015, 8, 9–17. [Google Scholar] [CrossRef]
- Piccionello, A.P.; Riccio, V.; Senesi, L.; Volta, A.; Pennasilico, L.; Botto, R.; Rossi, G.; Tambella, A.M.; Galosi, L.; Marini, C.; et al. Adipose Micro-Grafts Enhance Tendinopathy Healing in Ovine Model: An in Vivo Experimental Perspective Study. STEM CELLS Transl. Med. 2021, 10, 1544–1560. [Google Scholar] [CrossRef]
- Zumwalt, M.; Reddy, A.P. Stem Cells for Treatment of Musculoskeletal Conditions—Orthopaedic/Sports Medicine Applications. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165624. [Google Scholar] [CrossRef]
- Oshita, T.; Tobita, M.; Tajima, S.; Mizuno, H. Adipose-Derived Stem Cells Improve Collagenase-Induced Tendinopathy in a Rat Model. Am. J. Sports Med. 2016, 44, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.; Ferraro, G. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Mannucci, S.; Conti, G.; Dai Prè, E.; Sbarbati, A.; Riccio, M. A Non-Enzymatic Method to Obtain a Fat Tissue Derivative Highly Enriched in Adipose Stem Cells (ASCs) from Human Lipoaspirates: Preliminary Results. Int. J. Mol. Sci. 2018, 19, 2061. [Google Scholar] [CrossRef] [PubMed]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and Enzymatic Procedures to Isolate the Stromal Vascular Fraction from Adipose Tissue: Preliminary Results. Front Cell Dev. Biol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef]
- Behfar, M.; Sarrafzadeh-Rezaei, F.; Hobbenaghi, R.; Delirezh, N.; Dalir-Naghadeh, B. Adipose-derived stromal vascular fraction improves tendon healing in rabbits. Chin. J. Traumatol. 2011, 14, 329–335. [Google Scholar]
- Viganò, M.; Lugano, G.; Perucca Orfei, C.; Menon, A.; Ragni, E.; Colombini, A.; De Luca, P.; Randelli, P.; de Girolamo, L. Autologous microfragmented adipose tissue reduces inflammatory and catabolic markers in supraspinatus tendon cells derived from patients affected by rotator cuff tears. Int. Orthop. 2021, 45, 419–426. [Google Scholar] [CrossRef]
- Kunze, K.N.; Burnett, R.A.; Wright-Chisem, J.; Frank, R.M.; Chahla, J. Adipose-Derived Mesenchymal Stem Cell Treatments and Available Formulations. Curr. Rev. Musculoskelet. Med. 2020, 13, 264–280. [Google Scholar] [CrossRef]
- Schroeder, A.; Rubin, J.P.; Kokai, L.; Sowa, G.; Chen, J.; Onishi, K. Use of Adipose-Derived Orthobiologics for Musculoskeletal Injuries: A Narrative Review. PM&R 2020, 12, 805–816. [Google Scholar]
- Rivera-Izquierdo, M.; Cabeza, L.; Láinez-Ramos-Bossini, A.; Quesada, R.; Perazzoli, G.; Alvarez, P.; Prados, J.; Melguizo, C. An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expert Opin. Biol. Ther. 2019, 19, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Siennicka, K.; Zolocinska, A.; Stepien, K.; Lubina-Dabrowska, N.; Maciagowska, M.; Zolocinska, E.; Slysz, A.; Piusinska-Macoch, R.; Mazur, S.; Zdanowicz, U.; et al. Adipose-Derived Cells (Stromal Vascular Fraction) Transplanted for Orthopedical or Neurological Purposes: Are They Safe Enough? Stem Cells Int. 2016, 2016, 5762916. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kwon, B.; Lee, K.; Son, Y.H.; Chung, S.G. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am. J. Sports Med. 2017, 45, 1429–1439. [Google Scholar] [CrossRef]
- De Aro, A.A.; Carneiro, G.D.; Teodoro, L.F.R.; Da Veiga, F.C.; Ferrucci, D.L.; Simões, G.F.; Pimentel, E.R. Injured Achilles Tendons Treated with Adipose-Derived Stem Cells Transplantation and GDF-5. Cells 2018, 7, 127. [Google Scholar] [CrossRef]
- Chen, H.S.; Su, Y.T.; Chan, T.M.; Su, Y.J.; Syu, W.S.; Harn, H.J.; Lin, S.Z.; Chiu, S.C. Human adipose-derived stem cells accelerate the restoration of tensile strength of tendon and alleviate the progression of rotator cuff injury in a rat model. Cell Transplant. 2015, 24, 509–520. [Google Scholar] [CrossRef]
- Valencia Mora, M.; Antuña Antuña, S.; García Arranz, M.; Carrascal, M.T.; Barco, R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury 2014, 45 (Suppl. S4), S22–S27. [Google Scholar] [CrossRef]
- Shen, H.; Kormpakis, I.; Havlioglu, N.; Linderman, S.W.; Sakiyama-Elbert, S.E.; Erickson, I.E.; Zarembinski, T.; Silva, M.J.; Gelberman, R.H.; Thomopoulos, S. The effect of mesenchymal stromal cell sheets on the inflammatory stage of flexor tendon healing. Stem Cell Res. Ther. 2016, 7, 144. [Google Scholar] [CrossRef]
- Canapp, S.O., Jr.; Canapp, D.A.; Ibrahim, V.; Carr, B.J.; Cox, C.; Barrett, J.G. The Use of Adipose-Derived Progenitor Cells and Platelet-Rich Plasma Combination for the Treatment of Supraspinatus Tendinopathy in 55 Dogs: A Retrospective Study. Front Vet. Sci. 2016, 3, 61. [Google Scholar] [CrossRef]
- Vieira, M.H.; Oliveira, R.J.; Eça, L.P.; Pereira, I.S.; Hermeto, L.C.; Matuo, R.; Fernandes, W.S.; Silva, R.A.; Antoniolli, A.C. Therapeutic potential of mesenchymal stem cells to treat Achilles tendon injuries. Genet Mol. Res. 2014, 13, 10434–10449. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, Z.Y.; Lin, Y.H.; Chen, S.H.; Chou, P.Y.; Kao, H.K.; Lin, F.H. Extracellular Vesicles of Adipose-Derived Stem Cells Promote the Healing of Traumatized Achilles Tendons. Int. J. Mol. Sci. 2021, 22, 12373. [Google Scholar] [CrossRef] [PubMed]
- Uysal, C.A.; Tobita, M.; Hyakusoku, H.; Mizuno, H. Adipose-derived stem cells enhance primary tendon repair: Biomechanical and immunohistochemical evaluation. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1712–1729. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Ma, M.; Cai, J.F.; Yuan, F.; Zhou, W.; Luo, S.L.; Pan, Z.Y.; Zeng, W.; Zhong, N.; Yin, F. Effects of Local Application of Adipose-Derived Stromal Vascular Fraction on Tendon-Bone Healing after Rotator Cuff Tear in Rabbits. Chin. Med. J. (Engl.) 2018, 131, 2620–2622. [Google Scholar] [CrossRef] [PubMed]
- Behfar, M.; Sarrafzadeh-Rezaei, F.; Hobbenaghi, R.; Delirezh, N.; Dalir-Naghadeh, B. Enhanced mechanical properties of rabbit flexor tendons in response to intratendinous injection of adipose derived stromal vascular fraction. Curr. Stem Cell Res. Ther. 2012, 7, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Geburek, F.; Mundle, K.; Conrad, S.; Hellige, M.; Walliser, U.; van Schie, H.T.; van Weeren, R.; Skutella, T.; Stadler, P.M. Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions—A pilot study. Stem Cell Res. Ther. 2016, 7, 21. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Yamada, A.L.; Golim, M.A.; Álvarez, L.E.; Hussni, C.A.; Alves, A.L. Evaluation of mesenchymal stem cell migration after equine tendonitis therapy. Equine Vet. J. 2014, 46, 635–638. [Google Scholar] [CrossRef]
- Conze, P.; van Schie, H.T.; van Weeren, R.; Staszyk, C.; Conrad, S.; Skutella, T.; Hopster, K.; Rohn, K.; Stadler, P.; Geburek, F. Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen Med. 2014, 9, 743–757. [Google Scholar] [CrossRef]
- Polly, S.S.; Nichols, A.E.C.; Donnini, E.; Inman, D.J.; Scott, T.J.; Apple, S.M.; Werre, S.R.; Dahlgren, L.A. Adipose-Derived Stromal Vascular Fraction and Cultured Stromal Cells as Trophic Mediators for Tendon Healing. J. Orthop. Res. 2019, 37, 1429–1439. [Google Scholar] [CrossRef]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Kim, P.S.; Yoon, J.Y.; Yoon, K.S. Intratendinous Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Rotator Cuff Disease: A First-In-Human Trial. Stem Cells 2018, 36, 1441–1450. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sung, C.H.; Chung, S.H.; Kwak, S.J.; Koh, Y.G. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am. J. Sports Med. 2017, 45, 2010–2018. [Google Scholar] [CrossRef]
- Hurd, J.L.; Facile, T.R.; Weiss, J.; Hayes, M.; Hayes, M.; Furia, J.P.; Maffulli, N.; Winnier, G.E.; Alt, C.; Schmitz, C.; et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: A prospective, randomized, controlled first-in-human pilot study. J. Orthop. Surg. Res. 2020, 15, 122. [Google Scholar] [PubMed]
- Freitag, J.; Shah, K.; Wickham, J.; Tenen, A. Effect of autologous adipose-derived mesenchymal stem cell therapy in combination with autologous platelet-rich plasma in the treatment of elbow tendinopathy. BMJ Case Rep. 2020, 13, e234592. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Tabben, M.; Rolón, A.U.; Levi, L.; Chamari, K.; D’Hooghe, P. Promising improvement of chronic lateral elbow tendinopathy by using adipose derived mesenchymal stromal cells: A pilot study. J. Exp. Orthop. 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.A.; Chamari, K.; Tabben, M.; Alkhelaifi, K.; Ricardo, T.; Damián, C.; D’hooghe, P. Expanded adipose derived mesenchymal stromal cells are effective in treating chronic insertional patellar tendinopathy: Clinical and MRI evaluations of a pilot study. J. Exp. Orthop. 2021, 8, 49. [Google Scholar] [CrossRef]
- Striano, R.D.; Malanga, G.A.; Bilbool, N.; Khatira, A. Refractory shoulder pain with osteoarthritis, and rotator cuff tear, Treated with Micro Fragmented Adipose Tissue. J. Orthop. Spine Sports Med. 2018, 1, 1–5. [Google Scholar]
- Albano, D.; Messina, C.; Usuelli, F.G.; De Girolamo, L.; Grassi, M.; Maccario, C.; Bignotti, B.; Tagliafico, A.; Sconfienza, L.M. Magnetic resonance and ultrasound in achilles tendinopathy: Predictive role and response assessment to platelet-rich plasma and adipose-derived stromal vascular fraction injection. Eur. J. Radiol. 2017, 95, 130–135. [Google Scholar] [CrossRef]
- Usuelli, F.G.; Grassi, M.; Maccario, C.; Vigano’, M.; Lanfranchi, L.; Alfieri Montrasio, U.; de Girolamo, L. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: Results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2000–2010. [Google Scholar] [CrossRef]
- Kaizawa, Y.; Franklin, A.; Leyden, J.; Behn, A.W.; Tulu, U.S.; Sotelo Leon, D.; Wang, Z.; Abrams, G.D.; Chang, J.; Fox, P.M. Augmentation of chronic rotator cuff healing using adipose-derived stem cell-seeded human tendon-derived hydrogel. J. Orthop. Res. 2019, 37, 877–886. [Google Scholar] [CrossRef]
- Yang, G.; Rothrauff, B.B.; Lin, H.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 2013, 34, 9295–9306. [Google Scholar] [CrossRef]
- Subramanian, G.; Stasuk, A.; Elsaadany, M.; Yildirim-Ayan, E. Effect of Uniaxial Tensile Cyclic Loading Regimes on Matrix Organization and Tenogenic Differentiation of Adipose-Derived Stem Cells Encapsulated within 3D Collagen Scaffolds. Stem Cells Int. 2017, 2017, 6072406. [Google Scholar] [CrossRef] [PubMed]
- Martinello, T.; Bronzini, I.; Volpin, A.; Vindigni, V.; Maccatrozzo, L.; Caporale, G.; Bassetto, F.; Patruno, M. Successful recellularization of human tendon scaffolds using adipose-derived mesenchymal stem cells and collagen gel. J. Tissue Eng. Regen Med. 2014, 8, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.C.; Mizuno, H. Tendon regeneration and repair with adipose derived stem cells. Curr. Stem Cell Res. Ther. 2010, 5, 161–167. [Google Scholar] [CrossRef]
- Lipner, J.; Shen, H.; Cavinatto, L.; Liu, W.; Havlioglu, N.; Xia, Y.; Galatz, L.M.; Thomopoulos, S. In Vivo Evaluation of Adipose-Derived Stromal Cells Delivered with a Nanofiber Scaffold for Tendon-to-Bone Repair. Tissue Eng Part A. 2015, 21, 2766–2774. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.J.; Crowe, C.; McGoldrick, R.; Hui, K.; Pham, H.; Chang, J. Optimization of an injectable tendon hydrogel: The effects of platelet-rich plasma and adipose-derived stem cells on tendon healing in vivo. Tissue Eng Part A. 2015, 21, 1579–1586. [Google Scholar] [CrossRef]
- Deng, D.; Wang, W.; Wang, B.; Zhang, P.; Zhou, G.; Zhang, W.J.; Cao, Y.; Liu, W. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials 2014, 35, 8801–8809. [Google Scholar] [CrossRef]
- Franklin, A.; Gi Min, J.; Oda, H.; Kaizawa, Y.; Leyden, J.; Wang, Z.; Chang, J.; Fox, P.M. Homing of Adipose-Derived Stem Cells to a Tendon-Derived Hydrogel: A Potential Mechanism for Improved Tendon-Bone Interface and Tendon Healing. J. Hand Surg. Am. 2020, 45, 1180.e1–1180.e12. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Evans, C.H. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med. 1999, 28, 71–76. [Google Scholar] [CrossRef]
- Bedi, A.; Maak, T.; Walsh, C.; Rodeo, S.A.; Grande, D.; Dines, D.M.; Dines, J.S. Cytokines in rotator cuff degeneration and repair. J. Shoulder Elb. Surg. 2012, 21, 218–227. [Google Scholar] [CrossRef]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.; Tennyson, M.; Zhang, J.; Khan, W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tendon and Ligament Repair-A Systematic Review of In Vivo Studies. Cells 2021, 10, 2553. [Google Scholar] [CrossRef] [PubMed]
- Isola, A.; Chen, S. Exosomes: The messengers of health and disease. Curr. Neuropharmacol. 2016, 15, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef]
- Manning, C.N.; Martel, C.; Sakiyama-Elbert, S.E.; Silva, M.J.; Shah, S.; Gelberman, R.H.; Thomopoulos, S. Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res. Ther. 2015, 6, 74. [Google Scholar] [CrossRef]
- Bertozzi, N.; Simonacci, F.; Grieco, M.P.; Grignaffini, E.; Raposio, E. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann. Med. Surg. 2017, 20, 41–48. [Google Scholar] [CrossRef]
- Daher, S.R.; Johnstone, B.H.; Phinney, D.G.; March, K.L. Adipose stromal/stem cells: Basic and translational advances: The IFATS collection. Stem Cells 2008, 26, 2664–2665. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Büscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef]
- Dahl, J.A.; Duggal, S.; Coulston, N.; Millar, D.; Melki, J.; Shahdadfar, A.; Brinchmann, J.E.; Collas, P. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int. J. Dev. Biol. 2008, 52, 1033–1042. [Google Scholar] [CrossRef]
- Raposio, E.; Ciliberti, R. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. 2017, 24, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Picardo, M. Maximizing non-enzymatic methods for harvesting adipose-derived stem from lipoaspirate: Technical considerations and clinical implications for regenerative surgery. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mocini, F.; Monteleone, A.S.; Piazza, P.; Cardona, V.; Vismara, V.; Messinese, P.; Campana, V.; Sircana, G.; Maccauro, G.; Saccomanno, M.F. The role of adipose derived stem cells in the treatment of rotator cuff tears: From basic science to clinical application. Orthop. Rev. 2020, 12 (Suppl. S1), 8682. [Google Scholar] [CrossRef] [PubMed]
| Authors | Injury | ADSC-SVF Provenience | ADSC-SVF Delivery | Device Used | Investigation | Outcomes |
|---|---|---|---|---|---|---|
| Striano et al. [56] | Partial thickness Rotator cuff tear | Autologous from thigh area | Intralesional-injection | Lipogems | Clinical (ASES score, NPRT) | Significant improvements in pain, function and quality of life |
| Albano et al. [57] | Chronic achilles’ tendinopathy | Autologous, derived from abdomen | Intralesional-injection | Hy-tissue SVF (Fastkit) | Clinical (VAS) Radiological (MRI, US) | Significant decrease VAS and an increased tendon thickness |
| Usuelli et al. [58] | Chronic achilles’ tendinopathy | Autologous, derived from abdomen | Intralesional-injection | Hy-tissue SVF (Fastkit) | Clinical (VAS, Visa-A, AOFAS) | Immediate benefits for VAS, Visa-A, AOFAS score |
| Authors | Injury (Animal) | Biomaterial | Treatment | ADSC-SVF Provenience | Investigation | Outcomes |
|---|---|---|---|---|---|---|
| Lipner et al. [64] | Rotator Cuff tears (Sprague Dawley rats) | Nanofibrous poly lactic co-glycolic acid scaffold | Group 1: tendon suture Group 2: tendon suture + acellular scaffold Group 3: tendon suture + ADSC scaffold Group 4: tendon suture + ADSC + BMP2 scaffold | Allogenic subcutaneous adipose tissue mice | Histological, bone morphology, biomechanical outcomes | The acellular scaffold showed a delayed healing response. Cellular + BMP2 scaffold showed decreased mechanical properties. No difference in bone morphology. |
| Chiou et al. [65] | Achilles tendon midsubstance defect (Wistar rats) | Tendon hydrogel scaffold | Group 1: saline control Group 2: tendon hydrogel Group 3: tendon hydrogel scaffold + PRP Group 4: tendon hydrogel scaffold + ADSC + PRP | Allogenic subcutaneous adipose tissue mice | Histological, Biomechanical analysis | Group 4 higher fibres organization. No biomechanical differences |
| Deng et al. [66] | Achilles’tendon defect (new zeland rabbits) | polyglycolic acid (PGA)+ PLA (polylactic acid) | Group 1: scaffold Group 2: scaffold + ADSC | Autologous, derived from nuchal side | Gross view, Histological Biomechanical analysis | Significant difference in mechanical strength in group 2, improved histological score. |
| Franklin et al. [67] | Achilles’tendon defect and rotator cuff chronic tendinopahty (Sprague Dawley rat) | Human decellularized flexor tendon hydrogel (tHG) | Group 1: saline Group 2: tHG Group 3: tHG + ADSC Group 4: tHg + FB Group5 | In commerce | Flow citometry | Enhance homing of adsc cells administred ev. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senesi, L.; De Francesco, F.; Marchesini, A.; Pangrazi, P.P.; Bertolini, M.; Riccio, V.; Riccio, M. Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts. Medicina 2023, 59, 273. https://doi.org/10.3390/medicina59020273
Senesi L, De Francesco F, Marchesini A, Pangrazi PP, Bertolini M, Riccio V, Riccio M. Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts. Medicina. 2023; 59(2):273. https://doi.org/10.3390/medicina59020273
Chicago/Turabian StyleSenesi, Letizia, Francesco De Francesco, Andrea Marchesini, Pier Paolo Pangrazi, Maddalena Bertolini, Valentina Riccio, and Michele Riccio. 2023. "Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts" Medicina 59, no. 2: 273. https://doi.org/10.3390/medicina59020273
APA StyleSenesi, L., De Francesco, F., Marchesini, A., Pangrazi, P. P., Bertolini, M., Riccio, V., & Riccio, M. (2023). Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts. Medicina, 59(2), 273. https://doi.org/10.3390/medicina59020273








