Potential Drug-Drug Interactions among Patients with Schizophrenia Spectrum Disorders: Prevalence, Association with Risk Factors, and Replicate Analysis in 2021
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Source
2.3. Potential Drug—Drug Interactions Dataset
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Patients
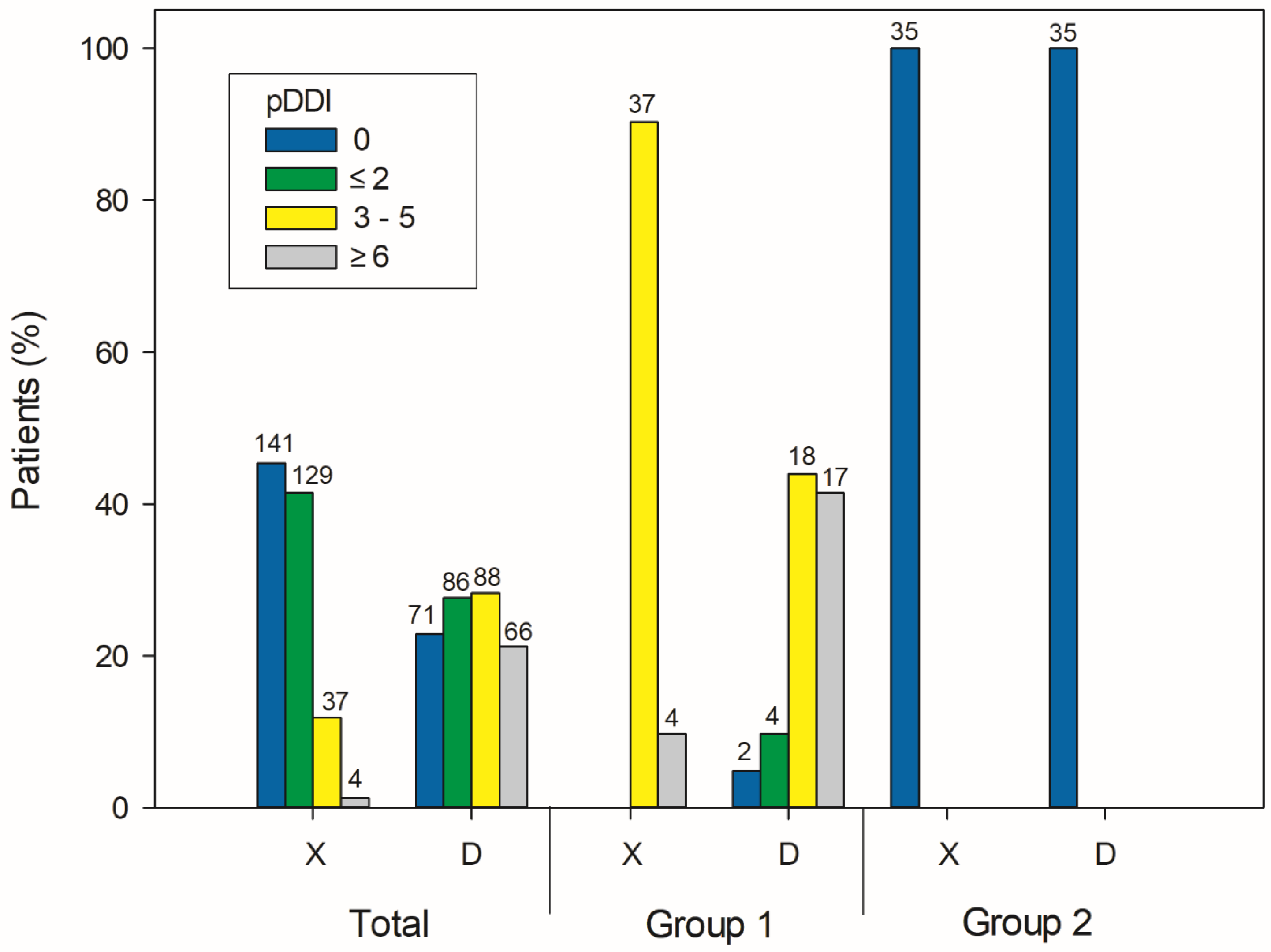
3.2. Prevalence of Potential Drug-Drug Interactions
3.3. Risk Factors for Type X Potential Drug—Drug Interactions
3.4. Risk Factors for the Development of Clinically Observed Symptoms and Signs
3.5. Replicate Analysis of Potential Drug-Drug Interactions in 2021
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: https://icd.who.int/browse10/2010/en (accessed on 8 April 2022).
- Stahl, S.M. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology: Antipsychotics and Mood Stabilizers; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Lakshmikuttyamma, A.; Haghparast, P.; Hajjar, E.; Smith, T.; Pooresfehani, P. Chapter 7—Antipsychotic agents. In Side Effects of Drugs Annual; Ray, S.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 42, pp. 81–89. [Google Scholar]
- Hasan, A.; Falkai, P.; Wobrock, T.; Lieberman, J.; Glenthoj, B.; Gattaz, W.F.; Thibaut, F.; Moller, H.J.; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychiatry 2012, 13, 318–378. [Google Scholar] [CrossRef]
- Barnes, T.R.; Drake, R.; Paton, C.; Cooper, S.J.; Deakin, B.; Ferrier, I.N.; Gregory, C.J.; Haddad, P.M.; Howes, O.D.; Jones, I.; et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2020, 34, 3–78. [Google Scholar] [CrossRef]
- Gallego, J.A.; Bonetti, J.; Zhang, J.; Kane, J.M.; Correll, C.U. Prevalence and correlates of antipsychotic polypharmacy: A systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr. Res. 2012, 138, 18–28. [Google Scholar] [CrossRef]
- Kim, J.J.; Pae, C.U.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S. Exploring Hidden Issues in the Use of Antipsychotic Polypharmacy in the Treatment of Schizophrenia. Clin. Psychopharmacol. Neurosci. 2021, 19, 600–609. [Google Scholar] [CrossRef]
- Viron, M.J.; Stern, T.A. The impact of serious mental illness on health and healthcare. Psychosomatics 2010, 51, 458–465. [Google Scholar] [CrossRef]
- Wolff, J.; Hefner, G.; Normann, C.; Kaier, K.; Binder, H.; Hiemke, C.; Toto, S.; Domschke, K.; Marschollek, M.; Klimke, A. Polypharmacy and the risk of drug-drug interactions and potentially inappropriate medications in hospital psychiatry. Pharmacoepidemiol. Drug Saf. 2021, 30, 1258–1268. [Google Scholar] [CrossRef]
- Castilho, E.C.D.; Reis, A.M.M.; Borges, T.L.; Siqueira, L.D.C.; Miasso, A.I. Potential drug-drug interactions and polypharmacy in institutionalized elderly patients in a public hospital in Brazil. J. Psychiatr. Ment. Health Nurs. 2018, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- English, B.A.; Dortch, M.; Ereshefsky, L.; Jhee, S. Clinically significant psychotropic drug-drug interactions in the primary care setting. Curr. Psychiatry Rep. 2012, 14, 376–390. [Google Scholar] [CrossRef]
- Hines, L.E.; Murphy, J.E. Potentially harmful drug-drug interactions in the elderly: A review. Am. J. Geriatr. Pharmacother. 2011, 9, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Mallet, L.; Spinewine, A.; Huang, A. The challenge of managing drug interactions in elderly people. Lancet 2007, 370, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.A.; Dima, L.; Correll, C.U.; Manu, P. Drug-drug interactions involving antipsychotics and antihypertensives. Expert Opin. Drug Metab. Toxicol. 2022, 18, 285–298. [Google Scholar] [CrossRef]
- Goodlet, K.J.; Zmarlicka, M.T.; Peckham, A.M. Drug-drug interactions and clinical considerations with co-administration of antiretrovirals and psychotropic drugs. CNS Spectr. 2019, 24, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Low, Y.; Setia, S.; Lima, G. Drug-drug interactions involving antidepressants: Focus on desvenlafaxine. Neuropsychiatr. Dis. Treat. 2018, 14, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Toto, S.; Hefner, G.; Hahn, M.; Hiemke, C.; Roll, S.C.; Wolff, J.; Klimke, A. Current use of anticholinergic medications in a large naturalistic sample of psychiatric patients. J. Neural Transm. 2021, 128, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hefner, G.; Hahn, M.; Hiemke, C.; Toto, S.; Wolff, J.; Roll, S.C.; Klimke, A. Pharmacodynamic Drug-Drug interactions of QT-prolonging drugs in hospitalized psychiatric patients. J. Neural Transm. 2021, 128, 243–252. [Google Scholar] [CrossRef]
- Das, B.; Rawat, V.S.; Ramasubbu, S.K.; Kumar, B. Frequency, characteristics and nature of risk factors associated with use of QT interval prolonging medications and related drug-drug interactions in a cohort of psychiatry patients. Therapie 2019, 74, 599–609. [Google Scholar] [CrossRef]
- Khan, Q.; Ismail, M.; Haider, I.; Khan, F. Prevalence of QT interval prolonging drug-drug interactions (QT-DDIs) in psychiatry wards of tertiary care hospitals in Pakistan: A multicenter cross-sectional study. Int. J. Clin. Pharm. 2017, 39, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Iqbal, Z.; Khattak, M.B.; Javaid, A.; Khan, M.I.; Khan, T.M.; Asim, S.M. Potential Drug-Drug Interactions in Psychiatric Ward of a Tertiary Care Hospital: Prevalence, Levels and Association with Risk Factors. Trop. J. Pharm. Res. 2012, 11, 83–94. [Google Scholar] [CrossRef]
- Rankovic, A.; Milentijevic, I.; Jankovic, S. Factors associated with potential drug-drug interactions in psychiatric inpatients. Eur. J. Hosp. Pharm. 2022. [Google Scholar] [CrossRef]
- Kirilochev, O.O.; Dorfman, I.P.; Umerova, A.R.; Bataeva, S.E. Potential drug-drug interactions in the psychiatric hospital: Frequency analysis. Res. Results Pharmacol. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Wolff, J.; Hefner, G.; Normann, C.; Kaier, K.; Binder, H.; Domschke, K.; Hiemke, C.; Marschollek, M.; Klimke, A. Predicting the risk of drug-drug interactions in psychiatric hospitals: A retrospective longitudinal pharmacovigilance study. BMJ Open 2021, 11, e045276. [Google Scholar] [CrossRef] [PubMed]
- Muhic, N.; Mrhar, A.; Brvar, M. Comparative analysis of three drug-drug interaction screening systems against probable clinically relevant drug-drug interactions: A prospective cohort study. Eur. J. Clin. Pharmacol. 2017, 73, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hatton, R.C.; Zhu, Y.; Hincapie-Castillo, J.M.; Bussing, R.; Barnicoat, M.; Winterstein, A.G. Consistency of psychotropic drug-drug interactions listed in drug monographs. J. Am. Pharm. Assoc. 2017, 57, 698–703. [Google Scholar] [CrossRef]
- Monteith, S.; Glenn, T.; Gitlin, M.; Bauer, M. Potential Drug interactions with Drugs used for Bipolar Disorder: A Comparison of 6 Drug Interaction Database Programs. Pharmacopsychiatry 2020, 53, 220–227. [Google Scholar] [CrossRef]
- Nguyen, T.; Liu, X.; Abuhashem, W.; Bussing, R.; Winterstein, A.G. Quality of Evidence Supporting Major Psychotropic Drug-Drug Interaction Warnings: A Systematic Literature Review. Pharmacotherapy 2020, 40, 455–468. [Google Scholar] [CrossRef]
- Magro, L.; Moretti, U.; Leone, R. Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin. Drug Saf. 2012, 11, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Paterno, M.D.; Maviglia, S.M.; Gorman, P.N.; Seger, D.L.; Yoshida, E.; Seger, A.C.; Bates, D.W.; Gandhi, T.K. Tiering drug-drug interaction alerts by severity increases compliance rates. J. Am. Med. Inform. Assoc. 2009, 16, 40–46. [Google Scholar] [CrossRef]
- Smithburger, P.L.; Buckley, M.S.; Bejian, S.; Burenheide, K.; Kane-Gill, S.L. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin. Drug Saf. 2011, 10, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Bačar Bole, C.; Pislar, M.; Mrhar, A.; Tavcar, R. Prescribing patterns for inpatients with schizophrenia spectrum disorders in a psychiatric hospital in Slovenia: Results of 16-month prospective, non-interventional clinical research. Psychiatr. Danub. 2017, 29, 155–161. [Google Scholar] [CrossRef]
- Bačar Bole, C.; Pislar, M.; Sen, M.; Tavcar, R.; Mrhar, A. Switching antipsychotics: Results of 16-month non-interventional, prospective, observational clinical research of inpatients with schizophrenia spectrum disorders. Acta Pharm. 2017, 67, 99–112. [Google Scholar] [CrossRef]
- Lexicomp Online. Lexicomp Drug Interactions Analysis. Available online: https://www.wolterskluwer.com/en/solutions/lexicomp/resources/lexicomp-user-academy/drug-interactions-analysis. (accessed on 8 July 2022).
- Jazbar, J.; Locatelli, I.; Horvat, N.; Kos, M. Clinically relevant potential drug-drug interactions among outpatients: A nationwide database study. Res. Social Adm. Pharm. 2018, 14, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Vegh, A.; Lanko, E.; Fittler, A.; Vida, R.G.; Miseta, I.; Takacs, G.; Botz, L. Identification and evaluation of drug-supplement interactions in Hungarian hospital patients. Int. J. Clin. Pharm. 2014, 36, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Namazi, S.; Pourhatami, S.; Borhani-Haghighi, A.; Roosta, S. Incidence of Potential Drug-Drug Interaction and Related Factors in Hospitalized Neurological Patients in two Iranian Teaching Hospitals. Iran. J. Med. Sci. 2014, 39, 515–521. [Google Scholar]
- Aburamadan, H.A.R.; Sridhar, S.B.; Tadross, T.M. Assessment of potential drug interactions among psychiatric inpatients receiving antipsychotic therapy of a secondary care hospital, United Arab Emirates. J. Adv. Pharm. Technol. Res. 2021, 12, 45–51. [Google Scholar] [CrossRef]
- Guo, J.J.; Wu, J.; Kelton, C.M.; Jing, Y.; Fan, H.; Keck, P.E.; Patel, N.C. Exposure to potentially dangerous drug-drug interactions involving antipsychotics. Psychiatr. Serv. 2012, 63, 1080–1088. [Google Scholar] [CrossRef]
- Ocana-Zurita, M.C.; Juarez-Rojop, I.E.; Genis, A.; Tovilla-Zarate, C.A.; Gonzalez-Castro, T.B.; Lilia Lopez-Narvaez, M.; de la O de la O, M.E.; Nicolini, H. Potential drug-drug interaction in Mexican patients with schizophrenia. Int. J. Psychiatry Clin. Pract. 2016, 20, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, Y.; Fujii, K.; Kurimoto, N.; Yamada, N.; Okawa, M.; Aoki, T.; Takahashi, J.; Ishida, N.; Horie, M.; Kunugi, H. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, S.K.; Mishra, A.; Mandal, S. Prevalence of QT-Prolonging Drug-Drug Interactions in Psychiatry: A Systematic Review and Meta Analysis. J. Pharm. Pract. 2022, 8971900221121371. [Google Scholar] [CrossRef]
- Nose, M.; Bighelli, I.; Castellazzi, M.; Martinotti, G.; Carra, G.; Lucii, C.; Ostuzzi, G.; Sozzi, F.; Barbui, C.; Star Network, G. Prevalence and correlates of QTc prolongation in Italian psychiatric care: Cross-sectional multicentre study. Epidemiol. Psychiatr. Sci. 2016, 25, 532–540. [Google Scholar] [CrossRef]
- Han, J.; Shen, M.; Wan, Q.; Lv, Z.; Xiao, L.; Wang, G. Risk factors for community-acquired pneumonia among inpatients with mental disorders in a tertiary general hospital. Front. Psychiatry 2022, 13, 941198. [Google Scholar] [CrossRef]
- Pankiewicz-Dulacz, M.; Stenager, E.; Chen, M.; Stenager, E.N. Risk factors of major infections in schizophrenia. A nationwide Danish register study. J. Psychosom. Res. 2019, 121, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Haga, T.; Ito, K.; Sakashita, K.; Iguchi, M.; Ono, M.; Tatsumi, K. Risk factors for pneumonia in patients with schizophrenia. Neuropsychopharmacol. Rep. 2018, 38, 204–209. [Google Scholar] [CrossRef]
- Bryant, A.D.; Fletcher, G.S.; Payne, T.H. Drug interaction alert override rates in the Meaningful Use era: No evidence of progress. Appl. Clin. Inform. 2014, 5, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Isaac, T.; Weissman, J.S.; Davis, R.B.; Massagli, M.; Cyrulik, A.; Sands, D.Z.; Weingart, S.N. Overrides of medication alerts in ambulatory care. Arch. Intern. Med. 2009, 169, 305–311. [Google Scholar] [CrossRef]
- Poly, T.N.; Islam, M.M.; Yang, H.C.; Li, Y.J. Appropriateness of Overridden Alerts in Computerized Physician Order Entry: Systematic Review. JMIR Med. Inform. 2020, 8, e15653. [Google Scholar] [CrossRef]
- Monteith, S.; Glenn, T. Comparison of potential psychiatric drug interactions in six drug interaction database programs: A replication study after 2 years of updates. Hum. Psychopharmacol. 2021, 36, e2802. [Google Scholar] [CrossRef] [PubMed]
- Stuhec, M.; Lah, L. Clinical pharmacist interventions in elderly patients with mental disorders in primary care focused on psychotropics: A retrospective pre-post observational study. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211011007. [Google Scholar] [CrossRef]

| Total No. (%) | Group 1 a | Group 2 b | |
|---|---|---|---|
| Patients, n | 311 (100) | 41 (13) | 35 (11) |
| Men, n | 179 (58) | 17 (42) | 23 (66) |
| Women, n | 132 (43) | 24 (59) | 12 (34) |
| Age [years], median (Q1–Q3) | 42 (31–52) | 44 (32–52) | 43 (27–51) |
| Smokers, n | 213 (68) | 30 (73) | 28 (80) |
| Time of hospitalization [days], median (Q1–Q3) | 34 (18–69) | 79 (50–142) | 31.0 (16–61) * |
| Number of hospitalizations, median (Q1–Q3) | 1 (1–2) | 1 (1–2) | 1 (1–1) * |
| Number of prescribed drugs, median (Q1–Q3) | 7 (5–10) | 12 (8–15) | 4 (2–6) * |
| Polypharmacotherapy, n (%) c | 242 (77.8) | 41 (100) | 13 (37.1) * |
| Number of antipsychotics, median (Q1–Q3) | 2 (2–3) | 4 (3–5) | 2 (1–2) * |
| Antipsychotic PDD/DDD, median (Q1–Q3) | / | 1.7 (1.3–2.1) | 0.93 (0.73–1.50) * |
| Type X Potential Drug—Drug Interaction | No. of Patients (%) | Summary | Patient Management | Interaction Severity | Reliability Rating | |
|---|---|---|---|---|---|---|
| Diazepam | Olanzapine | 59 (19.0) | Olanzapine may enhance the adverse/toxic effect of benzodiazepines. | Additive adverse effects: cardiorespiratory depression, excessive sedation. | major | good |
| Lorazepam | Olanzapine | 26 (8.4) | ||||
| Alprazolam | Olanzapine | 23 (7.4) | ||||
| Midazolam | Olanzapine | 12 (3.9) | ||||
| Haloperidol | Quetiapine | 24 (7.7) | QT-prolonging antipsychotics. | Monitor for QTc interval prolongation and ventricular arrhythmias. | major | good |
| Haloperidol | Flupenthixol | 9 (2.9) | ||||
| Clozapine | Flupenthixol | 12 (3.9) | Anticholinergic agents may enhance the adverse/toxic effect of other anticholinergic agents. | Additive anticholinergic effects: dry mouth, dry eyes, blurred vision, urinary retention, and constipation. | major | good |
| Clozapine | Quetiapine | 10 (3.2) | major | good | ||
| Quetiapine | Zuclopenthixol | 9 (2.9) | major | good | ||
| Amisulpride | Clozapine | 9 (2.9) | Amisulpride may enhance the adverse/toxic effect of antipsychotics. | Symptoms and signs of neuroleptic malignant syndrome. | major | fair |
| Variables | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | P-Value | |
| Age | 1.02 (0.98–1.06) | 0.293 | 0.97 (0.89–1.04) | 0.376 |
| Sex | 0.37 (0.14–0.93) | 0.037 | 0.48 (0.07–2.81) | 0.425 |
| Number of drugs | 2.67 (1.79–5.02) | <0.001 | 2.85 (1.84–5.73) | <0.001 |
| Number of antipsychotics | 6.62 (3.19–18.04) | <0.001 | / # | / # |
| PDD/DDD | 4.85 (1.75–14.73) | 0.003 | / # | / # |
| Symptoms and Signs | Multivariate Model | Variables | ||||
|---|---|---|---|---|---|---|
| Age | Sex | Number of Drugs | PDD/DDD | Type X pDDIs | ||
| Nervous system and psychiatric disorders | IRR (95% CI) | 1.00 (0.99–1.01) | 1.09 (0.86–1.39) | 1.06 (1.04–1.09) | 1.33 (1.12–1.58) | 1.46 (0.99–2.17) |
| p-value | 0.913 | 0.499 | <0.001 | 0.012 | 0.099 | |
| Gastrointestinal, hepatobiliary, metabolic, endocrine, renal and urinary disorders | IRR (95% CI) | 1.00 (0.99–1.01) | 0.66 (0.45–0.97) | 1.14 (1.09–1.19) | 1.10 (0.84–1.43) | 0.81 (0.48–1.38) |
| p-value | 0.799 | 0.031 | <0.001 | 0.540 | 0.562 | |
| Cardiac and vascular disorders | IRR (95% CI) | 1.03 (0.99–1.08) | 0.50 (0.18–1.36) | 1.22 (1.02–1.45) | 0.73 (0.40–1.35) | 0.55 (0.09–3.48) |
| p-value | 0.116 | 0.212 | 0.012 | 0.429 | 0.523 | |
| Respiratory disorders | IRR (95% CI) | 1.06 (1.01–1.09) | 0.67 (0.16–2.75) | 1.19 (1.04–1.37) | 1.26 (0.55–2.89) | / |
| p-value | 0.095 | 0.530 | <0.043 | 0.647 | / | |
| Others | IRR (95% CI) p-value | 1.00 (0.98–1.03) 0.705 | 0.78 (0.47–1.31) 0.335 | 1.22 (1.15–1.30) <0.001 | 1.42 (1.02–1.97) 0.102 | 0.28 (0.12–0.61) 0.006 |
| Analysis 2014 | Analysis 2021 | |
|---|---|---|
| pDDIs in all patients | ||
| Patients with type X pDDIs | 170 ** | 121 |
| Patients with type D pDDIs | 240 ** | 207 |
| Total number of type X pDDIs | 308 | 217 |
| Total number of type D pDDIs | 942 | 1043 |
| Number of different type X pDDIs | 78 | 54 |
| Number of different type D pDDIs | 166 | 158 |
| Max number of type X pDDIs per patient (type D) | 10 (21) | 9 (24) |
| pDDIs in specific group | ||
| Group 1 a | 41 | 17 |
| Group 2 b | 35 | 63 |
| Changes of pDDI in all patients | ||
| X → X | / | 38 |
| X → D | / | 5 |
| X → C, B, A, N | / | 35 |
| New X | / | 16 |
| D → D | / | 75 |
| D → X | / | 0 |
| D → C, B, A, N | / | 91 |
| New D | / | 83 |
| Most frequent pDDIs | ||
| 1. type X pDDIs (n) | Diazepam—Olanzapine (59) | Diazepam—Olanzapine (59) |
| 2. type X pDDIs (n) | Lorazepam—Olanzapine (26) | Lorazepam—Olanzapine (26) |
| 3. type X pDDIs (n) | Alprazolam—Olanzapine (23) | Alprazolam—Olanzapine (23) |
| 4. type X pDDIs (n) | Haloperidol—Quetiapine (24) | Midazolam—Olanzapine (12) |
| 5. type X pDDIs (n) | Midazolam—Olanzapine (12) | Amisulpride—Clozapine (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bačar Bole, C.; Nagode, K.; Pišlar, M.; Mrhar, A.; Grabnar, I.; Vovk, T. Potential Drug-Drug Interactions among Patients with Schizophrenia Spectrum Disorders: Prevalence, Association with Risk Factors, and Replicate Analysis in 2021. Medicina 2023, 59, 284. https://doi.org/10.3390/medicina59020284
Bačar Bole C, Nagode K, Pišlar M, Mrhar A, Grabnar I, Vovk T. Potential Drug-Drug Interactions among Patients with Schizophrenia Spectrum Disorders: Prevalence, Association with Risk Factors, and Replicate Analysis in 2021. Medicina. 2023; 59(2):284. https://doi.org/10.3390/medicina59020284
Chicago/Turabian StyleBačar Bole, Cvetka, Katja Nagode, Mitja Pišlar, Aleš Mrhar, Iztok Grabnar, and Tomaž Vovk. 2023. "Potential Drug-Drug Interactions among Patients with Schizophrenia Spectrum Disorders: Prevalence, Association with Risk Factors, and Replicate Analysis in 2021" Medicina 59, no. 2: 284. https://doi.org/10.3390/medicina59020284






