Surgical Treatment for Advanced Oropharyngeal Cancer: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Searching Protocol
2.2. Inclusion/Exclusion Criteria
3. Results
3.1. Overall Surgical Strategies
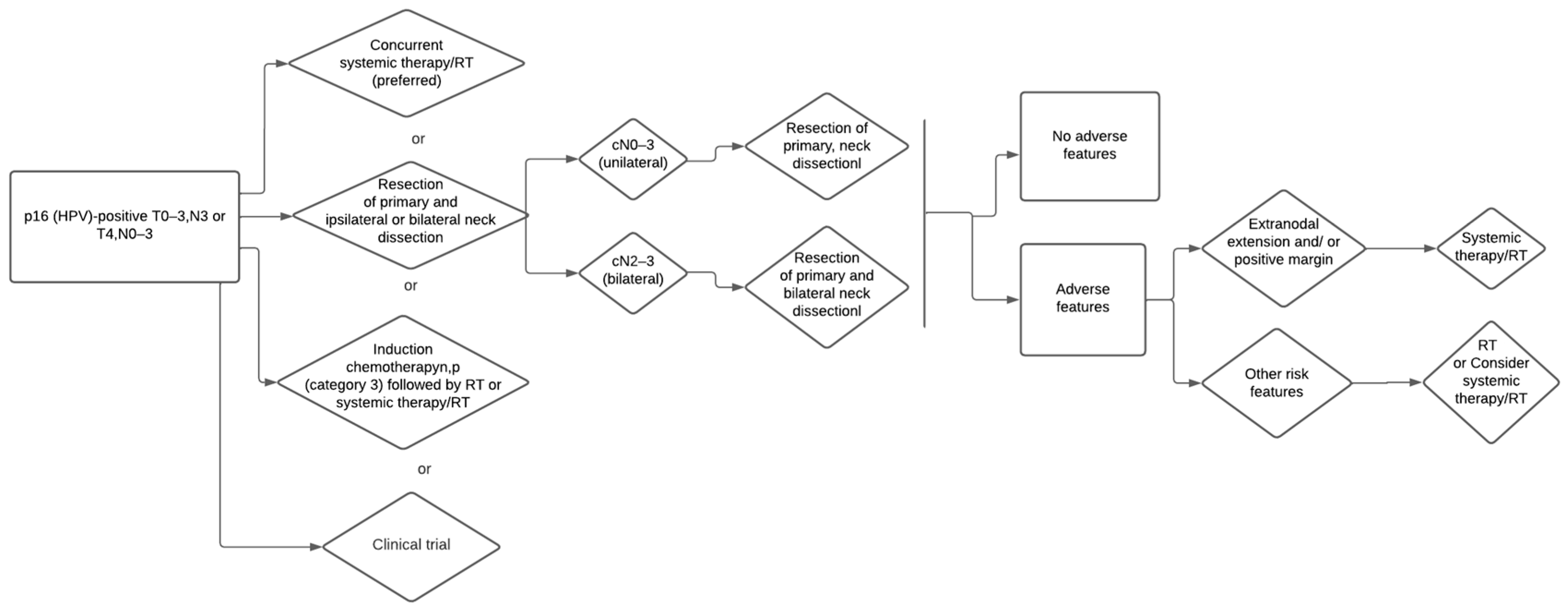
3.2. Primary Surgery for HPV/p16 Positive
3.3. Primary Surgery for HPV/p16 Negative
3.4. De-Intensification for Adjuvant Treatment
3.5. Salvage Surgical Treatment
3.6. Surgical Limits and Contraindications
3.7. Checkpoint-Inhibitors Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Schouten, C.S.; de Graaf, P.; Alberts, F.M.; Hoekstra, O.S.; Comans, E.F.; Bloemena, E.; Witte, B.I.; Sanchez, E.; Leemans, C.R.; Castelijns, J.A.; et al. Response evaluation after chemoradiotherapy for advanced nodal disease in head and neck cancer using diffusion-weighted MRI and 18F-FDG-PET-CT. Oral Oncol. 2015, 51, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Jones, O.S.; Breeze, C.E.; Gilson, R. Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect. Dis. 2019, 19, 131–132. [Google Scholar] [CrossRef]
- Faraji, F.; Rettig, E.M.; Tsai, H.; El Asmar, M.; Fung, N.; Eisele, D.W.; Fakhry, C. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer 2019, 125, 761–769. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hübbers, C.U.; Akgül, B. HPV and cancer of the oral cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Rietbergen, M.M.; Witte, B.I.; Velazquez, E.R.; Snijders, P.J.F.; Bloemena, E.; Speel, E.J.; Brakenhoff, R.H.; Kremer, B.; Lambin, P.; Leemans, C.R. Different prognostic models for different patient populations: Validation of a new prognostic model for patients with oropharyngeal cancer in Western Europe. Br. J. Cancer 2015, 112, 1733–1736. [Google Scholar] [CrossRef]
- Perrone, F.; Gloghini, A.; Cortelazzi, B.; Bossi, P.; Licitra, L.; Pilotti, S. Isolating p16-positive/HPV-negative oropharyngeal cancer: An effort worth making. Am. J. Surg. Pathol. 2011, 35, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Carlander, A.F.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Jensen, J.S.; Grønhøj, C.; von Buchwald, C. A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses 2021, 13, 1326. [Google Scholar] [CrossRef]
- Iyer, N.G.; Dogan, S.; Palmer, F.; Rahmati, R.; Nixon, I.; Lee, N.; Patel, S.G.; Shah, J.; Ganly, I. Detailed Analysis of Clinicopathologic Factors Demonstrate Distinct Difference in Outcome and Prognostic Factors Between Surgically Treated HPV-Positive and Negative Oropharyngeal Cancer. Ann. Surg. Oncol. 2015, 22, 4411–4421. [Google Scholar] [CrossRef] [PubMed]
- Tham, T.; Wotman, M.; Roche, A.; Kraus, D.; Costantino, P. The prognostic effect of anatomic subsite in HPV-positive oropharyngeal squamous cell carcinoma. Am. J. Otolaryngol. 2019, 40, 567–572. [Google Scholar] [CrossRef]
- Baumeister, P.; Reiter, M.; Welz, C.; Becker, S.; Betz, C.; Harréus, U. Surgically treated oropharyngeal cancer: Risk factors and tumor characteristics. J. Cancer Res. Clin. Oncol. 2014, 140, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Hitt, R.; Iglesias, L.; López-Pousa, A.; Berrocal-Jaime, A.; Grau, J.J.; García-Girón, C.; Martínez-Trufero, J.; Guix, M.; Lambea-Sorrosal, J.; del Barco-Morillo, E.; et al. Long-term outcomes of induction chemotherapy followed by chemoradiotherapy vs chemoradiotherapy alone as treatment of unresectable head and neck cancer: Follow-up of the Spanish Head and Neck Cancer Group (TTCC) 2503 Trial. Clin. Transl. Oncol. 2020, 23, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Shibata, M.; Hamauchi, S.; Shirasu, H.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Kawakami, T.; Furuta, M.; Inoue, H.; et al. Feasibility and efficacy of chemoradiotherapy with concurrent split-dose cisplatin after induction chemotherapy with docetaxel/cisplatin/5-fluorouracil for locally advanced head and neck cancer. Mol. Clin. Oncol. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Harsh, K.K.; Maharia, S.R.; Nirban, R.K.; Khatri, P.; Beniwal, S.; Kumar, H.S.; Jakhar, S.L. Metronomic palliative chemotherapy in locally advanced, recurrent and metastatic head-and-neck cancer: A single-arm, retrospective study of a regional cancer center of North India (Asia). J. Cancer Res. Ther. 2020, 16, 559–564. [Google Scholar] [PubMed]
- Clark, J.M.; Holmes, E.M.; O’Connell, D.A.; Harris, J.; Seikaly, H.; Biron, V.L. Long-term survival and swallowing outcomes in advanced stage oropharyngeal squamous cell carcinomas. Papillomavirus Res. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Dwivedi, R.C.; Masterson, L.K.; Kothari, P.; Quon, H.; Holsinger, F.C. De-intensified adjuvant (chemo)radiotherapy versus standard adjuvant chemoradiotherapy post transoral minimally invasive surgery for resectable HPV-positive oropharyngeal carcinoma. Cochrane Database Syst. Rev. 2018, 12, Cd012939. [Google Scholar]
- Sun, X.S.; Tao, Y.; Le Tourneau, C.; Pointreau, Y.; Sire, C.; Kaminsky, M.C.; Coutte, A.; Boisselier, P.; Martin, L.; Miroir, J.; et al. Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: A double-blind, multicentre, randomised, phase 2 study. Lancet Oncol. 2020, 21, 1173–1187. [Google Scholar] [CrossRef]
- Patil, V.; Noronha, V.; Dhumal, S.B.; Joshi, A.; Menon, N.; Bhattacharjee, A.; Kulkarni, S.; Ankathi, S.K.; Mahajan, A.; Sable, N.; et al. Low-cost oral metronomic chemotherapy versus intravenous cisplatin in patients with recurrent, metastatic, inoperable head and neck carcinoma: An open-label, parallel-group, non-inferiority, randomised, phase 3 trial. Lancet Glob. Health 2020, 8, e1213–e1222. [Google Scholar] [CrossRef]
- Tian, X.; Xuan, Y.; Wu, R.; Gao, S. Nimotuzumab Combined with Induction Chemotherapy and Concurrent Chemoradiotherapy in Unresectable Locally Advanced Hypopharyngeal Carcinoma: A Single Institution Experience in China. Cancer Manag. Res. 2020, 12, 3323–3329. [Google Scholar] [CrossRef]
- Zenga, J.; Wilson, M.; Adkins, D.R.; Gay, H.A.; Haughey, B.H.; Kallogjeri, D.; Michel, L.S.; Paniello, R.C.; Rich, J.T.; Thorstad, W.L.; et al. Treatment Outcomes for T4 Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol. Neck Surg. 2015, 141, 1118–1127. [Google Scholar] [CrossRef]
- Piazza, C.; Dessouky, O.; Peretti, G.; De Benedetto, L.; Nicolai, P. Narrow band imaging: A new tool for evaluation of head and neck squamous cell carcinomas. Review of the literature. Acta Otorhinolaryngol. Ital. 2008, 28, 49–54. [Google Scholar]
- Ni, X.-G.; Wang, G.-Q. The Role of Narrow Band Imaging in Head and Neck Cancers. Curr. Oncol. Rep. 2016, 18, 10. [Google Scholar] [CrossRef]
- Gono, K.; Yamazaki, K.; Doguchi, N.; Nonami, T.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; et al. Endoscopic observation of tissue by narrowband illumination. Opt. Rev. 2003, 10, 211–215. [Google Scholar] [CrossRef]
- Wang, W.-H.; Lin, Y.-C.; Lee, K.-F.; Weng, H.-H. Nasopharyngeal carcinoma detected by narrow-band imaging endoscopy. Oral Oncol. 2011, 47, 736–741. [Google Scholar] [CrossRef]
- Watanabe, A.; Taniguchi, M.; Tsujie, H.; Hosokawa, M.; Fujita, M.; Sasaki, S. The value of narrow band imaging for early detection of laryngeal cancer. Eur. Arch. Otorhinolaryngol. 2009, 266, 1017–1023. [Google Scholar] [CrossRef]
- De Vito, A.; Meccariello, G.; Vicini, C. Narrow band imaging as screening test for early detection of laryngeal cancer: A prospective study. Clin. Otolaryngol. 2017, 42, 347–353. [Google Scholar] [CrossRef]
- Ansari, U.H.; Wong, E.; Smith, M.; Singh, N.; Palme, C.E.; Smith, M.C.; Riffat, F. Validity of narrow band imaging in the detection of oral and oropharyngeal malignant lesions: A systematic review and meta-analysis. Head Neck 2019, 41, 2430–2440. [Google Scholar] [CrossRef]
- Matsuba, H.; Katada, C.; Masaki, T.; Nakayama, M.; Okamoto, T.; Hanaoka, N.; Tanabe, S.; Koizumi, W.; Okamoto, M.; Muto, M. Diagnosis of the extent of advanced oropharyngeal and hypopharyngeal cancers by narrow band imaging with magnifying endoscopy. Laryngoscope 2011, 121, 753–759. [Google Scholar] [CrossRef]
- Piazza, C.; Del Bon, F.; Paderno, A.; Grazioli, P.; Perotti, P.; Barbieri, D.; Majorana, A.; Bardellini, E.; Peretti, G.; Nicolai, P. The diagnostic value of narrow band imaging in different oral and oropharyngeal subsites. Eur. Arch. Otorhinolaryngol. 2016, 273, 3347–3353. [Google Scholar] [CrossRef]
- Tirelli, G.; Piovesana, M.; Gatto, A.; Torelli, L.; Di Lenarda, R.; Nata, F.B. NBI utility in the pre-operative and intra-operative assessment of oral cavity and oropharyngeal carcinoma. Am. J. Otolaryngol. 2017, 38, 65–71. [Google Scholar] [CrossRef]
- Tirelli, G.; Nata, F.B.; Gatto, A.; Bussani, R.; Spinato, G.; Zacchigna, S.; Piovesana, M. Intraoperative Margin Control in Transoral Approach for Oral and Oropharyngeal Cancer. Laryngoscope 2019, 129, 1810–1815. [Google Scholar] [CrossRef]
- Ottaviani, G.; Gobbo, M.; Rupel, K.; D’Ambros, M.; Perinetti, G.; Di Lenarda, R.; Martinelli, V.; Bussani, R.; Tirelli, G.; Lodi, G.; et al. The diagnostic performance parameters of narrow band imaging: A preclinical and clinical study. Oral Oncol. 2016, 60, 130–136. [Google Scholar] [CrossRef]
- Vicini, C.; Montevecchi, F.; D’Agostino, G.; De Vito, A.; Meccariello, G. A novel approach emphasising intra-operative superficial margin enhancement of head-neck tumours with narrow-band imaging in transoral robotic surgery. Acta Otorhinolaryngol. Ital. 2015, 35, 157–161. [Google Scholar]
- Meccariello, G.; Montevecchi, F.; D’Agostino, G.; Iannella, G.; Calpona, S.; Parisi, E.; Costantini, M.; Cammaroto, G.; Gobbi, R.; Firinu, E.; et al. Trans-oral robotic surgery for the management of oropharyngeal carcinomas: A 9-year institutional experience. Acta Otorhinolaryngol. Ital. 2019, 39, 75–83. [Google Scholar] [CrossRef]
- Carta, F.; Sionis, S.; Cocco, D.; Gerosa, C.; Ferreli, C.; Puxeddu, R. Enhanced contact endoscopy for the assessment of the neoangiogenetic changes in precancerous and cancerous lesions of the oral cavity and oropharynx. Eur. Arch. Otorhinolaryngol. 2016, 273, 1895–1903. [Google Scholar] [CrossRef]
- Abraham, J. Imaging for head and neck cancer. Surg. Oncol. Clin. N. Am. 2015, 24, 455–471. [Google Scholar] [CrossRef]
- Trotta, B.M.; Pease, C.S.; Rasamny, J.J.; Raghavan, P.; Mukherjee, S. Oral cavity and oropharyngeal squamous cell cancer: Key imaging findings for staging and treatment planning. Radiographics 2011, 31, 339–354. [Google Scholar] [CrossRef]
- Glastonbury, C.M. Head and Neck Squamous Cell Cancer: Approach to Staging and Surveillance. In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2020; Chapter 17. [Google Scholar] [PubMed]
- Wu, J.; Gensheimer, M.F.; Zhang, N.; Guo, M.; Liang, R.; Zhang, C.; Fischbein, N.; Pollom, E.L.; Beadle, B.; Le, Q.-T.; et al. Tumor Subregion Evolution-Based Imaging Features to Assess Early Response and Predict Prognosis in Oropharyngeal Cancer. J. Nucl. Med. 2020, 61, 327–336. [Google Scholar] [CrossRef]
- Faraji, F.; Coquia, S.F.; Wenderoth, M.B.; Padilla, E.S.; Blitz, D.; DeJong, M.R.; Aygun, N.; Hamper, U.M.; Fakhry, C. Evaluating oropharyngeal carcinoma with transcervical ultrasound, CT, and MRI. Oral Oncol. 2018, 78, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Muylle, K.; Castaigne, C.; Flamen, P. 18F-fluoro-2-deoxy-D-glucose positron emission tomographic imaging: Recent developments in head and neck cancer. Curr. Opin. Oncol. 2005, 17, 249–253. [Google Scholar] [CrossRef]
- Tahari, A.K.; Alluri, K.C.; Quon, H.; Koch, W.; Wahl, R.L.; Subramaniam, R.M. FDG PET/CT imaging of oropharyngeal squamous cell carcinoma: Characteristics of human papillomavirus-positive and -negative tumors. Clin. Nucl. Med. 2014, 39, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Golusiński, W.; Golusińska-Kardach, E. Current Role of Surgery in the Management of Oropharyngeal Cancer. Front. Oncol. 2019, 9, 388. [Google Scholar] [CrossRef]
- Ford, S.E.; Brandwein-Gensler, M.; Carroll, W.R.; Rosenthal, E.L.; Magnuson, J.S. Transoral robotic versus open surgical approaches to oropharyngeal squamous cell carcinoma by human papillomavirus status. Otolaryngol. Head Neck Surg. 2014, 151, 606–611. [Google Scholar] [CrossRef]
- Liederbach, E.; Lewis, C.M.; Yao, K.; Brockstein, B.E.; Wang, C.-H.; Lutfi, W.; Bhayani, M.K. A Contemporary Analysis of Surgical Trends in the Treatment of Squamous Cell Carcinoma of the Oropharynx from 1998 to 2012: A Report from the National Cancer Database. Ann. Surg. Oncol. 2015, 22, 4422–4431. [Google Scholar] [CrossRef]
- Moore, E.J.; Olsen, S.M.; Laborde, R.R.; García, J.J.; Walsh, F.J.; Price, D.L.; Janus, J.R.; Kasperbauer, J.L.; Olsen, K.D. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin. Proc. 2012, 87, 219–225. [Google Scholar] [CrossRef]
- Moore, E.J.; Hinni, M.L. Critical review: Transoral laser microsurgery and roboticassisted surgery for oropharynx cancer including human papilloma virusrelated cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1163–1167. [Google Scholar] [CrossRef]
- Hutcheson, K.A.; Holsinger, F.C.; Kupferman, M.F.; Lewin, J.S. Functional outcomes after TORS for oropharyngeal cancer, a systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 463–471. [Google Scholar] [CrossRef]
- Dowthwaite, S.A.; Franklin, J.H.; Palma, D.A.; Fung, K.; Yoo, J.; Nichols, A.C. The role of transoral robotic surgery in the management of oropharyngeal cancer: A review of the literature. ISRN Oncol. 2012, 2012, 945162. [Google Scholar] [CrossRef]
- Dhanireddy, B.; Burnett, N.P.; Sanampudi, S.; Wooten, C.E.; Slezak, J.; Shelton, B.; Shelton, L.; Shearer, A.; Arnold, S.; Kudrimoti, M.; et al. Outcomes in surgically resectable oropharynx cancer treated with transoral robotic surgery versus definitive chemoradiation. Am. J. Otolaryngol. 2019, 40, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Gangwani, K.; Shetty, L.; Seshagiri, R.; Kulkarni, D. Comparison of TORS with Conventional Surgery for Oropharyngeal Carcinomas in T1-T4 Lesions. Ann. Maxillofac. Surg. 2019, 9, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, G.; Bianchi, G.; Calpona, S.; Parisi, E.; Cammaroto, G.; Iannella, G.; Sgarzani, R.; Montevecchi, F.; De Vito, A.; Capaccio, P.; et al. Trans oral robotic surgery versus definitive chemoradiotherapy for oropharyngeal cancer: 10-year institutional experience. Oral Oncol. 2020, 110, 104889. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Costantino, A.; Mercante, G.; Petruzzi, G.; Sebastiani, D.; Franzese, C.; Scorsetti, M.; Pellini, R.; Malvezzi, L.; Spriano, G. Present and Future of De-intensification Strategies in the Treatment of Oropharyngeal Carcinoma. Curr. Oncol. Rep. 2020, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Skillington, S.A.; Kallogjeri, D.; Lewis, J.S.; Piccirillo, J.F. The Role of Adjuvant Chemotherapy in Surgically Managed, p16-Positive Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2017, 9, 253–259. [Google Scholar] [CrossRef]
- Tsukahara, K.; Shimizu, A.; Ito, T.; Yamashita, G.; Okamoto, I. Second postoperative hemorrhage five weeks after transoral robotic surgery. Auris Nasus Larynx 2020, 49, 304–307. [Google Scholar] [CrossRef]
- O’Malley, B.W., Jr.; Quon, H.; Leonhardt, F.D.; Chalian, A.A.; Weinstein, G.S. Transoral robotic surgery for parapharyngeal space tumors. J. Oto-Rhino-Laryngol. Its Relat. Spec. 2010, 72, 332–336. [Google Scholar] [CrossRef]
- Porcuna, D.V.; Guisasola, C.P.; Soria, C.V.; Cirauqui, B.C.; Muñoz, P.L.; Collurá, F.; Nin, R.M. Transoral robotic surgery for squamous cell carcinoma of the oropharynx in a primarily human papillomavirus-negative patient population. Clin. Transl. Oncol. 2020, 22, 1303–1311. [Google Scholar] [CrossRef]
- Price, J.M.; West, C.M.; Mistry, H.B.; Betts, G.; Bishop, P.; Kennedy, J.; Dixon, L.; Homer, J.J.; Garcez, K.P.; Lee, L.W.; et al. Thomson DJ Improved survival prediction for oropharyngeal cancer beyond TNMv8. Oral Oncol. 2021, 115, 105140. [Google Scholar] [CrossRef]
- Würdemann, N.; Wagner, S.; Sharma, S.J.; Prigge, E.-S.; Reuschenbach, M.; Gattenlöhner, S.; Klussmann, J.P.; Wittekindt, C. Prognostic Impact of AJCC/UICC 8th Edition New Staging Rules in Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2017, 7, 129. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Gava, A.; Baggio, V.; Marchiori, C.; Stellin, M.; Fuson, R.; Lamon, S.; Mosto, M.C.D. Matched survival analysis in patients with locoregionally advanced resectable oropharyngeal carcinoma: Platinum-based induction and concurrent chemoradiotherapy versus primary surgical resection. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, C.; Jeffery, C.; Clark, J.M.; O’Connell, D.; Harris, J.; Seikaly, H. Transoral Robotic Surgery Versus Chemoradiation Treatment in Oropharyngeal Cancer: Case-matched Comparison of Survival and Swallowing Outcomes. Res. Sq. 2019. [Google Scholar] [CrossRef]
- Stokes, W.; Ramadan, J.; Lawson, G.; Ferris, F.R.L.; Holsinger, F.C.; Turner, M.T. Bleeding complications after transoral robotic surgery: A meta-analysis and systematic review. Laryngoscope 2021, 131, 95–105. [Google Scholar] [CrossRef]
- Baskin, R.M.; Boyce, B.J.; Amdur, R.; Mendenhall, W.M.; Hitchcock, K.; Silver, N.; Dziegielewski, P.T. Transoral robotic surgery for oropharyngeal cancer: Patient selection and special considerations. Cancer Manag. Res. 2018, 10, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Pipkorn, P.; Zenga, J.; Haughey, B.H. The Hybrid Transoral-Pharyngotomy Approach to Oropharyngeal Carcinoma: Technique and Outcome. Ann. Otol. Rhinol. Laryngol. 2017, 126, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, L.; Baltres, A.; Fiani, D.J.; Zrounba, P.; Buiret, G.; Fleury, B.; Benzerdjeb, N.; Grégoire, V. Prevalence and distribution of cervical lymph node metastases in HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma. Radiother. Oncol. 2021, 157, 122–129. [Google Scholar] [CrossRef]
- Korsten, L.H.A.; Jansen, F.; Lissenberg-Witte, B.I.; Vergeer, M.; Brakenhoff, R.H.; Leemans, C.R.; Verdonck-de Leeuw, I.M. The course of health-related quality of life from diagnosis to two years follow-up in patients with oropharyngeal cancer: Does HPV status matter? Support Care Cancer 2021, 29, 4473–4483. [Google Scholar] [CrossRef]
- Marks, J.A.; Switchenko, J.M.; Steuer, C.E.; Ryan, M.; Patel, M.R.; McDonald, M.W.; Higgins, K.; Beitler, J.J.; Shin, D.M.; Gillespie, T.W.; et al. Socioeconomic Factors Influence the Impact of Tumor HPV Status on Outcome of Patients with Oropharyngeal Squamous Cell Carcinoma. JCO Oncol. Pract. 2021, 17, e313–e322. [Google Scholar] [CrossRef]
- Jiarpinitnun, C.; Larbcharoensub, N.; Pattaranutaporn, P.; Chureemas, T.; Juengsamarn, J.; Trachu, N.; Lukerak, S.; Chansriwong, P.; Ngamphaiboon, N. Characteristics and Impact of HPV-Associated p16 Expression on Head and Neck Squamous Cell Carcinoma in Thai Patients. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1679–1687. [Google Scholar] [CrossRef]
- Culié, D.; Viotti, J.; Modesto, A.; Schiappa, R.; Chamorey, E.; Dassonville, O.; Poissonnet, G.; Guelfucci, B.; Bizeau, A.; Vergez, S.; et al. Upfront surgery or definitive radiotherapy for patients with p16-negative oropharyngeal squamous cell carcinoma. A GETTEC multicentric study. Eur. J. Surg. Oncol. 2021, 47, 367–374. [Google Scholar] [CrossRef]
- Dabas, S.; Gupta, K.; Sharma, A.K.; Shukla, H.; Ranjan, R.; Sharma, D.K. Oncological outcome following initiation of treatment for stage III and IV HPV negative oropharyngeal cancers with transoral robotic surgery (TORS). Eur. J. Surg. Oncol. 2019, 45, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.R.; Noel, C.W.; Veigas, M.; Martino, R.; Chepeha, D.B.; Bratman, S.V.; Goldstein, D.P.; Hansen, A.R.; Yu, E.; Metser, U.; et al. Finding/identifying primaries with neck disease (FIND) clinical trial protocol: A study integrating transoral robotic surgery, histopathological localisation and tailored deintensification of radiotherapy for unknown primary and small oropharyngeal head and neck squamous cell carcinoma. BMJ Open 2019, 9, e035431. [Google Scholar] [PubMed] [Green Version]
- Moore, E.J.; Van Abel, K.M.; Routman, D.M.; Ms, C.M.L.; Price, K.A.R.; Neben-Wittich, M.; Chintakuntlawar, A.V.; Price, D.L.; Kasperbauer, J.L.; Garcia, J.J.; et al. Human papillomavirus oropharynx carcinoma: Aggressive de-escalation of adjuvant therapy. Head Neck 2021, 43, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.A.; Weinstein, G.S.; O’Malley, B.W.; Feldman, M.; Quon, H. Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck 2011, 33, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Sload, R.; Silver, N.; Jawad, B.A.; Gross, N.D. The Role of Transoral Robotic Surgery in the Management of HPV Negative Oropharyngeal Squamous Cell Carcinoma. Curr. Oncol. Rep. 2016, 18, 53. [Google Scholar] [CrossRef]
- De Almeida, J.R.; Li, R.; Magnuson, J.S.S.; Smith, R.V.; Moore, E.; Lawson, G.; Remacle, M.; Ganly, I.; Kraus, D.H.; Teng, M.S.; et al. Oncologic outcomes after transoral robotic surgery: A multi-institutional study. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 1043–1051. [Google Scholar] [CrossRef]
- Dogan, S.; Xu, B.; Middha, S.; Vanderbilt, C.M.; Bowman, A.S.; Migliacci, J.; Morris, L.G.; Seshan, V.E.; Ganly, I. Identification of prognostic molecular biomarkers in 157 HPV-positive and HPV-negative squamous cell carcinomas of the oropharynx. Int. J. Cancer 2019, 145, 3152–3162. [Google Scholar] [CrossRef]
- Guo, T.; Qualliotine, J.R.; Ha, P.K.; Califano, J.A.; Kim, Y.; Saunders, J.R.; Blanco, R.G.; D’Souza, G.; Zhang, Z.; Chung, C.H.; et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 2015, 121, 1977–1984. [Google Scholar] [CrossRef]
- Ang, K.; Trotti, A.; Brown, B.W.; Garden, A.S.; Foote, R.L.; Morrison, W.H.; Geara, F.B.; Klotch, D.W.; Goepfert, H.; Peters, L.J. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int. J. Radiat. Oncol. 2001, 51, 571–578. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; Van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.-K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (# 22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar]
- Siegel, R.S.; Joshi, A.; Taheri, R.; Sadeghi, N.; Thakkar, P.; Vongpachith, A.; Goodman, J. Phase II study: Induction chemotherapy and transoral surgery as definitive treatment (Tx) for locally advanced oropharyngeal squamous cell carcinoma (OPSCC)—An update and retrospective review of non-study patients. J. Clin. Oncol. 2019, 37 (Suppl. S15), 6072. [Google Scholar] [CrossRef]
- Sadeghi, N.; Khalife, S.; Mascarella, M.A.; Ramanakumar, A.V.; Richardson, K.; Joshi, A.S.; Bouganim, N.; Taheri, R.; Fuson, A.; Siegel, R. Pathologic response to neoadjuvant chemotherapy in HPV-associated oropharynx cancer. Head Neck 2020, 42, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Price, K.A.R.; Nichols, A.; Shen, C.J.; Rammal, A.; Lang, P.; Palma, D.A.; Rosenberg, A.J.; Chera, B.S.; Agrawal, N. Novel strategies to effectively de-escalate curative-intent therapy for patients with HPV-associated oropharyngeal cancer: Current and future directions. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Luciani, A.; Ghidini, A.; Cherri, S.; Gamba, P.; Maddalo, M.; Bossi, P.; Zaniboni, A. Treatment de-escalation for HPV+ oropharyngeal cancer: A systematic review and meta-analysis. Head Neck 2022, 44, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, K.M.; Ghosh, A.; Mohamed, A.; Kamal, M.; Gunn, G.B.; Dale, T.; Kalpathy-Cramer, J.; Messer, J.; Garden, A.S.; Elhalawani, H.; et al. Chronic radiation-associated dysphagia in oropharyngeal cancer survivors: Towards age-adjusted dose constraints for deglutitive muscles. Clin. Transl. Radiat. Oncol. 2019, 18, 16–22. [Google Scholar] [CrossRef]
- Parvathaneni, U.; Lavertu, P.; Gibson, M.K.; Glastonbury, C.M. Advances in diagnosis and multidisciplinary management of oropharyngeal squamous cell carcinoma: State of the art. Radiographics 2019, 39, 2055–2068. [Google Scholar] [CrossRef]
- Ferris, R. Transoral Surgery Followed by Low-Dose or Standard-Dose Radiation Therapy with or Without Chemotherapy in Treating Patients with HPV Positive Stage III-IVA Oropharyngeal Cancer. p. NCT01898494. Available online: https://clinicaltrials.gov/ct2/show/NCT01898494 (accessed on 25 March 2021).
- Miles, B.; Posner, M.; Teng, M.; Yao, M.; Chai, R.; Misiukiewicz, K.; Gupta, V.; Bakst, R.; Sharma, S.; Zhang, D.; et al. De-Escalated Adjuvant Therapy after Transoral Robotic Surgery for HPV related Oropharyngeal Carcinoma: The SiRS Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1123. [Google Scholar] [CrossRef]
- Strohl, M.P.; Wai, K.C.; Ha, P.K. De-intensification strategies in HPV-related oropharyngeal squamous cell carcinoma—A narrative review. Ann. Transl. Med. 2020, 8, 1601. [Google Scholar] [CrossRef]
- Gleich, L.L.; Ryzenman, J.; Gluckman, J.L.; Wilson, K.M.; Barrett, W.L.; Redmond, K.P. Recurrent advanced (T3 or T4) head and neck squamous cell carcinoma: Is salvage possible? Arch. Otolaryngol. Head Neck Surg. 2004, 130, 35–38. [Google Scholar] [CrossRef]
- Goodwin, W.J., Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: When do the ends justify the means? Laryngoscope 2000, 110, 1–18. [Google Scholar] [CrossRef]
- Gonçalves Agra, I.M.; Lopes Carvalho, A.; Ulbrich, F.S.; de Campos, O.D.; Martins, E.P.; Magrin, J.; Kowalski, L.P. Prognostic factors in salvage surgery for recurrent oral and oropharyngeal cancer. Head Neck 2006, 28, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zafereo, M.E.; Hanasono, M.M.; Rosenthal, D.I.; Sturgis, E.M.; Lewin, J.S.; Roberts, D.B.; Weber, R.S. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer 2009, 115, 5723–5733. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Cohen, M.A.; Givi, B.; Dixon, B.J.; Gilbert, R.W.; Gullane, P.J.; Brown, D.H.; Irish, J.C.; Msc, J.R.D.A.; Higgins, K.M.; et al. Salvage surgery for locally recurrent oropharyngeal cancer. Head Neck 2016, 38, E658–E664. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Abel, S.; Hasan, S.; Verma, V.; Greenberg, L.; Colonias, A.; Wegner, R.E. Practice patterns and outcomes following radiation dose de-escalation for oropharyngeal cancer. Laryngoscope 2020, 130, E171–E176. [Google Scholar] [CrossRef]
- Sweeny, L.; Rosenthal, E.L.; Clemons, L.; Stevens, T.M.; McIntosh, E.R.C.; Carroll, W.R. Outcomes after surgical salvage for recurrent oropharyngeal squamous cell carcinoma. Oral Oncol. 2016, 60, 118–124. [Google Scholar] [CrossRef]
- Santoro, L.; Tagliabue, M.; Massaro, M.A.; Ansarin, M.; Calabrese, L.; Dm, G.G.; Alterio, D.; Rocca, M.C.; Grosso, E.; Plànicka, M.; et al. Algorithm to predict postoperative complications in oropharyngeal and oral cavity carcinoma. Head Neck 2015, 37, 548–556. [Google Scholar] [CrossRef]
- Moro, J.D.S.; Maroneze, M.C.; Ardenghi, T.; Barin, L.M.; Danesi, C.C. Oral and oropharyngeal cancer: Epidemiology and survival analysis. Einstein 2018, 16, eAO4248. [Google Scholar] [CrossRef]
- Albergotti, W.G.; Jordan, J.; Anthony, K.; Abberbock, S.; Wasserman-Wincko, T.; Kim, S.; Ferris, R.L.; Duvvuri, U. A prospective evaluation of short-term dysphagia after transoral robotic surgery for squamous cell carcinoma of the oropharynx. Cancer 2017, 123, 3132–3140. [Google Scholar] [CrossRef]
- Tateya, I.; Shiotani, A.; Satou, Y.; Tomifuji, M.; Morita, S.; Muto, M.; Ito, J. Transoral surgery for laryngo-pharyngeal cancer—The paradigm shift of the head and cancer treatment. Auris Nasus Larynx 2016, 43, 21–32. [Google Scholar] [CrossRef]
- Mowry, S.E.; Ho, A.; LoTempio, M.M.; Sadeghi, A.; Blackwell, K.E.; Wang, M.B. Quality of life in advanced oropharyngeal carcinoma after chemoradiation versus surgery and radiation. Laryngoscope 2006, 116, 1589–1593. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; Teknos, T.N.; Durmus, K.; Old, M.; Agrawal, A.; Kakarala, K.; Marcinow, A.; Ozer, E. Transoral robotic surgery for oropharyngeal cancer: Long-term quality of life and functional outcomes. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.H.; Townsend, M.; Ba, H.Y.H.; Miller, E.; Kallogjeri, D.; Zenga, J.; Pipkorn, P.; Jackson, R.; Haughey, B.; Rich, J.T. Predictors of swallow function after transoral surgery for locally advanced oropharyngeal cancer. Laryngoscope 2020, 130, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Netscher, D.T.; Meade, R.A.; Goodman, C.M.; Alford, E.L.; Stewart, M.G. Quality of life and disease-specific functional status following microvascular reconstruction for advanced (T3 and T4) oropharyngeal cancers. Plast. Reconstr. Surg. 2000, 105, 1628–1634. [Google Scholar] [CrossRef]
- Wax, M.K.; Myers, L.L.; Andersen, P.E.; Cohen, J.I. The effect of head and neck reconstruction on quality of life. Curr. Opin. Otolaryngol. Head Neck Surg. 1999, 7, 180. [Google Scholar] [CrossRef]
- Weinstein, G.S.; O’Malley, B.W.; Rinaldo, A.; Silver, C.E.; Werner, J.A.; Ferlito, A. Understanding contraindications for transoral robotic surgery (TORS) for oropharyngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2015, 271, 1551–1552. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.H.; Lee, J.M.C.; Choi, J.W.; Jung, D.; Cho, H.; Kang, H.; Hong, M.H.; Heo, S.J.; Kim, S.H.; et al. Molecular subtypes of oropharyngeal cancer show distinct immune microenvironment related with immune checkpoint blockade response. Br. J. Cancer 2020, 122, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.E.; Cohen, E.; Ferris, R.L.; Adelstein, D.J.; Brizel, D.; Ridge, J.A.; O’Sullivan, B.; Burtness, B.A.; Butterfield, L.; Carson, I.W.E.; et al. Immunotherapy of head and neck cancer: Emerging clinical trials from a National Cancer Institute Head and Neck Cancer Steering Committee Planning Meeting. Cancer 2017, 123, 1259–1271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniaci, A.; Hao, S.-P.; Cancemi, F.; Giardini, D.; Checcoli, E.; Soprani, F.; Iannella, G.; Vicini, C.; Cocuzza, S.; La Mantia, I.; et al. Surgical Treatment for Advanced Oropharyngeal Cancer: A Narrative Review. Medicina 2023, 59, 304. https://doi.org/10.3390/medicina59020304
Maniaci A, Hao S-P, Cancemi F, Giardini D, Checcoli E, Soprani F, Iannella G, Vicini C, Cocuzza S, La Mantia I, et al. Surgical Treatment for Advanced Oropharyngeal Cancer: A Narrative Review. Medicina. 2023; 59(2):304. https://doi.org/10.3390/medicina59020304
Chicago/Turabian StyleManiaci, Antonino, Sheng-Po Hao, Francesco Cancemi, Damiano Giardini, Emanuele Checcoli, Francesco Soprani, Giannicola Iannella, Claudio Vicini, Salvatore Cocuzza, Ignazio La Mantia, and et al. 2023. "Surgical Treatment for Advanced Oropharyngeal Cancer: A Narrative Review" Medicina 59, no. 2: 304. https://doi.org/10.3390/medicina59020304






