Temperature-Sensitive Auditory Neuropathy: Report of a Novel Variant of OTOF Gene and Review of Current Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical History
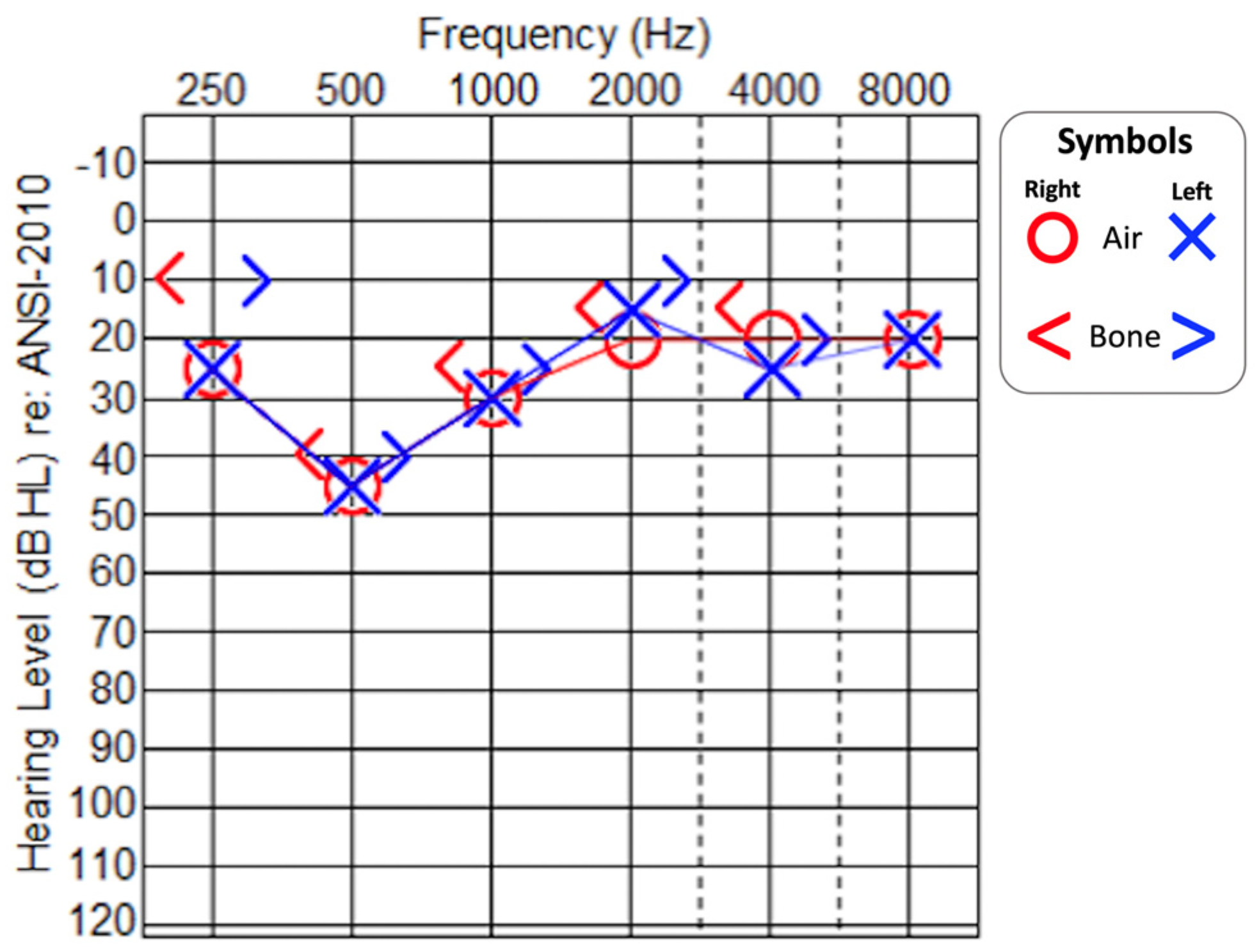
2.2. Audiological Evaluation
2.3. Imaging
2.4. Genetic Testing
2.4.1. NGS Analysis
2.4.2. Bioinformatics Analysis
2.4.3. Array-Comparative Genomic Hybridization (Array-CGH)
2.5. Genetic Results
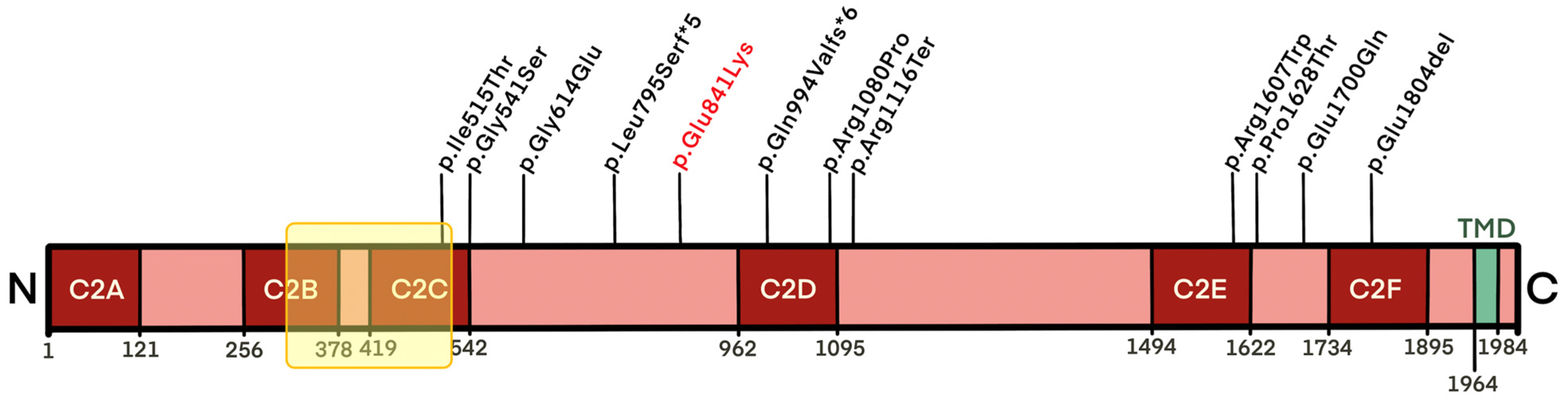
- c.2521G>A, p.(Glu841Lys);
- c.(897+1_898−1)_(1579+1_1580−1)del.
- c.−22−2A>C in intron 1 of the GJB2 (Gap Junction Protein Beta 2/Connexin 26) gene (RefSeq: NM_004004) (rs201895089), classified as a likely pathogenic variant;
- c.2717A>G, p.(Tyr906Cys) in exon 9 of the TECTA gene (Alpha-Tectorin) (RefSeq: NM_005422), classified as a variant with an uncertain significance.
2.6. Therapeutic Approach
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Starr, A.; Sininger, Y.; Winter, M.; Derebery, M.J.; Oba, S.; Michalewski, H.J. Transient Deafness Due To Temperature-Sensitive Auditory Neuropathy. Ear Hear. 1998, 19, 169–179. [Google Scholar] [CrossRef]
- Pangršič, T.; Reisinger, E.; Moser, T. Otoferlin: A multi-C2 domain protein essential for hearing. Trends Neurosci. 2012, 35, 671–680. [Google Scholar] [CrossRef]
- Pangršič, T.; Lasarow, L.; Reuter, K.; Takago, H.; Schwander, M.; Riedel, D.; Frank, T.; Tarantino, L.M.; Bailey, J.S.; Strenzke, N.; et al. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nat. Neurosci. 2010, 13, 869–876. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Wang, M.; Wang, H. Protection of Spiral Ganglion Neurons and Prevention of Auditory Neuropathy. Hear. Loss: Mech. Prev. Cure 2019, 1130, 93–107. [Google Scholar] [CrossRef]
- Rutherford, M.A.; von Gersdorff, H.; Goutman, J.D. Encoding sound in the cochlea: From receptor potential to afferent discharge. J. Physiol. 2021, 599, 2527–2557. [Google Scholar] [CrossRef]
- Yasunaga, S.; Grati, M.; Cohen-Salmon, M.; El-Amraoui, A.; Mustapha, M.; Salem, N.; El-Zir, E.; Loiselet, J.; Petit, C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 1999, 21, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, H.; Booth, K.T.; Ephraim, S.S.; Crone, B.; Black-Ziegelbein, E.A.; Marini, R.J.; Shearer, A.E.; Sloan-Heggen, C.M.; Kolbe, D.; Casavant, T.; et al. Genomic Landscape and Mutational Signatures of Deafness-Associated Genes. Am. J. Hum. Genet. 2018, 103, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Jónsson, H.; Sulem, P.; Kehr, B.; Kristmundsdottir, S.; Zink, F.; Hjartarson, E.; Hardarson, M.T.; Hjorleifsson, K.E.; Eggertsson, H.P.; Gudjonsson, S.A.; et al. Parental influence on human germline de novo mutations in 1548 trios from Iceland. Nature 2017, 549, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Mutai, H.; Kunishima, S.; Namba, K.; Morimoto, N.; Shinjo, Y.; Arimoto, Y.; Kataoka, Y.; Shintani, T.; Morita, N.; et al. A prevalent founder mutation and genotype-phenotype correlations of OTOF in Japanese patients with auditory neuropathy. Clin. Genet. 2012, 82, 425–432. [Google Scholar] [CrossRef]
- Del Castillo, F.J.; Del Castillo, I. Genetics of isolated auditory neuropathies. Front. Biosci. 2012, 17, 1251–1265. [Google Scholar] [CrossRef]
- Rodríguez-Ballesteros, M.; Reynoso, R.; Olarte, M.; Villamar, M.; Morera, C.; Santarelli, R.; Arslan, E.; Medá, C.; Curet, C.; Völter, C.; et al. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum. Mutat. 2008, 29, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Almontashiri, N.A.; Alswaid, A.; Oza, A.; Al-Mazrou, K.A.; Elrehim, O.; Tayoun, A.A.; Rehm, H.L.; Amr, S.S. Recurrent variants in OTOF are significant contributors to prelingual nonsydromic hearing loss in Saudi patients. Genet. Med. 2018, 20, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lan, L.; Shi, W.; Yu, L.; Xie, L.-Y.; Xiong, F.; Zhao, C.; Li, N.; Yin, Z.; Zong, L.; et al. Temperature sensitive auditory neuropathy. Hear. Res. 2016, 335, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, H.; Thorpe, R.K.; Smith, R.J.H. OTOF-Related Deafness; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; GeneReviews®: Seattle, WA, USA, 2008; pp. 1993–2022. [Google Scholar]
- Starr, A.; Picton, T.W.; Sininger, Y.; Hood, L.J.; Berlin, C.I. Auditory neuropathy. Brain 1996, 119, 741–753. [Google Scholar] [CrossRef]
- Berlin, C.I.; Hood, L.J.; Morlet, T.; Wilensky, D.; Li, L.; Mattingly, K.R.; Taylor-Jeanfreau, J.; Keats, B.J.; John, P.S.; Montgomery, E.; et al. Multi-site diagnosis and management of 260 patients with Auditory Neuropathy/Dys-synchrony (Auditory Neuropathy Spectrum Disorder*). Int. J. Audiol. 2010, 49, 30–43. [Google Scholar] [CrossRef]
- Madden, C.; Rutter, M.; Hilbert, L.; Greinwald, J.J.H.; Choo, D.I. Clinical and Audiological Features in Auditory Neuropathy. Arch. Otolaryngol. Neck Surg. 2002, 128, 1026–1030. [Google Scholar] [CrossRef]
- Puglisi, G.; di Berardino, F.; Montuschi, C.; Sellami, F.; Albera, A.; Zanetti, D.; Albera, R.; Astolfi, A.; Kollmeier, B.; Warzybok, A. Evaluation of Italian Simplified Matrix Test for Speech-Recognition Measurements in Noise. Audiol. Res. 2021, 11, 73–88. [Google Scholar] [CrossRef]
- VarSome. Available online: https://varsome.com (accessed on 17 December 2022).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Deafness Variation Database. Available online: https://deafnessvariationdatabase.org (accessed on 17 December 2022).
- Guidelines of the Italian Society of Human Genetics. Available online: https://www.sigu.net (accessed on 17 December 2022).
- Kearney, H.M.; Thorland, E.C.; Brown, K.K.; Quintero-Rivera, F.; South, S.T. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet. Med. 2011, 13, 680–685. [Google Scholar] [CrossRef]
- Chang, M.Y.; Kim, A.R.; Kim, N.K.; Lee, C.; Park, W.-Y.; Choi, B.Y. Refinement of Molecular Diagnostic Protocol of Auditory Neuropathy Spectrum Disorder. Medicine 2015, 94, e1996. [Google Scholar] [CrossRef]
- Kim, B.J.; Jang, J.H.; Han, J.H.; Park, H.-R.; Oh, D.Y.; Lee, S.; Kim, M.Y.; Kim, A.R.; Lee, C.; Kim, N.K.D.; et al. Mutational and phenotypic spectrum of OTOF-related auditory neuropathy in Koreans: Eliciting reciprocal interaction between bench and clinics. J. Transl. Med. 2018, 16, 330. [Google Scholar] [CrossRef]
- Gorga, M.P.; Stelmachowicz, P.G.; Barlow, S.M.; Brookhouser, P.E. Case of recurrent, reversible, sudden sensorineural hearing loss in a child. J. Am. Acad. Audiol. 1995, 6, 163–172. [Google Scholar]
- Varga, R.; Avenarius, M.R.; Kelley, P.M.; Keats, B.J.; Berlin, C.I.; Hood, L.J.; Morlet, T.G.; Brashears, S.M.; Starr, A.; Cohn, E.S.; et al. OTOF mutations revealed by genetic analysis of hearing loss families including a potential temperature sensitive auditory neuropathy allele. J. Med. Genet. 2005, 43, 576–581. [Google Scholar] [CrossRef]
- Strenzke, N.; Chakrabarti, R.; Al-Moyed, H.; Müller, A.; Hoch, G.; Pangrsic, T.; Yamanbaeva, G.; Lenz, C.; Pan, K.; Auge, E.; et al. Hair cell synaptic dysfunction, auditory fatigue and thermal sensitivity in otoferlin Ile515Thr mutants. EMBO J. 2016, 35, 2519–2535. [Google Scholar] [CrossRef]
- Cianfrone, G.; Turchetta, R.; Mazzei, F.; Bartolo, M.; Parisi, L. Temperature-Dependent Auditory Neuropathy: Is it an Acoustic Uhthoff-like Phenomenon? A Case Report. Ann. Otol. Rhinol. Laryngol. 2006, 115, 518–527. [Google Scholar] [CrossRef]
- Madanoglu, N.; Derinsu, U. Familial temperature-sensitive auditory neuropathy/auditory dyssynchrony. J. Int. Adv. Otol. 2007, 3, 69–74. [Google Scholar]
- Romanos, J.; Kimura, L.; Fávero, M.L.; Izarra, F.A.R.; Auricchio, M.T.B.D.M.; Batissoco, A.C.; Lezirovitz, K.; Abreu-Silva, R.S.; Mingroni-Netto, R.C. Novel OTOF mutations in Brazilian patients with auditory neuropathy. J. Hum. Genet. 2009, 54, 382–385. [Google Scholar] [CrossRef]
- Marlin, S.; Feldmann, D.; Nguyen, Y.; Rouillon, I.; Loundon, N.; Jonard, L.; Bonnet, C.; Couderc, R.; Garabedian, E.N.; Petit, C.; et al. Temperature-sensitive auditory neuropathy associated with an otoferlin mutation: Deafening fever! Biochem. Biophys. Res. Commun. 2010, 394, 737–742. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; Starr, A.; Bhatt, S.; Michalewski, H.J.; Zeng, F.-G.; Pratt, H. Auditory cortical N100 in pre- and post-synaptic auditory neuropathy to frequency or intensity changes of continuous tones. Clin. Neurophysiol. 2011, 122, 594–604. [Google Scholar] [CrossRef]
- Wynne, D.P.; Zeng, F.-G.; Bhatt, S.; Michalewski, H.J.; Dimitrijevic, A.; Starr, A. Loudness adaptation accompanying ribbon synapse and auditory nerve disorders. Brain 2013, 136, 1626–1638. [Google Scholar] [CrossRef]
- Kaga, K. Auditory nerve disease and auditory neuropathy spectrum disorders. Auris Nasus Larynx 2016, 43, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Wang, Y.-C.; Weil, D.; Zhao, Y.-L.; Rao, S.-Q.; Zong, L.; Ji, Y.-B.; Liu, Q.; Li, J.-Q.; Yang, H.-M.; et al. Screening mutations of OTOF gene in Chinese patients with auditory neuropathy, including a familial case of temperature-sensitive auditory neuropathy. BMC Med. Genet. 2010, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.-M.; Li, Q.; Gao, X.; Li, Y.-F.; Liu, Y.-L.; Dai, P.; Li, X.-P. Familial Temperature-Sensitive Auditory Neuropathy: Distinctive Clinical Courses Caused by Variants of the OTOF Gene. Front. Cell Dev. Biol. 2021, 9, 732930. [Google Scholar] [CrossRef]
- Vona, B.; Rad, A.; Reisinger, E. The Many Faces of DFNB9: Relating OTOF Variants to Hearing Impairment. Genes 2020, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Zadro, C.; Ciorba, A.; Fabris, A.; Morgutti, M.; Trevisi, P.; Gasparini, P.; Martini, A. Five new OTOF gene mutations and auditory neuropathy. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.A.; Berman, M.A.; Conlin, L.K.; Rehm, H.L.; Francey, L.J.; Deardorff, M.A.; Holst, J.; Kaur, M.; Gallant, E.; Clark, D.M.; et al. PECONPI: A novel software for uncovering pathogenic copy number variations in non-syndromic sensorineural hearing loss and other genetically heteroge-neous disorders. Am. J. Med. Genet. Part A 2013, 161, 2134–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Reference | Starr, 1998 [1] Varga, 2006 [27] Strenzke, 2016 [28] | Romanos, 2009 [31] | Marlin, 2010 [32] | Matsunaga, 2012 [9]; Kaga, 2016 [35] | Wang, 2010 [36]; Zhang, 2016 [13] | Zhang, 2016 [13] | Zhang, 2016 [13] | Zhu, 2021 [37] | Present Study | |
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Nucleotide change | c.1544T>C + c.3346C>T | c.1841G>A + c. 3239G>C | c.5410_5412delGAG + c.5410_5412delGAG | c.1621G>A + c.1621G>A | c.4819C>T + c.2975_2978delAG | c.1621G>A + c.2382_2383delC | c.4819C>T + c.4819C>T | c.4882C>A + c.5098G>C | c.2521G>A+ c.(897+1_898−1)_(1579+1_1580−1)del |
| Predicted protein change | p.Ile515Thr + p.Arg1116Ter | p.Gly614Glu + p.Arg1080Pro | p.Glu1804del + p.Glu1804del | p.Gly541Ser + p.Gly541Ser | p.Arg1607Trp + p.Gln994ValfsX6 | p.Gly541Ser + p.Leu795SerfsX5 | p.Arg1607Trp + p.Arg1607Trp | p.Pro1628Thr + p.Glu1700Gln | p.Glu841Lys | |
| Phenotype | No. patients | 2 | 1 | 3 | 1 | 1 | 1 | 1 | 4 | 1 |
| Origin | United States | Brazil | Scotland | Japan | China | China | China | China | Italy | |
| Sex | M (1), F (1) | F | M (1), F (1) | M | M | M | M | M | M | |
| Age at onset | 2 years | - | 2 years | 10 years | 13 months | 30 months | 6 years | 8–15 years | 2 years | |
| Afebrile | PTA | Mild HL at low frequencies | Mild HL | Normal/mild HL | Mild HL | Moderate †/mild HL | Moderate HL | Moderate HL †/normal | Normal | Moderate HL at low frequencies |
| SDS (%) | 88–100 | - | ≤ 80 | ≤ 80 | †/16 | 93–98 (after CI) | 96 | 88–100 | - | |
| DWRS | - | - | - | - | - | - | - | - | 100% (quiet) 70% (noise) | |
| Ty | A | - | - | - | A | A | A | A | A | |
| SR | Absent | - | - | - | Absent | Absent | Absent | Absent | Absent | |
| OAEs | Present | Present | Present | Present | Present | Present | Present | Present | Present | |
| ABR | Abnormal | Abnormal | Abnormal | Absent | Absent | Absent | Absent | Normal | Absent | |
| Febrile | Body T (°C) | 38.1/37.8 | - | > 38 | 37.2 | 36.6 †/36.5 | - | 36.9 | 38–40.2 | 38.7 |
| PTA | Profound/mild HL | Severe | Profound/severe HL | Profound HL | Severe †/moderate HL | - | Mild HL | Mild HL | Moderate HL | |
| SDS (%) | 0/0 | - | < 100 | 15 | - †/16–20 | - | 88–80 | 0–20 | - | |
| DWRS | - | - | - | - | - | - | - | - | 60% (quiet) 10% (noise) | |
| Ty | A | - | - | - | A | A | A | Ax3/Cx1 | A | |
| SR | Absent | - | - | - | Absent | Absent | Absent | Absent | Absent | |
| OAEs | Present | - | Present | Present | Present | Present | Present | Present x 3/ absent x 1 | - | |
| ABR | Absent | - | Absent | Absent | Absent | Absent | Absent | Absent | - | |
| Therapy | - | - | Hearing aids | - | Improved with age | Cochlear implantation (CI) | Improved with age | - | Hearing aids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forli, F.; Capobianco, S.; Berrettini, S.; Bruschini, L.; Romano, S.; Fogli, A.; Bertini, V.; Lazzerini, F. Temperature-Sensitive Auditory Neuropathy: Report of a Novel Variant of OTOF Gene and Review of Current Literature. Medicina 2023, 59, 352. https://doi.org/10.3390/medicina59020352
Forli F, Capobianco S, Berrettini S, Bruschini L, Romano S, Fogli A, Bertini V, Lazzerini F. Temperature-Sensitive Auditory Neuropathy: Report of a Novel Variant of OTOF Gene and Review of Current Literature. Medicina. 2023; 59(2):352. https://doi.org/10.3390/medicina59020352
Chicago/Turabian StyleForli, Francesca, Silvia Capobianco, Stefano Berrettini, Luca Bruschini, Silvia Romano, Antonella Fogli, Veronica Bertini, and Francesco Lazzerini. 2023. "Temperature-Sensitive Auditory Neuropathy: Report of a Novel Variant of OTOF Gene and Review of Current Literature" Medicina 59, no. 2: 352. https://doi.org/10.3390/medicina59020352






