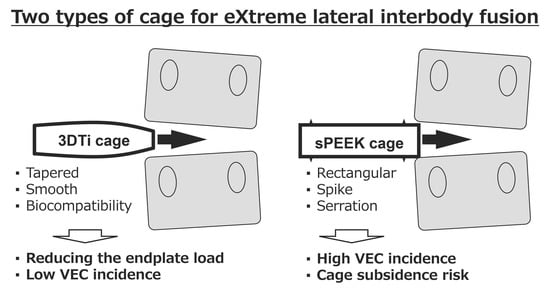
Vertebral Endplate Concavity in Lateral Lumbar Interbody Fusion: Tapered 3D-Printed Porous Titanium Cage versus Squared PEEK Cage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
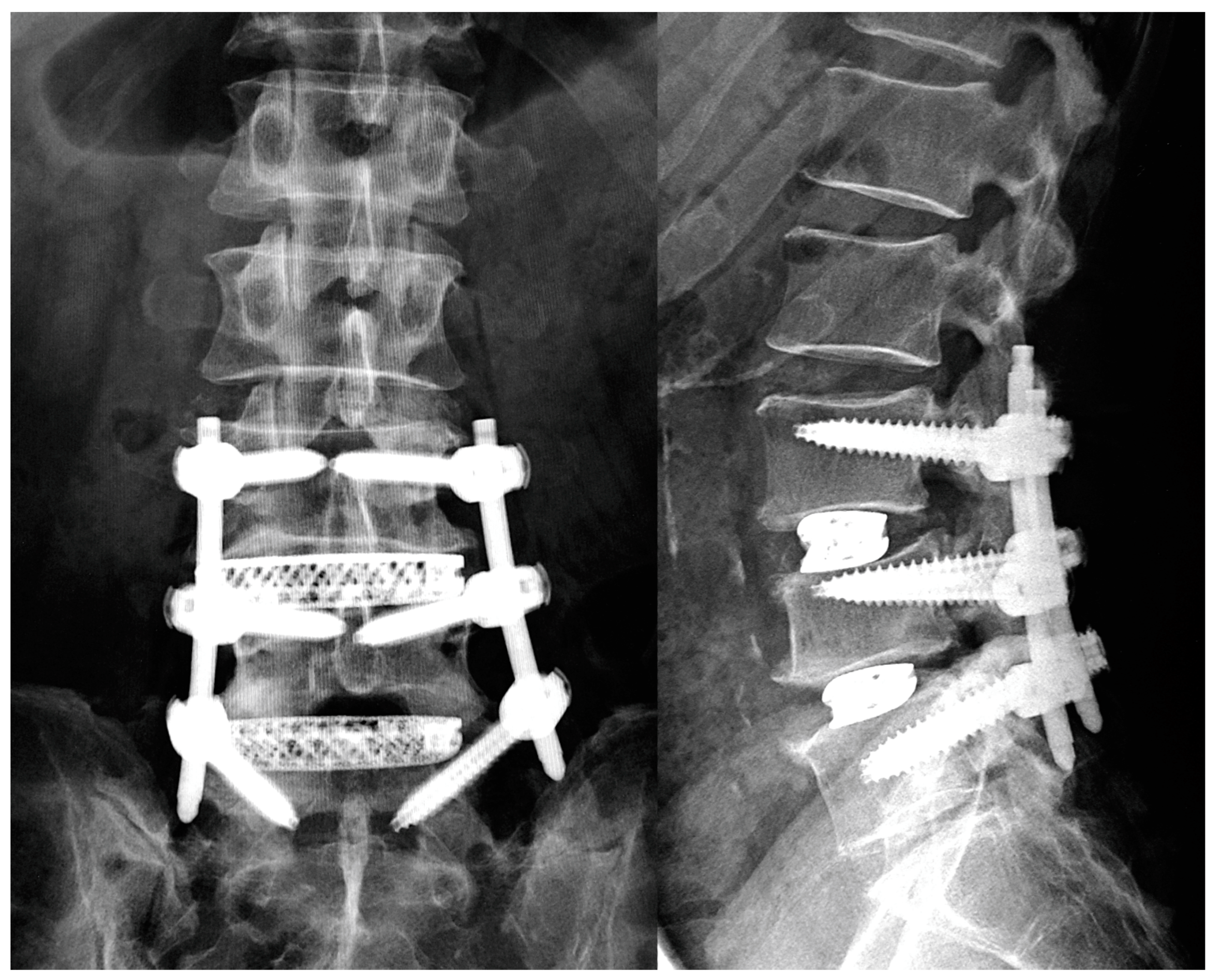
2.2. Surgical Procedure
2.3. Patients’ Demographic and Operative Data
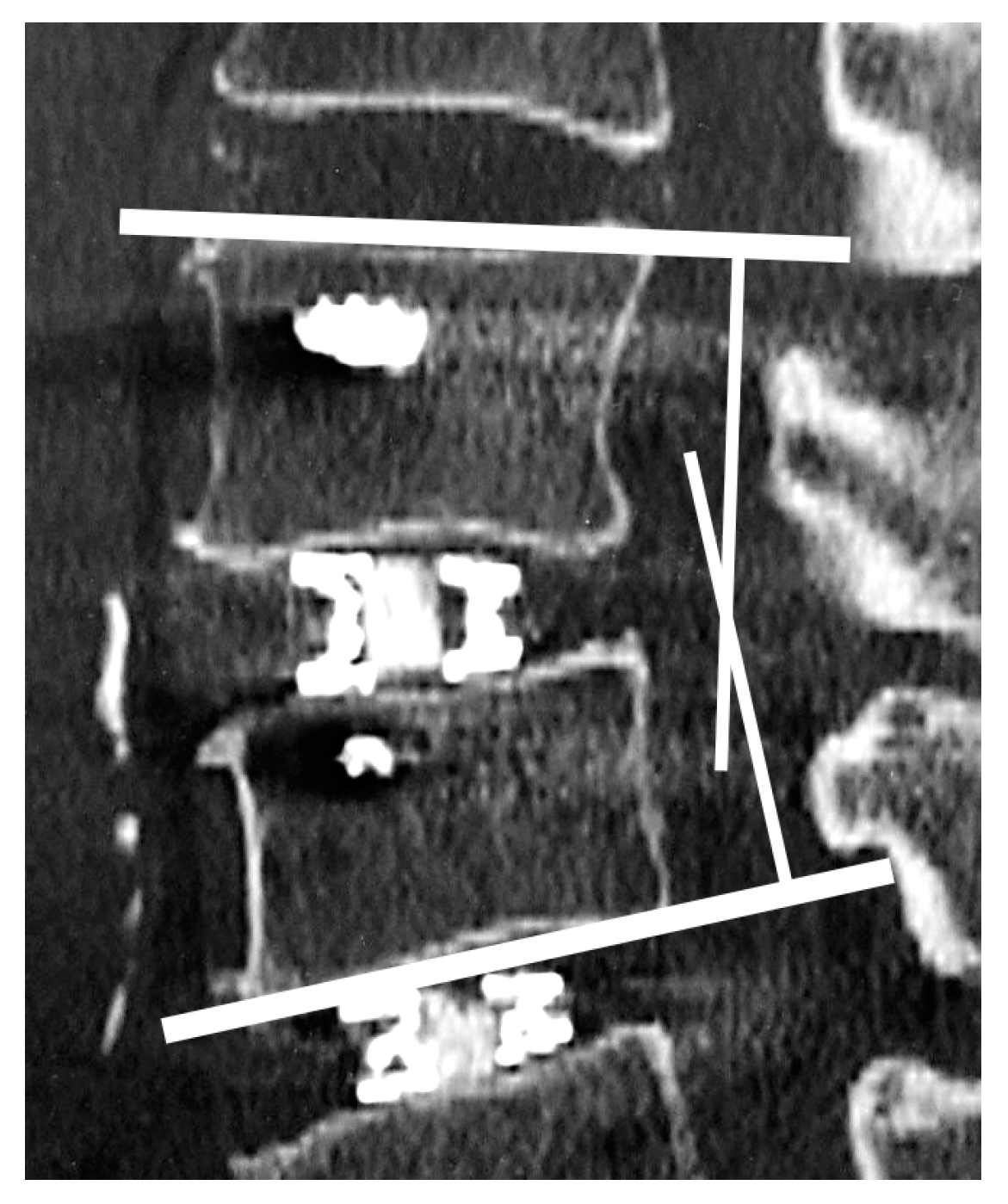
2.4. Radiological Assessments
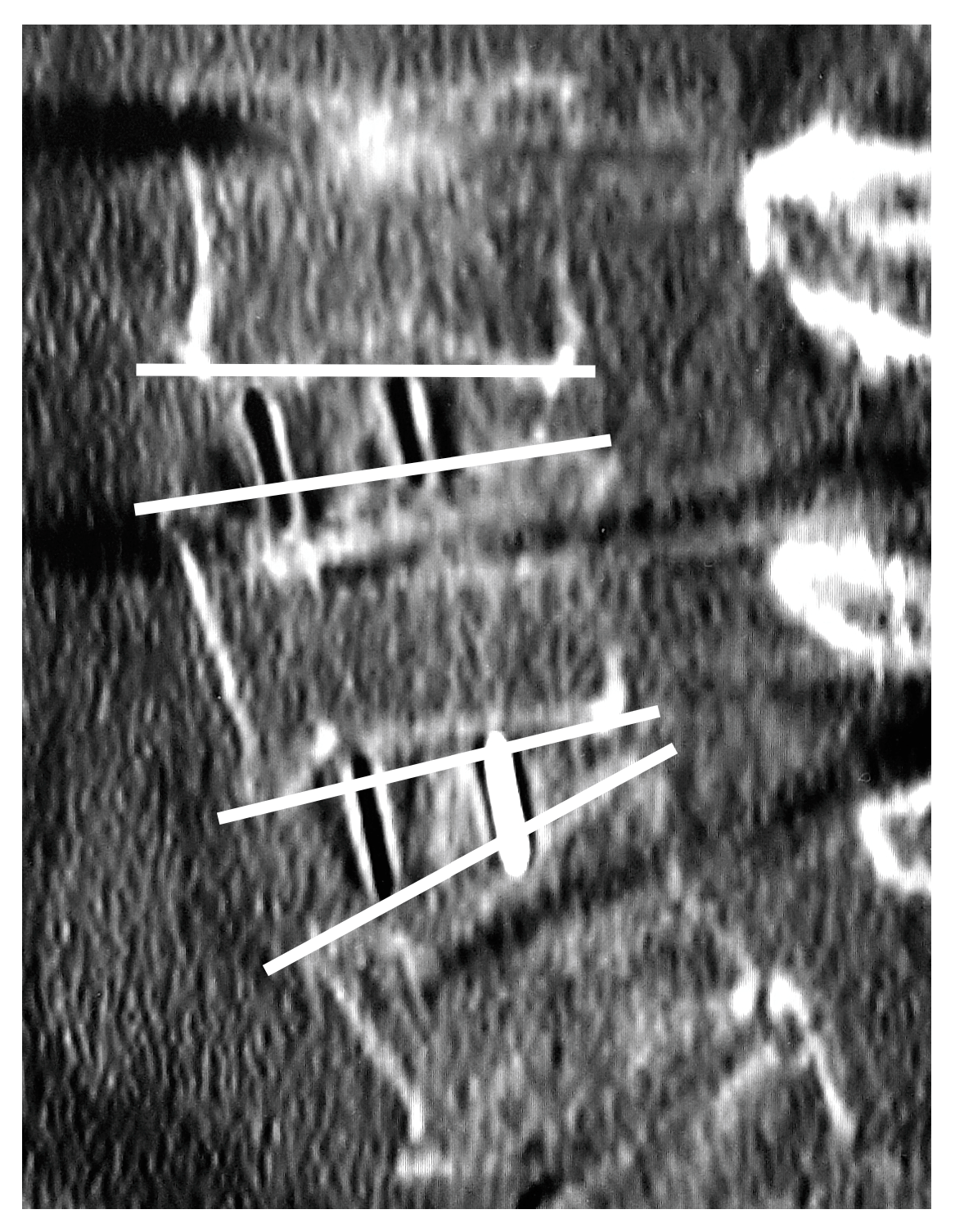
2.5. Vertebral Endplate Concavity (VEC) and Cage Subsidence
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shan, Z.; Zhao, F.; Cheung, J.P.Y. Poor Bone Quality, Multilevel Surgery, and Narrow and Tall Cages Are Associated with Intraoperative Endplate Injuries and Late-onset Cage Subsidence in Lateral Lumbar Interbody Fusion: A Systematic Review. Clin. Orthop. Relat. Res. 2022, 480, 163–188. [Google Scholar] [CrossRef]
- Tohmeh, A.G.; Khorsand, D.; Watson, B.; Zielinski, X. Radiographical and Clinical Evaluation of Extreme Lateral Interbody Fusion: Effects of Cage Size and Instrumentation Type with a Minimum of 1-Year Follow-up. Spine 2014, 39, E1582. [Google Scholar] [CrossRef]
- Satake, K.; Kanemura, T.; Yamaguchi, H.; Segi, N.; Ouchida, J. Predisposing Factors for Intraoperative Endplate Injury of Extreme Lateral Interbody Fusion. Asian Spine J. 2016, 10, 907–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-H.; Ha, K.-Y.; Kim, K.-T.; Chang, D.-G.; Park, H.-Y.; Yoon, E.-J.; Kim, S.-I. Risk factors for intraoperative endplate injury during minimally-invasive lateral lumbar interbody fusion. Sci. Rep. 2021, 11, 20149. [Google Scholar] [CrossRef] [PubMed]
- Heimbrook, A.; Kelly, C.; Gall, K. Effects of 3D printed surface topography and normal force on implant expulsion. J. Mech. Behav. Biomed. Mater. 2022, 130, 105208. [Google Scholar] [CrossRef]
- Krafft, P.R.; Osburn, B.; Vivas, A.C.; Rao, G.; Alikhani, P. Novel Titanium Cages for Minimally Invasive Lateral Lumbar Interbody Fusion: First Assessment of Subsidence. Spine Surg. Relat. Res. 2020, 4, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Fogel, G.; Martin, N.; Williams, G.M.; Unger, J.; Yee-Yanagishita, C.; Pelletier, M.; Walsh, W.; Peng, Y.; Jekir, M. Choice of Spinal Interbody Fusion Cage Material and Design Influences Subsidence and Osseointegration Performance. World Neurosurg. 2022, 162, e626–e634. [Google Scholar] [CrossRef]
- Adl Amini, D.; Okano, I.; Oezel, L.; Zhu, J.; Chiapparelli, E.; Shue, J.; Sama, A.A.; Cammisa, F.P.; Girardi, F.P.; Hughes, A.P. Evaluation of cage subsidence in standalone lateral lumbar interbody fusion: Novel 3D-printed titanium versus polyetheretherketone (PEEK) cage. Eur. Spine J. 2021, 30, 2377–2384. [Google Scholar] [CrossRef]
- Adl Amini, D.; Moser, M.; Oezel, L.; Zhu, J.; Okano, I.; Shue, J.; Sama, A.A.; Cammisa, F.P.; Girardi, F.P.; Hughes, A.P. Early Outcomes of Three-Dimensional–Printed Porous Titanium versus Polyetheretherketone Cage Implantation for Stand-Alone Lateral Lumbar Interbody Fusion in the Treatment of Symptomatic Adjacent Segment Degeneration. World Neurosurg. 2022, 162, e14–e20. [Google Scholar] [CrossRef]
- Adl Amini, D.A.; Moser, M.; Oezel, L.; Shue, J.; Pumberger, M.; Sama, A.A.; Cammisa, F.P.; Girardi, F.P.; Hughes, A.P. Fusion assessment in standalone lateral lumbar interbody fusion: 3D-printed titanium versus polyetheretherketone (PEEK) cages. J. Spine Surg. 2022, 8, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Blondel, B.; Chay, E.; Demakakos, J.; Lenke, L.; Tropiano, P.; Ames, C.; Smith, J.S.; Shaffrey, C.I.; Glassman, S.; et al. The Comprehensive Anatomical Spinal Osteotomy Classification. Neurosurgery 2014, 74, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchi, L.; Abdala, N.; Oliveira, L.; Amaral, R.; Coutinho, E.; Pimenta, L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion: Clinical article. J. Neurosurg. Spine 2013, 19, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kienle, A.; Graf, N.; Wilke, H.-J. Does impaction of titanium-coated interbody fusion cages into the disc space cause wear debris or delamination? Spine J. 2016, 16, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rancourt, D.; Shirazi-Adl, A.; Drouin, G.; Paiement, G. Friction properties of the interface between porous-surfaced metals and tibial cancellous bone. J. Biomed. Mater. Res. 1990, 24, 1503–1519. [Google Scholar] [CrossRef]
- Shirazi-Adl, A.; Dammak, M.; Paiement, G. Experimental determination of friction characteristics at the trabecular bone/porous-coated metal interface in cementless implants. J. Biomed. Mater. Res. 1993, 27, 167–175. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Vara, J.C.; Delgado, J.; Estrada-Martínez, A.; Pérez-Pevida, E.; Brizuela, A.; Bosch, B.; Pérez, R.; Gil, J. Effect of the Nature of the Particles Released from Bone Level Dental Implants: Physicochemical and Biological Characterization. Coatings 2022, 12, 219. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Jiao, C.; Liang, H.; Xie, D.; Shen, L.; Liu, Z. Analysis of Mechanical Properties and Permeability of Trabecular-Like Porous Scaffold by Additive Manufacturing. Front. Bioeng. Biotechnol. 2021, 9, 779854. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Rubshtein, A.P.; Trakhtenberg, I.S.; Makarova, E.B.; Triphonova, E.B.; Bliznets, D.G.; Yakovenkova, L.I.; Vladimirov, A.B. Porous material based on spongy titanium granules: Structure, mechanical properties, and osseointegration. Mater. Sci. Eng. C 2014, 35, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satake, K.; Kanemura, T.; Nakashima, H.; Yamaguchi, H.; Segi, N.; Ouchida, J. Cage subsidence in lateral interbody fusion with transpsoas approach: Intraoperative endplate injury or late-onset settling. Spine Surg. Relat. Res. 2017, 1, 203–210. [Google Scholar] [CrossRef] [PubMed]


| 3DTi N = 11 | SPEEK N = 21 | p-Value | |
|---|---|---|---|
| Age, years | 74.7 (6.3) | 73.8 (7.1) | 0.65 |
| Sex, female | 6 (55%) | 16 (76%) | 0.25 |
| BMD, g/cm2 | 0.692 (0.088) | 0.693 (0.122) | >0.99 |
| Surgery | >0.99 | ||
| 1-staged | 7 (64%) | 12 (57%) | |
| 2-staged | 4 (36%) | 9 (43%) | |
| Spinal alignment, pre-op | |||
| Lumbar Cobb angle (°) | 12.9 (8.6) | 12.7 (9.4) | 0.90 |
| PI (°) | 50.3 (8.1) | 49.7 (8.3) | 0.96 |
| LL (°) | 14.0 (25.3) | 23.8 (12.8) | 0.34 |
| PI-LL (°) | 36.3 (23.5) | 25.9 (10.5) | 0.28 |
| PT (°) | 30.1 (13.5) | 26.0 (8.0) | 0.36 |
| SVA (mm) | 114.3 (79.6) | 76.0 (52.8) | 0.28 |
| Spinal alignment, post-op | |||
| LL (°) | 32.7 (14.4) | 41.6 (15.3) | 0.34 |
| PI-LL (°) | 13.7 (9.9) | 8.6 (8.9) | 0.39 |
| PT (°) | 20.7 (8.2) | 22.6 (5.4) | 0.78 |
| SVA (mm) | 30.2 (40.5) | 31.1 (25.0) | 0.46 |
| 3DTi N = 11 | SPEEK N = 21 | p-Value | |
|---|---|---|---|
| Number of XLIF segment | >0.99 | ||
| 1 | 1 (9.1%) | 2 (9.5%) | |
| 2 | 6 (55%) | 10 (48%) | |
| 3 | 4 (36%) | 8 (38%) | |
| 4 | 0 (0%) | 1 (4.8%) | |
| UIV | 0.54 | ||
| Upper thoracic | 2 (18%) | 1 (4.8%) | |
| Lower thoracic | 1 (9.1%) | 2 (9.5%) | |
| Lumbar | 8 (73%) | 18 (86%) | |
| LIV | 0.63 | ||
| Lumbar | 6 (55%) | 15 (71%) | |
| Sacrum | 2 (18%) | 2 (9.5%) | |
| Pelvis | 3 (27%) | 4 (19%) | |
| XLIF complication | |||
| Thigh symptom | 1 (33%) | 2 (22%) | >0.99 |
| 3DTi Cage N = 25 | SPEEK Cage N = 38 | p-Value | |
|---|---|---|---|
| Cage height | 0.11 | ||
| <10 mm | 21 (84%) | 25 (66%) | |
| ≥10 mm | 4 (16%) | 13 (34%) | |
| Cage angle | |||
| 10° | 25 (100%) | 38 (100%) | |
| Cage level | 0.29 | ||
| L1–2 | 0 (0%) | 2 (5.3%) | |
| L2–3 | 5 (20%) | 14 (37%) | |
| L3–4 | 10 (40%) | 13 (34%) | |
| L4–5 | 10 (40%) | 9 (24%) | |
| Interbody angle * | |||
| Pre-op (°) | 4.3 (7.4) | 4.7 (6.5) | 0.90 |
| Post-op (°) | 12.3 (6.0) | 9.1 (5.5) | 0.029 |
| 3 months (°) | 11.6 (6.4) | 8.2 (5.7) | 0.013 |
| VEC | |||
| Post-op | 2 (8.0%) | 17 (45%) | 0.002 |
| 3 months progression | 0 (0%) | 8 (21%) | 0.019 |
| Marchi classification | 0.51 | ||
| Grade 0 | 25 (100%) | 36 (95%) | |
| Grade I | 0 (0%) | 2 (5.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segi, N.; Nakashima, H.; Shinjo, R.; Kagami, Y.; Machino, M.; Ito, S.; Ouchida, J.; Morishita, K.; Oishi, R.; Yamauchi, I.; et al. Vertebral Endplate Concavity in Lateral Lumbar Interbody Fusion: Tapered 3D-Printed Porous Titanium Cage versus Squared PEEK Cage. Medicina 2023, 59, 372. https://doi.org/10.3390/medicina59020372
Segi N, Nakashima H, Shinjo R, Kagami Y, Machino M, Ito S, Ouchida J, Morishita K, Oishi R, Yamauchi I, et al. Vertebral Endplate Concavity in Lateral Lumbar Interbody Fusion: Tapered 3D-Printed Porous Titanium Cage versus Squared PEEK Cage. Medicina. 2023; 59(2):372. https://doi.org/10.3390/medicina59020372
Chicago/Turabian StyleSegi, Naoki, Hiroaki Nakashima, Ryuichi Shinjo, Yujiro Kagami, Masaaki Machino, Sadayuki Ito, Jun Ouchida, Kazuaki Morishita, Ryotaro Oishi, Ippei Yamauchi, and et al. 2023. "Vertebral Endplate Concavity in Lateral Lumbar Interbody Fusion: Tapered 3D-Printed Porous Titanium Cage versus Squared PEEK Cage" Medicina 59, no. 2: 372. https://doi.org/10.3390/medicina59020372
APA StyleSegi, N., Nakashima, H., Shinjo, R., Kagami, Y., Machino, M., Ito, S., Ouchida, J., Morishita, K., Oishi, R., Yamauchi, I., & Imagama, S. (2023). Vertebral Endplate Concavity in Lateral Lumbar Interbody Fusion: Tapered 3D-Printed Porous Titanium Cage versus Squared PEEK Cage. Medicina, 59(2), 372. https://doi.org/10.3390/medicina59020372