Evaluation of Artificial Intelligence-Calculated Hepatorenal Index for Diagnosing Mild and Moderate Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
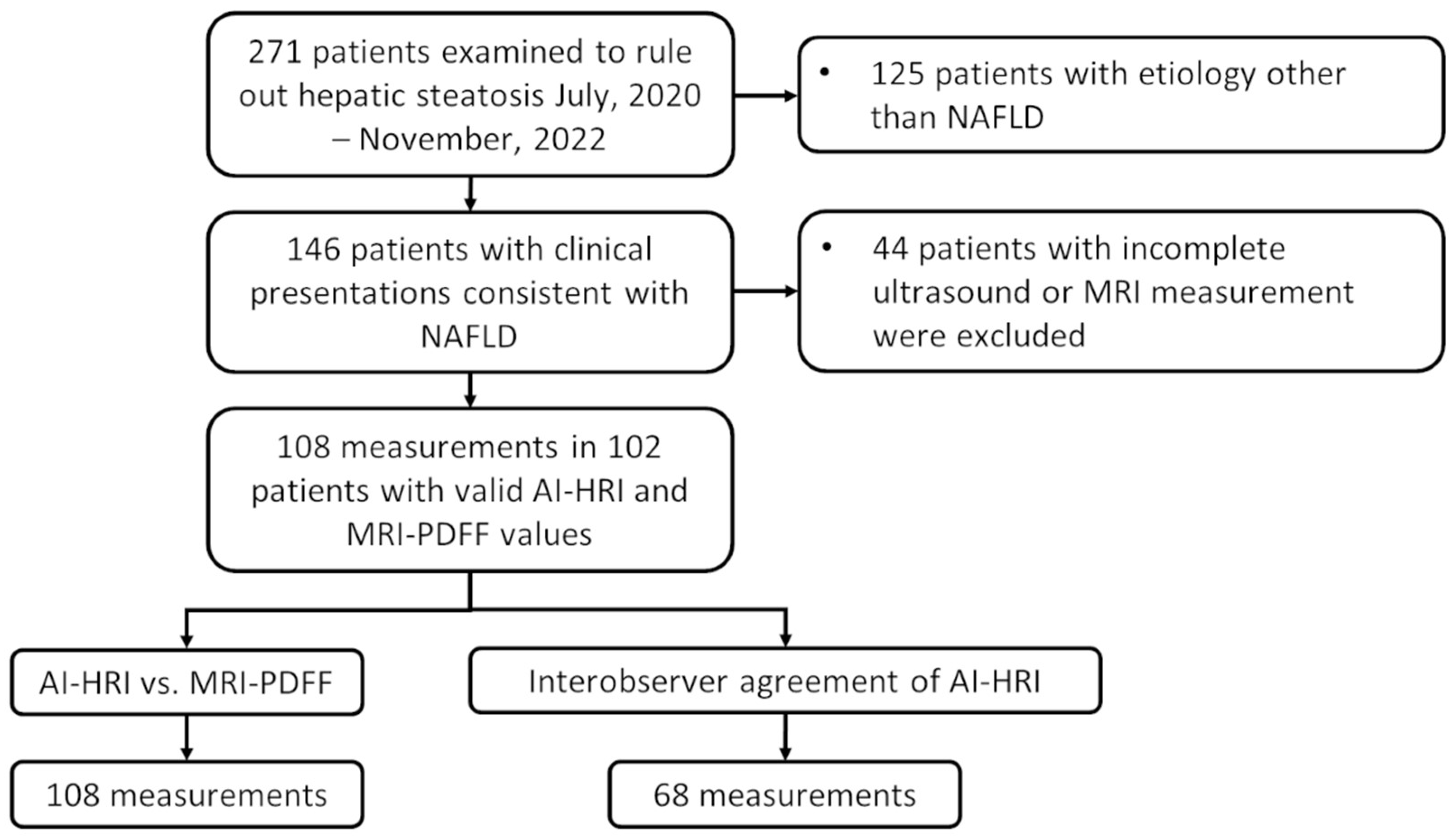
2.1. Patients
2.2. Ultrasound Scanning and AI-HRI Measurements
2.3. Measurement of AC, SC, and Semi-Quantitative Scoring of Hepatic Steatosis on US Images
2.4. Measurement of the MRI-PDFF
2.5. Statistical Analysis
3. Results
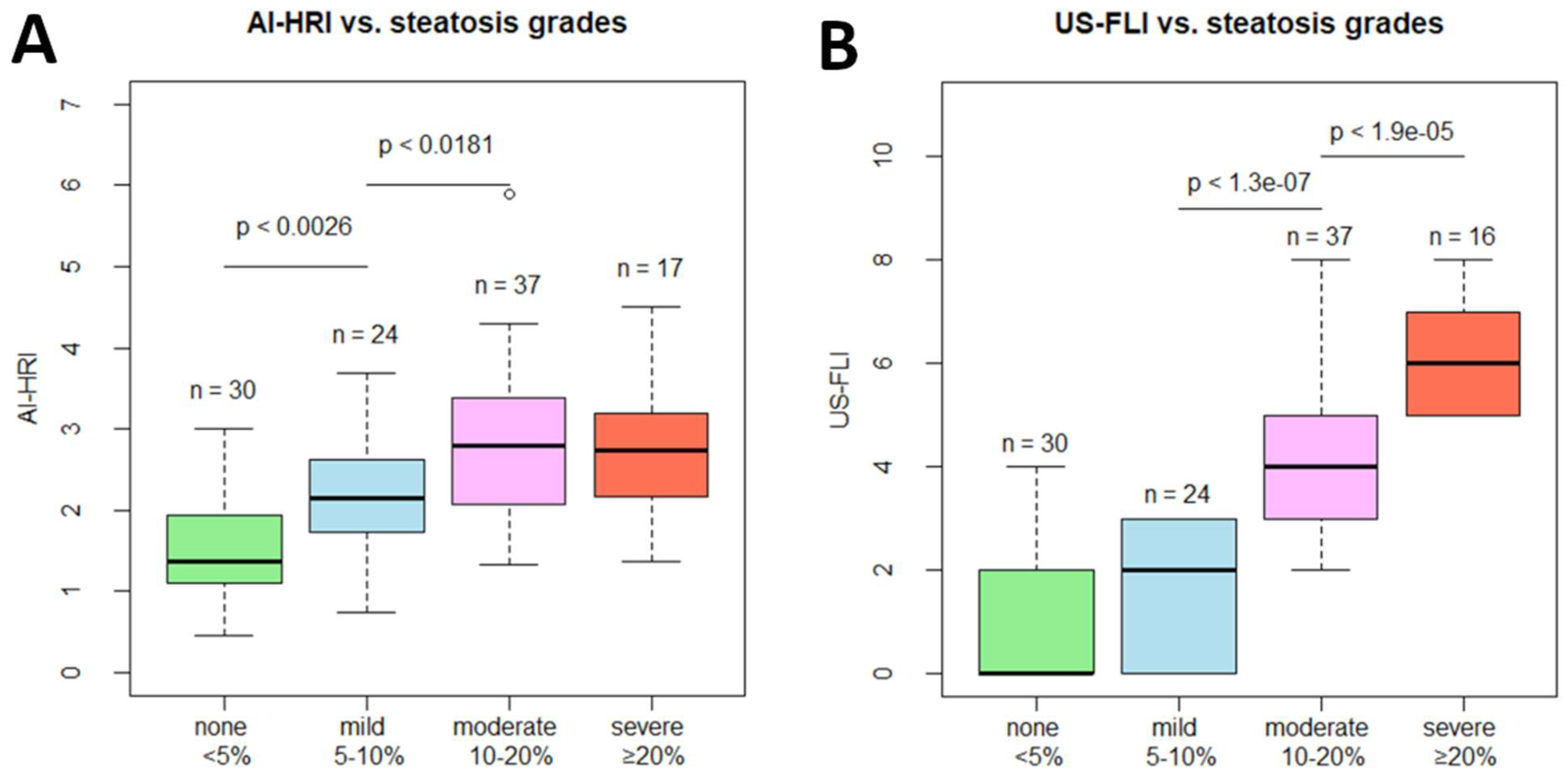
3.1. Comparison of AI-HRI and Other QUS Parameters between Different Grades of Steatosis
3.2. Evaluation of AI-HRI for Detection of Different Grades of Hepatic Steatosis
3.3. Correlation of AI-HRI with Other Methods
3.4. Interobserver Agreement of AI-HRI Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Kumar, V.; Ozturk, A.; Nam, K.; de Korte, C.L.; Barr, R.G. US Attenuation for Liver Fat Quantification: An AIUM-RSNA QIBA Pulse-Echo Quantitative Ultrasound Initiative. Radiology 2022, 302, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Blake, G.M.; Graffy, P.M.; Sandfort, V.; Elton, D.C.; Perez, A.A.; Summers, R.M. Liver Steatosis Categorization on Contrast-Enhanced CT Using a Fully Automated Deep Learning Volumetric Segmentation Tool: Evaluation in 1204 Healthy Adults Using Unenhanced CT as a Reference Standard. Am. J. Roentgenol. 2021, 217, 359–367. [Google Scholar] [CrossRef]
- Gu, J.; Liu, S.; Du, S.; Zhang, Q.; Xiao, J.; Dong, Q.; Xin, Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: A meta-analysis. Eur. Radiol. 2019, 29, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Lonardo, A.; Romagnoli, D.; Carulli, L.; Losi, L.; Day, C.P.; Loria, P. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012, 32, 1242–1252. [Google Scholar] [CrossRef]
- Petzold, G.; Lasser, J.; Rühl, J.; Bremer, S.C.B.; Knoop, R.F.; Ellenrieder, V.; Kunsch, S.; Neesse, A. Diagnostic accuracy of B-Mode ultrasound and Hepatorenal Index for graduation of hepatic steatosis in patients with chronic liver disease. PLoS ONE 2020, 15, e0231044. [Google Scholar] [CrossRef]
- Marshall, R.H.; Eissa, M.; Bluth, E.I.; Gulotta, P.M.; Davis, N.K. Hepatorenal Index as an Accurate, Simple, and Effective Tool in Screening for Steatosis. Am. J. Roentgenol. 2012, 199, 997–1002. [Google Scholar] [CrossRef]
- Van Tran, B.; Ujita, K.; Taketomi-Takahashi, A.; Hirasawa, H.; Suto, T.; Tsushima, Y. Reliability of ultrasound hepatorenal index and magnetic resonance imaging proton density fat fraction techniques in the diagnosis of hepatic steatosis, with magnetic resonance spectroscopy as the reference standard. PLoS ONE 2021, 16, e0255768. [Google Scholar] [CrossRef]
- Cha, D.I.; Kang, T.W.; Min, J.H.; Joo, I.; Sinn, D.H.; Ha, S.Y.; Kim, K.; Lee, G.; Yi, J. Deep learning-based automated quantification of the hepatorenal index for evaluation of fatty liver by ultrasonography. Ultrasonography 2021, 40, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kaposi, P.N.; Unger, Z.; Fejér, B.; Kucsa, A.; Tóth, A.; Folhoffer, A.; Szalay, F.; Bérczi, V. Interobserver agreement and diagnostic accuracy of shearwave elastography for the staging of hepatitis C virus-associated liver fibrosis. J. Clin. Ultrasound 2019, 48, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Folhoffer, A.; Rónaszéki, A.; Budai, B.; Borsos, P.; Orbán, V.; Győri, G.; Szalay, F.; Kaposi, P. Follow-Up of Liver Stiffness with Shear Wave Elastography in Chronic Hepatitis C Patients in Sustained Virological Response Augments Clinical Risk Assessment. Processes 2021, 9, 753. [Google Scholar] [CrossRef]
- Rónaszéki, A.D.; Budai, B.K.; Csongrády, B.; Stollmayer, R.; Hagymási, K.M.; Werling, K.M.; Fodor, T.; Folhoffer, A.M.; Kalina, I.M.; Győri, G.; et al. Tissue attenuation imaging and tissue scatter imaging for quantitative ultrasound evaluation of hepatic steatosis. Medicine 2022, 101, e29708. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.M.; Lee, G.; Jeon, S.K.; Joo, I. Quantitative Evaluation of Hepatic Steatosis Using Advanced Imaging Techniques: Focusing on New Quantitative Ultrasound Techniques. Korean J. Radiol. 2022, 23, 13–29. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Park, S.-J. Quantitative Ultrasound Radiofrequency Data Analysis for the Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Using Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Korean J. Radiol. 2021, 22, 1077–1086. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Henninger, B.; Alustiza, J.; Garbowski, M.; Gandon, Y. Practical guide to quantification of hepatic iron with MRI. Eur. Radiol. 2019, 30, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, G.; Yokoo, T.; Bydder, M.; Cruite, I.; Schroeder, M.E.; Sirlin, C.B.; Middleton, M.S. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2010, 24, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Berglund, J.; Skorpil, M. Multi-scale graph-cut algorithm for efficient water-fat separation. Magn. Reson. Med. 2016, 78, 941–949. [Google Scholar] [CrossRef]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2017, 67, 1348–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obuchowski, N.A.; Lieber, M.L.; Wians, F.H. ROC Curves in Clinical Chemistry: Uses, Misuses, and Possible Solutions. Clin. Chem. 2004, 50, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-J.; Kim, K.W.; Jung, J.-H.; Choi, S.H.; Jeong, W.K.; Kim, B.; Song, G.-W.; Lee, S.-G. Noninvasive quantitative estimation of hepatic steatosis by ultrasound: A comparison of the hepato-renal index and ultrasound attenuation index. Med. Ultrason. 2016, 18, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Fort, D.; Shortt, K.; Therapondos, G.; Galliano, G.; Nguyen, T.; Bluth, E. Ultrasound Stratification of Hepatic Steatosis Using Hepatorenal Index. Diagnostics 2021, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Pickhardt, P.J.; Kliewer, M.A.; Hernando, D.; Chen, G.-H.; Zagzebski, J.A.; Reeder, S.B. Accuracy of Liver Fat Quantification with Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison with MR Spectroscopy. Am. J. Roentgenol. 2017, 208, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H. Imaging evaluation of non-alcoholic fatty liver disease: Focused on quantification. Clin. Mol. Hepatol. 2017, 23, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmentieri, B.; Sio, I.D.; LA Mura, V.; Masarone, M.; Vecchione, R.; Bruno, S.; Torella, R.; Persico, M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig. Liver Dis. 2006, 38, 485–489. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, S.H.; Kim, H.J.; Kim, S.Y.; Kim, M.-Y.; Kim, D.Y.; Suh, D.J.; Kim, K.M.; Bae, M.H.; Lee, J.Y.; et al. Non-invasive assessment of hepatic steatosis: Prospective comparison of the accuracy of imaging examinations. J. Hepatol. 2010, 52, 579–585. [Google Scholar] [CrossRef]



| Patient Number: | 102 |
|---|---|
| Females/males: | 50/52 |
| * Age (years): | 55 ± 13 |
| * BMI: | 28.95 ± 4.63 |
| T2DM: | 23/102 (22.5%) |
| * Platelet(×109/L): | 245.17 ± 67.35 |
| * Albumin (g/L): | 43.82 ± 3.66 |
| * AST (IU/L): | 37.38 ± 26.57 |
| * ALT (IU/L): | 48.80 ± 39.84 |
| * ALP (IU/L): | 86.93 ± 48.62 |
| * Total bilirubin (µmol/L): | 13.89 ± 7.60 |
| * Sodium (mmol/L): | 139.89 ± 2.24 |
| * Creatinine (µmol/L): | 78.10 ± 21.35 |
| *$ APRI: | 0.41 ± 0.28 |
| *$ Fibrosis-4 Index: | 1.38 ± 0.85 |
| *$ NAFLD Fibrosis Score: | 1.57 ± 1.65 |
| ** HSI: | 37.83 ± 6.21 |
| * Steatosis Grade: | None | Mild | Moderate | Severe |
|---|---|---|---|---|
| AI-HRI | 1.480 ± 0.607 | 2.155 ± 0.776 | 2.777 ± 0.923 | 2.711 ± 0.822 |
| ** p-value< | NA | 0.003 | 0.018 | 0.787 |
| *** AC (dB/cm/Mhz) | 0.674 ± 0.084 | 0.797 ± 0.089 | 0.895 ± 0.097 | 1.004 ± 0.139 |
| ** p-value< | NA | 0.001 | 0.002 | 0.003 |
| *** SC | 91.75 ± 11.03 | 101.37 ± 7.15 | 105.98 ± 5.40 | 106.17 ± 4.81 |
| ** p-value< | NA | 0.001 | 0.115 | 1.00 |
| *** US-FLI | 0.900 ± 1.398 | 1.542 ± 1.318 | 4.000 ± 1.509 | 5.941 ± 0.966 |
| ** p-value< | NA | 0.074 | 0.001 | 0.001 |
| Method | Thresh. | Spec. | Sens. | PPV | NPV | Acc. |
|---|---|---|---|---|---|---|
| * For differentiation between normal liver (<5%) and mild (≥5%) steatosis | ||||||
| AI-HRI (AUC = 0.85) | 1.23 | 0.367 | 0.875 | 0.525 | 0.786 | 0.593 |
| ** 1.53 | 0.667 | 0.833 | 0.667 | 0.833 | 0.741 | |
| 1.85 | 0.733 | 0.750 | 0.692 | 0.786 | 0.741 | |
| AC (AUC = 0.922) | 0.74 | 0.828 | 0.750 | 0.783 | 0.800 | 0.792 |
| ** 0.77 | 0.897 | 0.708 | 0.850 | 0.788 | 0.811 | |
| 0.79 | 0.931 | 0.583 | 0.875 | 0.730 | 0.774 | |
| SC (AUC = 0.860) | 90.47 | 0.414 | 0.958 | 0.575 | 0.923 | 0.660 |
| ** 93.93 | 0.552 | 0.875 | 0.618 | 0.842 | 0.698 | |
| 94.87 | 0.586 | 0.792 | 0.613 | 0.773 | 0.679 | |
| US-FLI (AUC = 0.85) | *** 2 | 0.733 | 0.583 | 0.636 | 0.688 | 0.667 |
| * For differentiation between absent/mild (<10%) and moderate/severe (≥10%) steatosis | ||||||
| AI-HRI (AUC = 0.803) | 2.21 | 0.778 | 0.722 | 0.765 | 0.737 | 0.750 |
| ** 2.25 | 0.796 | 0.704 | 0.776 | 0.729 | 0.750 | |
| 2.29 | 0.815 | 0.685 | 0.787 | 0.721 | 0.750 | |
| AC (AUC = 0.895) | 0.81 | 0.792 | 0.840 | 0.792 | 0.840 | 0.816 |
| ** 0.83 | 0.849 | 0.800 | 0.833 | 0.818 | 0.825 | |
| 0.85 | 0.868 | 0.760 | 0.844 | 0.793 | 0.816 | |
| SC (AUC = 0.805) | 98.37 | 0.547 | 0.940 | 0.662 | 0.906 | 0.738 |
| ** 100.22 | 0.623 | 0.920 | 0.697 | 0.892 | 0.767 | |
| 101.35 | 0.642 | 0.860 | 0.694 | 0.829 | 0.748 | |
| US-FLI (AUC = 0.937) | *** 4 | 0.944 | 0.667 | 0.923 | 0.739 | 0.806 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsombor, Z.; Rónaszéki, A.D.; Csongrády, B.; Stollmayer, R.; Budai, B.K.; Folhoffer, A.; Kalina, I.; Győri, G.; Bérczi, V.; Maurovich-Horvat, P.; et al. Evaluation of Artificial Intelligence-Calculated Hepatorenal Index for Diagnosing Mild and Moderate Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease. Medicina 2023, 59, 469. https://doi.org/10.3390/medicina59030469
Zsombor Z, Rónaszéki AD, Csongrády B, Stollmayer R, Budai BK, Folhoffer A, Kalina I, Győri G, Bérczi V, Maurovich-Horvat P, et al. Evaluation of Artificial Intelligence-Calculated Hepatorenal Index for Diagnosing Mild and Moderate Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease. Medicina. 2023; 59(3):469. https://doi.org/10.3390/medicina59030469
Chicago/Turabian StyleZsombor, Zita, Aladár D. Rónaszéki, Barbara Csongrády, Róbert Stollmayer, Bettina K. Budai, Anikó Folhoffer, Ildikó Kalina, Gabriella Győri, Viktor Bérczi, Pál Maurovich-Horvat, and et al. 2023. "Evaluation of Artificial Intelligence-Calculated Hepatorenal Index for Diagnosing Mild and Moderate Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease" Medicina 59, no. 3: 469. https://doi.org/10.3390/medicina59030469






