A Pilot Experiment to Measure the Initial Mechanical Stability of the Femoral Head Implant in a Cadaveric Model of Osteonecrosis of Femoral Head Involving up to 50% of the Remaining Femoral Head
Abstract
:1. Introduction
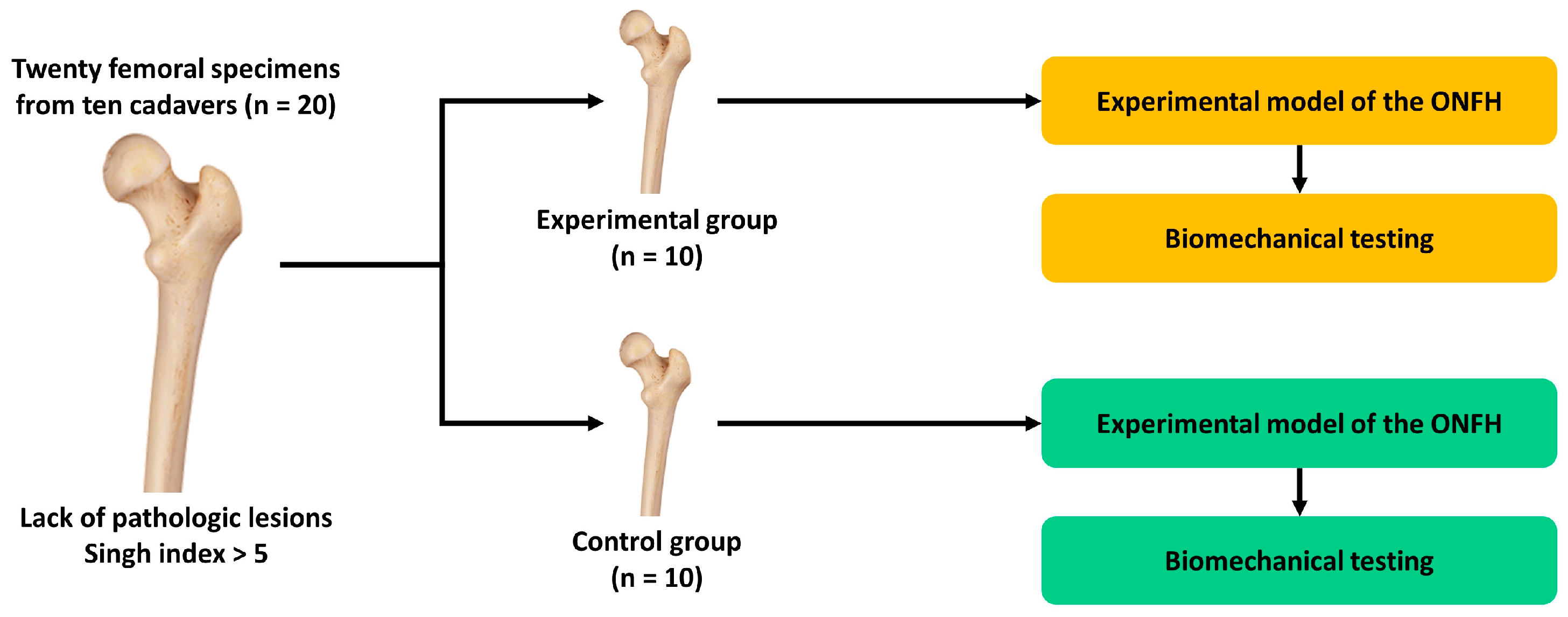
2. Materials and Methods
2.1. Experimental Materials and Setting
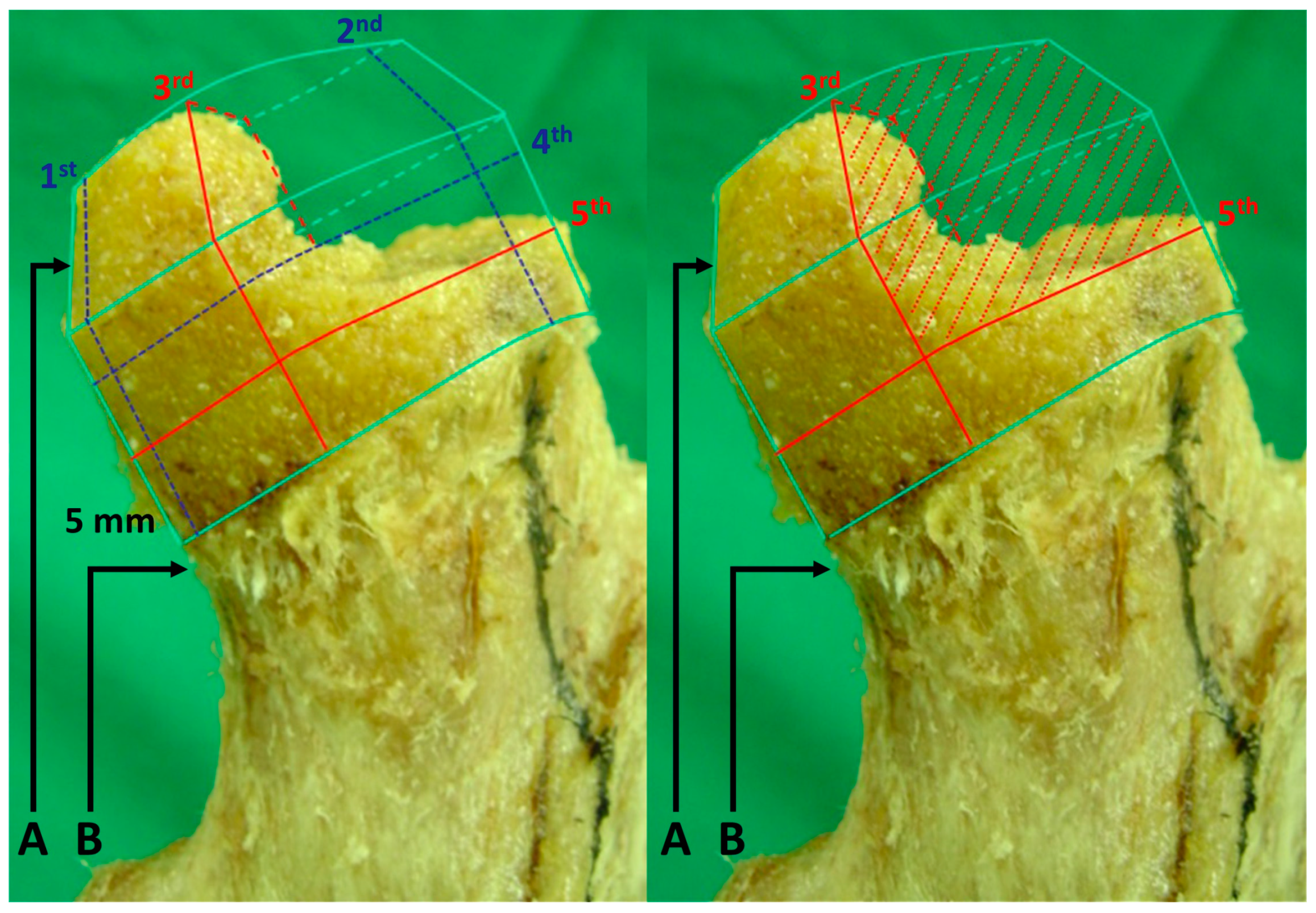
2.2. Creation of an Experimental Model of ONFH
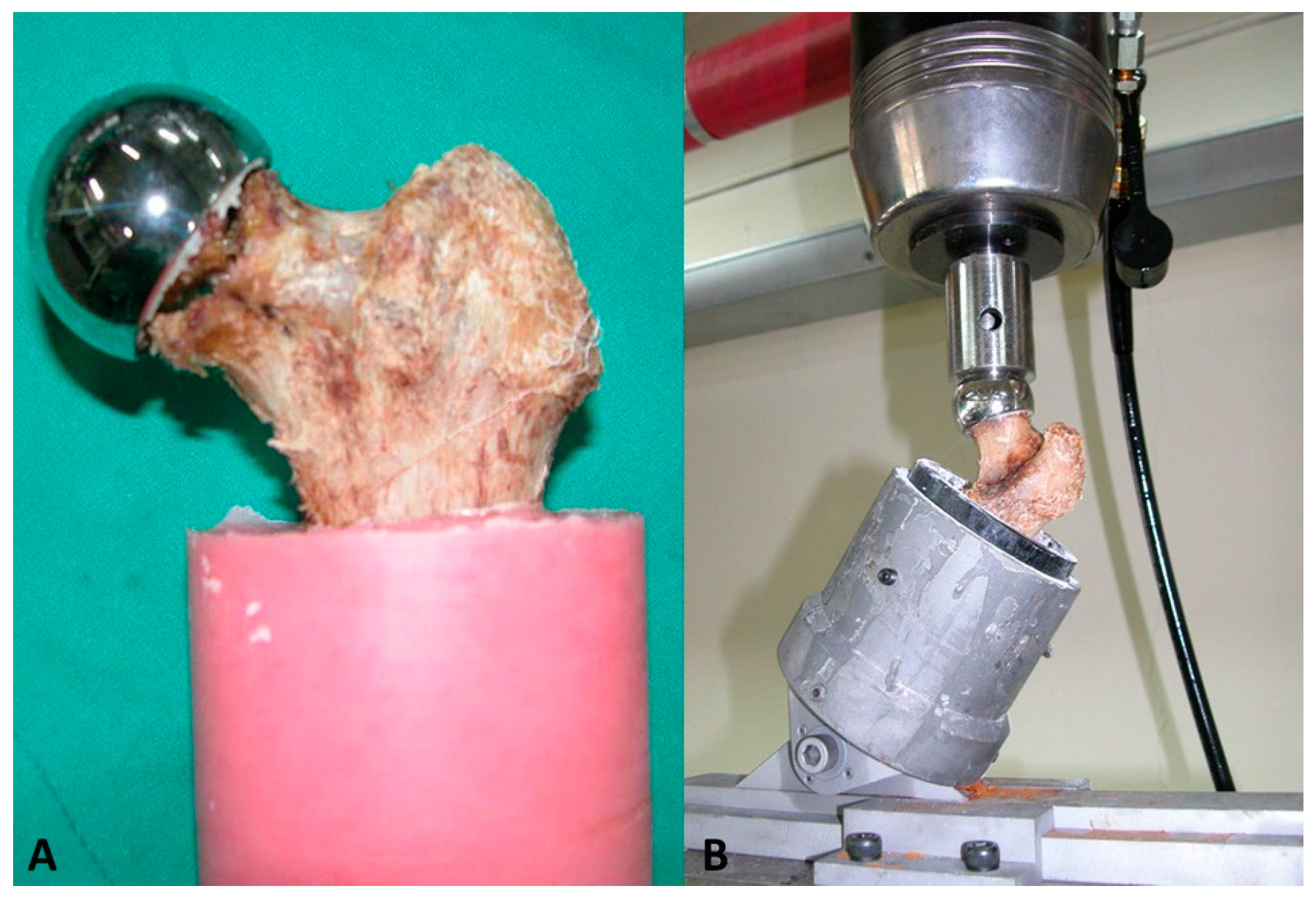
2.3. Assessment of the Biomechanical Stability of the Specimen
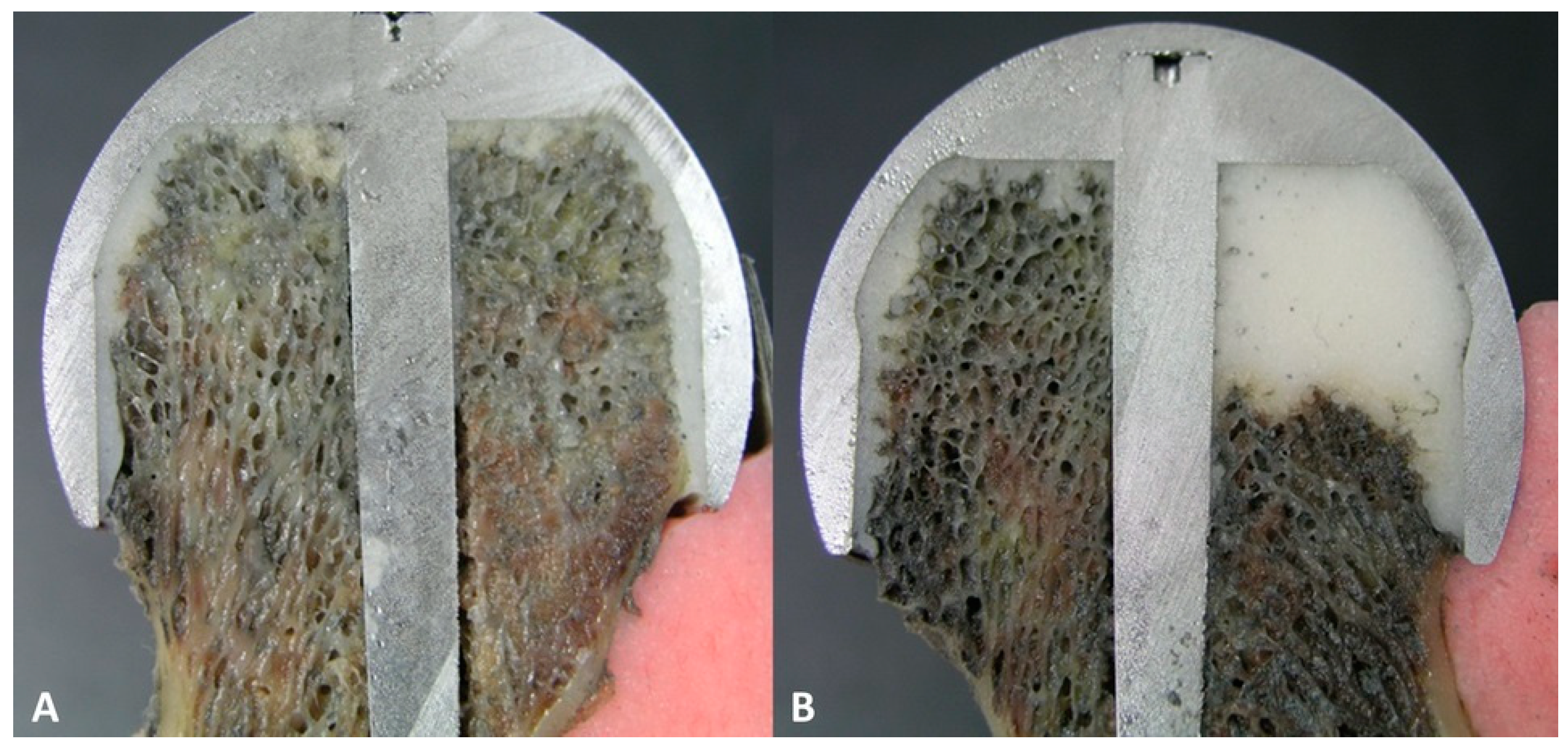
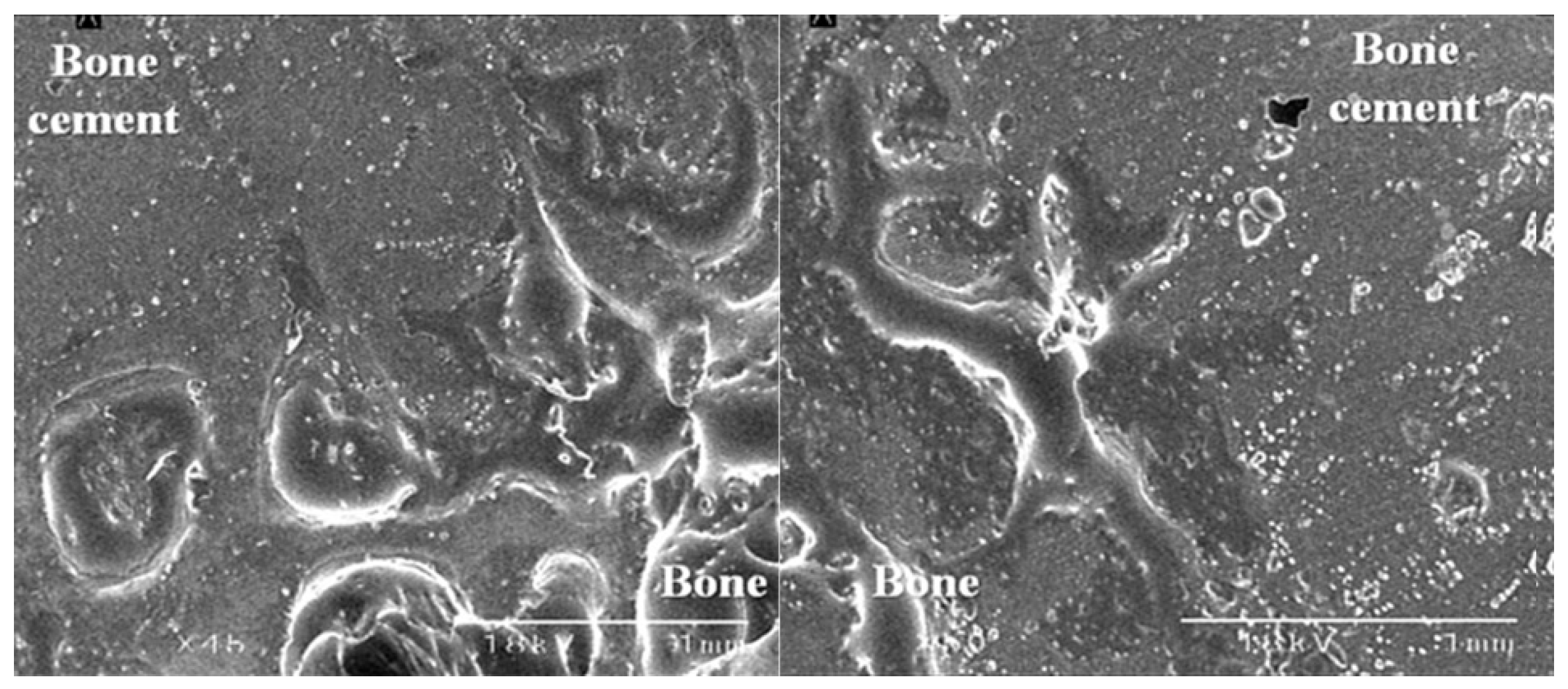
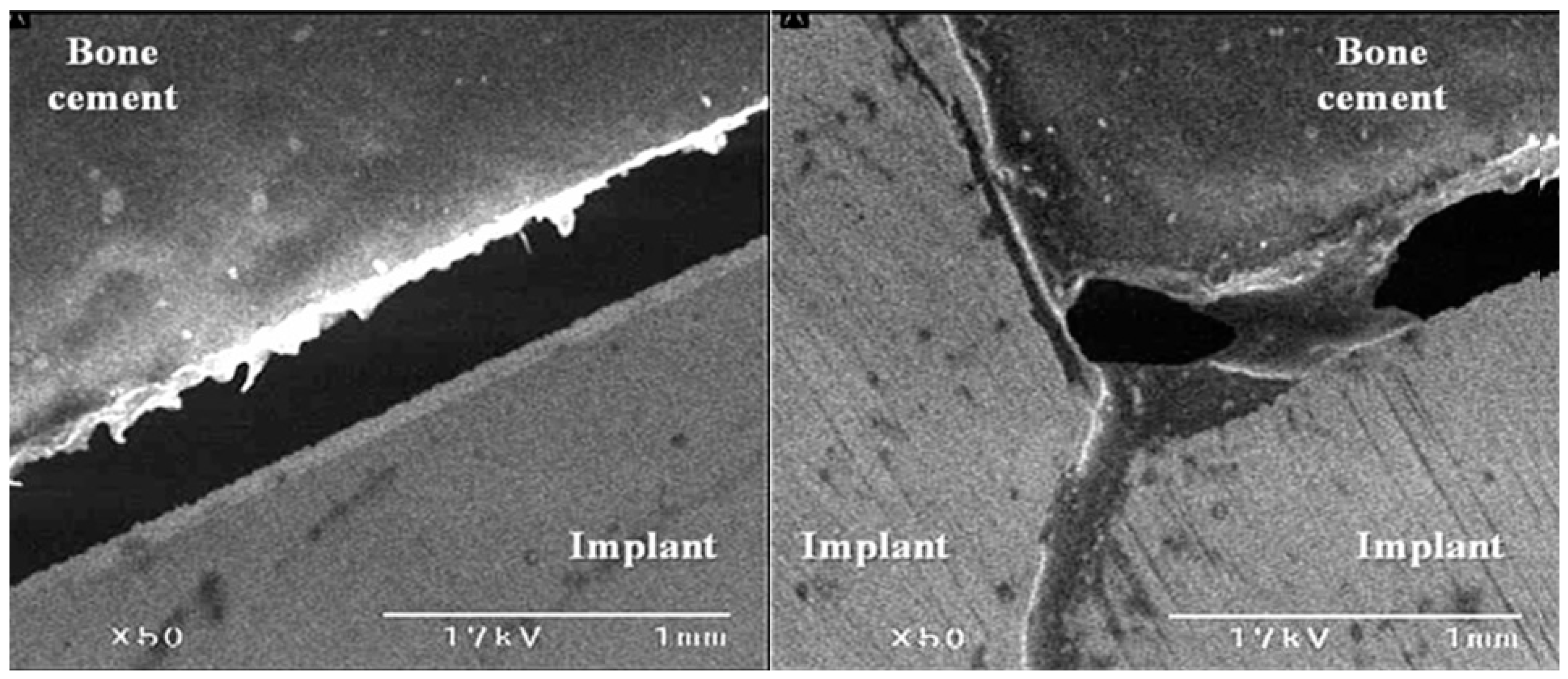
2.4. Scanning Electron Microscopy (SEM)
2.5. Statistical Analysis
3. Results
3.1. Size of the FHI
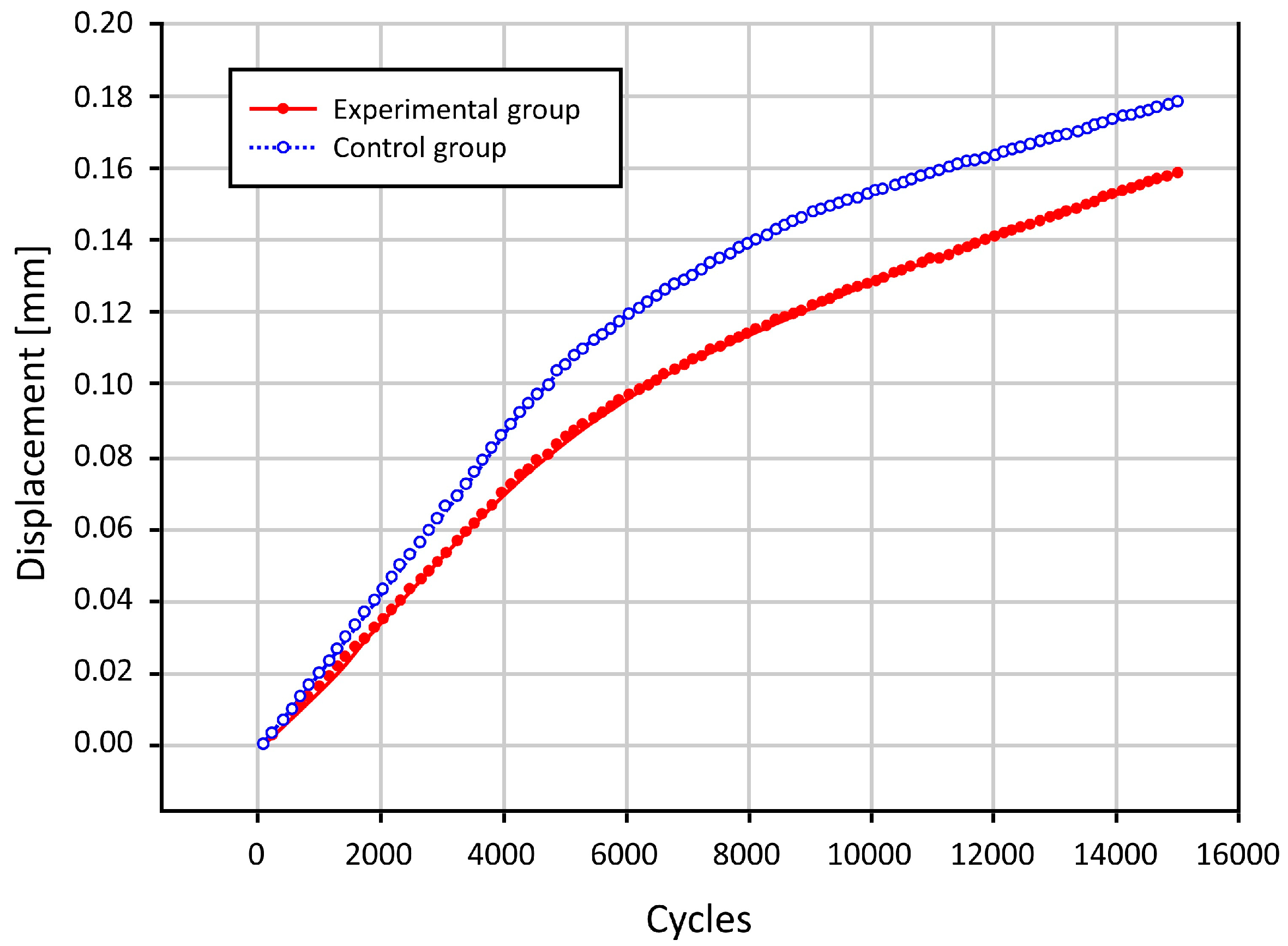
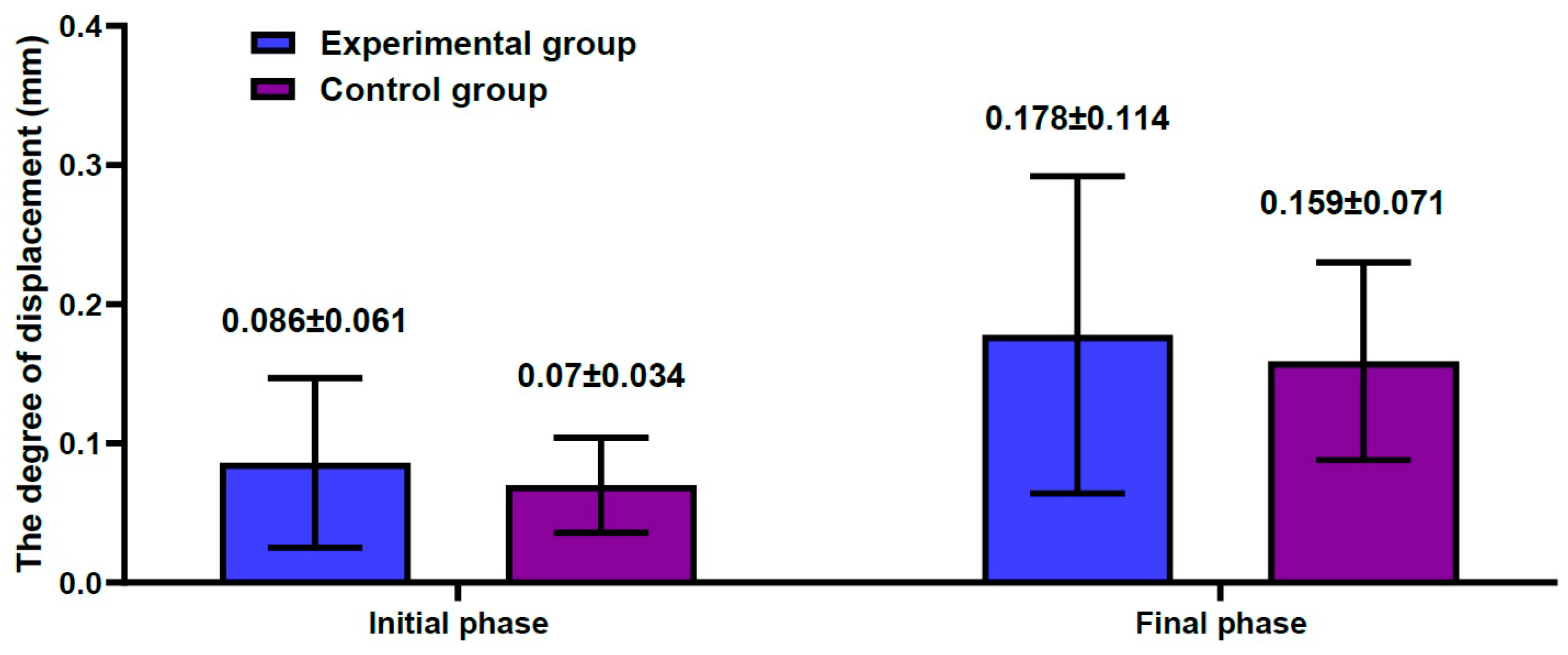
3.2. Results of the Biomechanical Study
3.3. The Bond Strength at the Bone–Cement Interface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardín-Pereda, A.; García-Sánchez, D.; Terán-Villagrá, N.; Alfonso-Fernández, A.; Fakkas, M.; Garcés-Zarzalejo, C.; Pérez-Campo, F.M. Osteonecrosis of the Femoral Head: A Multidisciplinary Approach in Diagnostic Accuracy. Diagnostics 2022, 12, 1731. [Google Scholar] [CrossRef]
- Murab, S.; Hawk, T.; Snyder, A.; Herold, S.; Totapally, M.; Whitlock, P.W. Tissue Engineering Strategies for Treating Avascular Necrosis of the Femoral Head. Bioengineering 2021, 8, 200. [Google Scholar] [CrossRef]
- Konarski, W.; Poboży, T.; Śliwczyński, A.; Kotela, I.; Krakowiak, J.; Hordowicz, M.; Kotela, A. Avascular Necrosis of Femoral Head—Overview and Current State of the Art. Int. J. Environ. Res. Public Health 2022, 19, 7348. [Google Scholar] [CrossRef] [PubMed]
- Petek, D.; Hannouche, D.; Suva, D. Osteonecrosis of the femoral head: Pathophysiology and current concepts of treatment. EFORT Open Rev. 2019, 4, 85–97. [Google Scholar] [CrossRef]
- Bejar, J.; Peled, E.; Boss, J.H. Vasculature deprivation-induced osteonecrosis of the rat femoral head as a model for therapeutic trials. Theor. Biol. Med. Model 2005, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.R.; Steinberg, M.E.; Y Cheng, E. Osteonecrosis of the femoral head: Diagnosis and classification systems. Curr. Rev. Musculoskelet. Med. 2015, 8, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Rezus, E.; Tamba, B.I.; Badescu, M.C.; Popescu, D.; Bratoiu, I.; Rezus, C. Osteonecrosis of the Femoral Head in Patients with Hypercoagulability—From Pathophysiology to Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6801. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Qin, K.; Yang, S.; Lu, J.; Lian, H.; Zhao, D. Proper mechanical stress promotes femoral head recovery from steroid-induced osteonecrosis in rats through the OPG/RANK/RANKL system. BMC Musculoskelet. Disord. 2020, 21, 281. [Google Scholar] [CrossRef]
- Paderno, E.; Zanon, V.; Vezzani, G.; Giacon, T.A.; Bernasek, T.L.; Camporesi, E.M.; Bosco, G. Evidence-Supported HBO Therapy in Femoral Head Necrosis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 2888. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ren, M.; Wang, J. The pathogenesis of steroid-induced osteonecrosis of the femoral head: A systematic review of the literature. Gene 2018, 671, 103–109. [Google Scholar] [CrossRef]
- Tsai, S.W.; Wu, P.K.; Chen, C.F.; Chiang, C.C.; Huang, C.K.; Chen, T.H.; Liu, C.L.; Chen, W.M. Etiologies and outcome of osteonecrosis of the femoral head: Etiology and outcome study in a Taiwan population. J. Chin. Med. Assoc. 2016, 79, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunze, K.N.; Sullivan, S.W.; Nwachukwu, B.U. Updates on Management of Avascular Necrosis Using Hip Arthroscopy for Core Decompression. Front Surg. 2022, 9, 662722. [Google Scholar] [CrossRef] [PubMed]
- Karasuyama, K.; Motomura, G.; Ikemura, S.; Fukushi, J.I.; Hamai, S.; Sonoda, K.; Kubo, Y.; Yamamoto, T.; Nakashima, Y. Risk factor analysis for postoperative complications requiring revision surgery after transtrochanteric rotational osteotomy for osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2018, 13, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, M.; Nabeshima, A.; Pan, C.C.; Behn, A.W.; Thio, T.; Lin, T.; Pajarinen, J.; Kawai, T.; Takagi, M.; Goodman, S.B.; et al. The effects of a functionally-graded scaffold and bone marrow-derived mononuclear cells on steroid-induced femoral head osteonecrosis. Biomaterials 2018, 187, 39–46. [Google Scholar] [CrossRef]
- Bakircioglu, S.; Atilla, B. Hip preserving procedures for osteonecrosis of the femoral head after collapse. J. Clin. Orthop. Trauma 2021, 23, 101636. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Goyal, T.; Sen, R.K. Management of femoral head osteonecrosis: Current concepts. Indian J. Orthop. 2015, 49, 28–45. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Li, Z.R.; Gao, F.Q.; Sun, W. Pericollapse Stage of Osteonecrosis of the Femoral Head: A Last Chance for Joint Preservation. Chin. Med. J. 2018, 131, 2589–2598. [Google Scholar] [CrossRef]
- Kuroda, Y.; Nankaku, M.; Okuzu, Y.; Kawai, T.; Goto, K.; Matsuda, S. Percutaneous autologous impaction bone graft for advanced femoral head osteonecrosis: A retrospective observational study of unsatisfactory short-term outcomes. J. Orthop. Surg. Res. 2021, 16, 141. [Google Scholar] [CrossRef]
- Jie, K.; Feng, W.; Li, F.; Wu, K.; Chen, J.; Zhou, G.; Zeng, H.; Zeng, Y. Long-term survival and clinical outcomes of non-vascularized autologous and allogeneic fibular grafts are comparable for treating osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2021, 16, 109. [Google Scholar] [CrossRef]
- Ma, J.; Sun, W.; Gao, F.; Guo, W.; Wang, Y.; Li, Z. Porous Tantalum Implant in Treating Osteonecrosis of the Femoral Head: Still a Viable Option? Sci. Rep. 2016, 6, 28227. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Boyd, K.; Kaste, S.C.; Kamdem Kamdem, L.; Rahija, R.J.; Relling, M.V. A mouse model for glucocorticoid-induced osteonecrosis: Effect of a steroid holiday. J. Orthop. Res. 2009, 27, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Sugano, N.; Kubo, T.; Takaoka, K.; Ohzono, K.; Hotokebuchi, T.; Matsumoto, T.; Igarashi, H.; Ninomiya, S. Diagnostic criteria for non-traumatic osteonecrosis of the femoral head. A multicentre study. J. Bone Joint Surg. Br. 1999, 81, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, K.; Oda, Y.; Kaneshiro, Y.; Iwaki, H.; Masada, T.; Kobayashi, A.; Asada, A.; Takaoka, K. Effect of simvastatin on steroid-induced osteonecrosis evidenced by the serum lipid level and hepatic cytochrome P4503A in a rabbit model. J. Orthop. Sci. 2008, 13, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Ichiseki, T.; Matsumoto, T.; Nishino, M.; Kaneuji, A.; Katsuda, S. Oxidative stress and vascular permeability in steroid-induced osteonecrosis model. J. Orthop. Sci. 2004, 9, 509–515. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hirano, K.; Tsutsui, H.; Sugioka, Y.; Sueishi, K. Corticosteroid enhances the experimental induction of osteonecrosis in rabbits with Shwartzman reaction. Clin. Orthop. Relat. Res. 1995, 316, 235–243. [Google Scholar] [CrossRef]
- Wu, X.; Yang, S.; Duan, D.; Zhang, Y.; Wang, J. Experimental osteonecrosis induced by a combination of low-dose lipopolysaccharide and high-dose methylprednisolone in rabbits. Jt. Bone Spine 2008, 75, 573–578. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, G.; Sheng, H.; Yeung, K.W.; Yeung, H.Y.; Chan, C.W.; Cheung, W.H.; Griffith, J.; Chiu, K.H.; Leung, K.S. Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone 2006, 39, 863–871. [Google Scholar] [CrossRef]
- Calkins, T.E.; Suleiman, L.I.; Culvern, C.; Alazzawi, S.; Kazarian, G.S.; Barrack, R.L.; Haddad, F.S.; Della Valle, C.J. Hip resurfacing arthroplasty and total hip arthroplasty in the same patient: Which do they prefer? Hip Int. 2021, 31, 328–334. [Google Scholar] [CrossRef]
- Clough, E.J.; Clough, T.M. Metal on metal hip resurfacing arthroplasty: Where are we now? J. Orthop. 2020, 23, 123–127. [Google Scholar] [CrossRef]
- Park, C.W.; Lim, S.J.; Kim, J.H.; Park, Y.S. Hip resurfacing arthroplasty for osteonecrosis of the femoral head: Implant-specific outcomes and risk factors for failure. J. Orthop. Translat. 2020, 21, 41–48. [Google Scholar] [CrossRef]
- Kabata, T.; Maeda, T.; Tanaka, K.; Yoshida, H.; Kajino, Y.; Horii, T.; Yagishita, S.; Tsuchiya, H. Hemi-resurfacing versus total resurfacing for osteonecrosis of the femoral head. J. Orthop. Surg. 2011, 19, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Sodhi, N.; Acuna, A.; Etcheson, J.; Mohamed, N.; Davila, I.; Ehiorobo, J.O.; Jones, L.C.; Delanois, R.E.; Mont, M.A. Management of osteonecrosis of the femoral head. Bone Jt. J. 2020, 102-B, 122–128. [Google Scholar] [CrossRef]
- Ball, S.T.; Le Duff, M.J.; Amstutz, H.C. Early results of conversion of a failed femoral component in hip resurfacing arthroplasty. J. Bone Joint Surg. Am. 2007, 89, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.W.; Grammatopoulos, G.; Gundle, R.; Gibbons, C.L.; Whitwell, D.; Taylor, A.; Glyn-Jones, S.; Pandit, H.G.; Ostlere, S.; Gill, H.S.; et al. Hip resurfacing and pseudotumour. Hip Int. 2011, 21, 279–283. [Google Scholar] [CrossRef]
- Tai, C.L.; Chen, Y.C.; Hsieh, P.H. The effects of necrotic lesion size and orientation of the femoral component on stress alterations in the proximal femur in hip resurfacing—A finite element simulation. BMC Musculoskelet. Disord. 2014, 15, 262. [Google Scholar] [CrossRef] [Green Version]
- Su, E.P.; Ho, H.; Bhal, V.; Housman, L.R.; Masonis, J.L.; Noble, J.W., Jr.; Hopper, R.H., Jr.; Engh, C.A., Jr. Results of the First U.S. FDA-Approved Hip Resurfacing Device at 10-Year Follow-up. J. Bone Joint Surg. Am. 2021, 103, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. P040033: Birmingham Hip Resurfacing (BHR) System. 2006. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf4/p040033a.pdf (accessed on 9 February 2023).
- Gross, T.P.; Liu, F.; Webb, L.A. Clinical outcome of the metal-on-metal hybrid Corin Cormet 2000 hip resurfacing system: An up to 11-year follow-up study. J. Arthroplast. 2012, 27, 533–538.e1. [Google Scholar] [CrossRef]
- Mogensen, S.L.; Jakobsen, T.; Christoffersen, H.; Krarup, N. High Re-Operation Rates Using Conserve Metal-On-Metal Total Hip Articulations. Open Orthop. J. 2016, 10, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, A.M.; Rathi, V.K.; Grauer, J.N.; Ross, J.S. How do Orthopaedic Devices Change After Their Initial FDA Premarket Approval? Clin. Orthop. Relat. Res. 2016, 474, 1053–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waewsawangwong, W.; Ruchiwit, P.; Huddleston, J.I.; Goodman, S.B. Hip arthroplasty for treatment of advanced osteonecrosis: Comprehensive review of implant options, outcomes and complications. Orthop. Res. Rev. 2016, 8, 13–29. [Google Scholar]
- Yamamoto, N.; Sukegawa, S.; Kitamura, A.; Goto, R.; Noda, T.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H.; Kawasaki, K.; et al. Deep Learning for Osteoporosis Classification Using Hip Radiographs and Patient Clinical Covariates. Biomolecules 2020, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, G. Experimental Study on Biaxial Dynamic Compressive Properties of ECC. Materials 2021, 14, 1257. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, T.; Nuutinen, J.P.; Koistinen, A.P.; Kröger, H.; Lappalainen, R. Biomechanical evaluation of bone screw fixation with a novel bone cement. Biomed. Eng. Online 2015, 14, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faris, M.A.; Abdullah, M.M.A.B.; Muniandy, R.; Abu Hashim, M.F.; Błoch, K.; Jeż, B.; Garus, S.; Palutkiewicz, P.; Mohd Mortar, N.A.; Ghazali, M.F. Comparison of Hook and Straight Steel Fibers Addition on Malaysian Fly Ash-Based Geopolymer Concrete on the Slump, Density, Water Absorption and Mechanical Properties. Materials 2021, 14, 1310. [Google Scholar] [CrossRef]
- Xu, H.Z.; Wang, X.Y.; Chi, Y.L.; Zhu, Q.A.; Lin, Y.; Huang, Q.S.; Dai, L.Y. Biomechanical evaluation of a dynamic pedicle screw fixation device. Clin. Biomech. 2006, 21, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Nourisa, J.; Rouhi, G. Biomechanical evaluation of intramedullary nail and bone plate for the fixation of distal metaphyseal fractures. J. Mech. Behav. Biomed. Mater. 2016, 56, 34–44. [Google Scholar] [CrossRef]
- Motavalli, M.; Whitney, G.A.; Dennis, J.E.; Mansour, J.M. Investigating a continuous shear strain function for depth-dependent properties of native and tissue engineering cartilage using pixel-size data. J. Mech. Behav. Biomed. Mater. 2013, 28, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, A.D.; Zou, H.; Shiu, Y.T.; Hsu, E.W. Characterization of regional deformation and material properties of the intact explanted vein by microCT and computational analysis. Cardiovasc. Eng. Technol. 2014, 5, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Shao, W.; Lv, X.; Wang, B.; Han, L.; Gong, S.; Wang, P.; Feng, Y. Advances in experimental models of osteonecrosis of the femoral head. J. Orthop. Translat. 2023, 39, 88–99. [Google Scholar] [CrossRef]
- Xu, J.; Gong, H.; Lu, S.; Deasey, M.J.; Cui, Q. Animal models of steroid-induced osteonecrosis of the femoral head-a comprehensive research review up to 2018. Int. Orthop. 2018, 42, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Z.; Liu, Z.; Lei, M.; Peng, J.; He, Y.X.; Xie, X.H.; Man, C.W.; Huang, L.; Wang, X.L.; Fong, D.T.; et al. Steroid-associated hip joint collapse in bipedal emus. PLoS ONE 2013, 8, e76797. [Google Scholar] [CrossRef] [PubMed]
- Dal Fabbro, G.; Agostinone, P.; Lucidi, G.A.; Pizza, N.; Maitan, N.; Grassi, A.; Zaffagnini, S. The Cadaveric Studies and the Definition of the Antero-Lateral Ligament of the Knee: From the Anatomical Features to the Patient-Specific Reconstruction Surgical Techniques. Int. J. Environ. Res. Public Health 2021, 18, 12852. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.-S.; Kim, Y.D.; Cho, N.; In, Y.; Kim, M.S.; Lim, D.; Koh, I.J. Restoration of the Joint Line Configuration Reproduces Native Mid-Flexion Biomechanics after Total Knee Arthroplasty: A Matched-Pair Cadaveric Study. Bioengineering 2022, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Sanderson, W.J.; Ingham, E.; Fisher, J.; Jennings, L.M. Development of a specimen-specific in vitro pre-clinical simulation model of the human cadaveric knee with appropriate soft tissue constraints. PLoS ONE 2020, 15, e0238785. [Google Scholar] [CrossRef]
- Vanaclocha, A.; Vanaclocha, V.; Atienza, C.M.; Clavel, P.; Jordá-Gómez, P.; Barrios, C.; Saiz-Sapena, N.; Vanaclocha, L. Bionate Lumbar Disc Nucleus Prosthesis: Biomechanical Studies in Cadaveric Human Spines. ACS Omega 2022, 7, 46501–46514. [Google Scholar] [CrossRef] [PubMed]
- Mont, M.A.; Zywiel, M.G.; Marker, D.R.; McGrath, M.S.; Delanois, R.E. The natural history of untreated asymptomatic osteonecrosis of the femoral head: A systematic literature review. J. Bone Joint Surg. Am. 2010, 92, 2165–2170. [Google Scholar] [CrossRef]
- Kaushik, A.P.; Das, A.; Cui, Q. Osteonecrosis of the femoral head: An update in year 2012. World J. Orthop. 2012, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, C.J.; Nunley, R.M.; Raterman, S.J.; Barrack, R.L. Initial American experience with hip resurfacing following FDA approval. Clin. Orthop. Relat. Res. 2009, 467, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Liacouras, P.C.; Wayne, J.S. Computational modeling to predict mechanical function of joints: Application to the lower leg with simulation of two cadaver studies. J. Biomech. Eng. 2007, 129, 811–817. [Google Scholar] [CrossRef]
- Weickenmeier, J.; Butler, C.A.M.; Young, P.G.; Goriely, A.; Kuhl, E. The mechanics of decompressive craniectomy: Personalized simulations. Comput. Methods Appl. Mech. Eng. 2017, 314, 180–195. [Google Scholar] [CrossRef] [Green Version]
- Scifert, C.F.; Noble, P.C.; Brown, T.D.; Bartz, R.L.; Kadakia, N.; Sugano, N.; Johnston, R.C.; Pedersen, D.R.; Callaghan, J.J. Experimental and computational simulation of total hip arthroplasty dislocation. Orthop. Clin. North Am. 2001, 32, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.J.; Chen, H.Y.; Hu, Y.; Ju, X.; Bai, B. Finite Element Analysis of porously punched prosthetic short stem virtually designed for simulative uncemented Hip Arthroplasty. BMC Musculoskelet. Disord. 2017, 18, 295. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Tian, Q.; Dai, Z.; Liu, X. Mechanical behaviour of umbrella-shaped, Ni-Ti memory alloy femoral head support device during implant operation: A finite element analysis study. PLoS ONE 2014, 9, e100765. [Google Scholar] [CrossRef] [Green Version]
- Goel, V.K.; Nyman, E. Computational Modeling and Finite Element Analysis. Spine 2016, 41 (Suppl. 7), S6–S7. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing Risk of Failure from Ceramic-on-Ceramic Total Hip Prosthesis by Selecting Ceramic Materials Based on Tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Hu, C.Y.; Yoon, T.R. Recent Updates for Biomaterials Used in Total Hip Arthroplasty. Biomater. Res. 2018, 22, 33. [Google Scholar] [CrossRef] [Green Version]
- Harun, M.N.; Wang, F.C.; Jin, Z.M.; Fisher, J. Long-Term Contact-CoupledWear Prediction for Metal-on-Metal Total Hip Joint Replacement. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2009, 223, 993–1001. [Google Scholar] [CrossRef]
- Choudhury, D.; Lackner, J.; Fleming, R.A.; Goss, J.; Chen, J.; Zou, M. Diamond-like Carbon Coatings with Zirconium-Containing Interlayers for Orthopedic Implants. J. Mech. Behav. Biomed. Mater. 2017, 68, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Basri, H.; Syahrom, A.; Ramadhoni, T.S.; Prakoso, A.T.; Ammarullah, M.I. The Analysis of the Dimple Arrangement of the Artificial Hip Joint to the Performance of Lubrication. IOP Conf. Ser. Mater. Sci. Eng. 2019, 620, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Basri, H.; Syahrom, A.; Prakoso, A.T.; Wicaksono, D.; Amarullah, M.I.; Ramadhoni, T.S.; Nugraha, R.D. The Analysis of Dimple Geometry on Artificial Hip Joint to the Performance of Lubrication. J. Phys. Conf. Ser. 2019, 1198, 1–10. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Saad, A.P.; Syahrom, A. Contact Pressure Analysis of Acetabular Cup Surface with Dimple Addition on Total Hip Arthroplasty Using Finite Element Method. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1034, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Li, Y.; Wei, B.; Hu, Y.; Luo, J. Investigation on Inner Flow Field Characteristics of Groove Textures in Fully Lubricated Thrust Bearings. Ind. Lubr. Tribol. 2018, 70, 754–763. [Google Scholar] [CrossRef]
- Pratap, T.; Patra, K. Mechanical Micro-Texturing of Ti-6Al-4V Surfaces for Improved Wettability and Bio-Tribological Performances. Surf. Coat. Technol. 2018, 349, 71–81. [Google Scholar] [CrossRef]
- Choudhury, D.; Vrbka, M.; Bin Mamat, A.; Stavness, I.; Roy, C.K.; Mootanah, R.; Krupka, I. The impact of surface and geometry on coefficient of friction of artificial hip joints. J. Mech. Behav. Biomed. Mater. 2017, 72, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of Bottom Profile Dimples on the Femoral Head on Wear in Metal-on-Metal Total Hip Arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K. Research in orthopedics: A necessity. Indian J. Orthop. 2009, 43, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Buckley, J.M.; Colnot, C.; Marcucio, R.; Miclau, T. Basic research in orthopedic surgery: Current trends and future directions. Indian J. Orthop. 2009, 43, 318–323. [Google Scholar] [PubMed]
- Nayar, S.K.; Dein, E.J.; Bernard, J.A.; Zikria, B.A.; Spiker, A.M. Basic Science Research Trends in Orthopedic Surgery: An Analysis of the Top 100 Cited Articles. HSS J. 2018, 14, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Grässel, S.; Nöth, U.; Relja, B.; Bernstein, A.; Docheva, D.; Kauther, M.D.; Katthagen, J.C.; Bader, R.; van Griensven, M.; et al. The future of basic science in orthopaedics and traumatology: Cassandra or Prometheus? Eur. J. Med. Res. 2021, 26, 56. [Google Scholar] [CrossRef]
- Maletsky, L.; Shalhoub, S.; Fitzwater, F.; Eboch, W.; Dickinson, M.; Akhbari, B.; Louie, E. In Vitro Experimental Testing of the Human Knee: A Concise Review. J. Knee Surg. 2016, 29, 138–148. [Google Scholar] [PubMed]
| # | Values | |||||
|---|---|---|---|---|---|---|
| Experimental Group (n = 10) | Control Group (n = 10) | |||||
| Initial Phase | Final Phase | Δ | Initial Phase | Final Phase | Δ | |
| 1 | 0.237 | 0.450 | 0.213 | 0.051 | 0.121 | 0.07 |
| 2 | 0.032 | 0.066 | 0.034 | 0.078 | 0.190 | 0.112 |
| 3 | 0.069 | 0.151 | 0.082 | 0.055 | 0.140 | 0.085 |
| 4 | 0.031 | 0.061 | 0.03 | 0.109 | 0.240 | 0.131 |
| 5 | 0.123 | 0.251 | 0.128 | 0.025 | 0.073 | 0.048 |
| 6 | 0.098 | 0.199 | 0.01 | 0.140 | 0.308 | 0.168 |
| 7 | 0.042 | 0.090 | 0.048 | 0.075 | 0.146 | 0.071 |
| 8 | 0.077 | 0.172 | 0.095 | 0.045 | 0.104 | 0.059 |
| 9 | 0.088 | 0.201 | 0.113 | 0.068 | 0.162 | 0.094 |
| 10 | 0.062 | 0.143 | 0.081 | 0.052 | 0.103 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.; Lee, Y.; Sun, D. A Pilot Experiment to Measure the Initial Mechanical Stability of the Femoral Head Implant in a Cadaveric Model of Osteonecrosis of Femoral Head Involving up to 50% of the Remaining Femoral Head. Medicina 2023, 59, 508. https://doi.org/10.3390/medicina59030508
Woo S, Lee Y, Sun D. A Pilot Experiment to Measure the Initial Mechanical Stability of the Femoral Head Implant in a Cadaveric Model of Osteonecrosis of Femoral Head Involving up to 50% of the Remaining Femoral Head. Medicina. 2023; 59(3):508. https://doi.org/10.3390/medicina59030508
Chicago/Turabian StyleWoo, Seungha, Youngho Lee, and Doohoon Sun. 2023. "A Pilot Experiment to Measure the Initial Mechanical Stability of the Femoral Head Implant in a Cadaveric Model of Osteonecrosis of Femoral Head Involving up to 50% of the Remaining Femoral Head" Medicina 59, no. 3: 508. https://doi.org/10.3390/medicina59030508
APA StyleWoo, S., Lee, Y., & Sun, D. (2023). A Pilot Experiment to Measure the Initial Mechanical Stability of the Femoral Head Implant in a Cadaveric Model of Osteonecrosis of Femoral Head Involving up to 50% of the Remaining Femoral Head. Medicina, 59(3), 508. https://doi.org/10.3390/medicina59030508






