Effects of Selective Serotonin Reuptake Inhibitor Treatment on Ovarian Reserves in Patients with Depression
Abstract
:1. Introduction
2. Materials and Methods
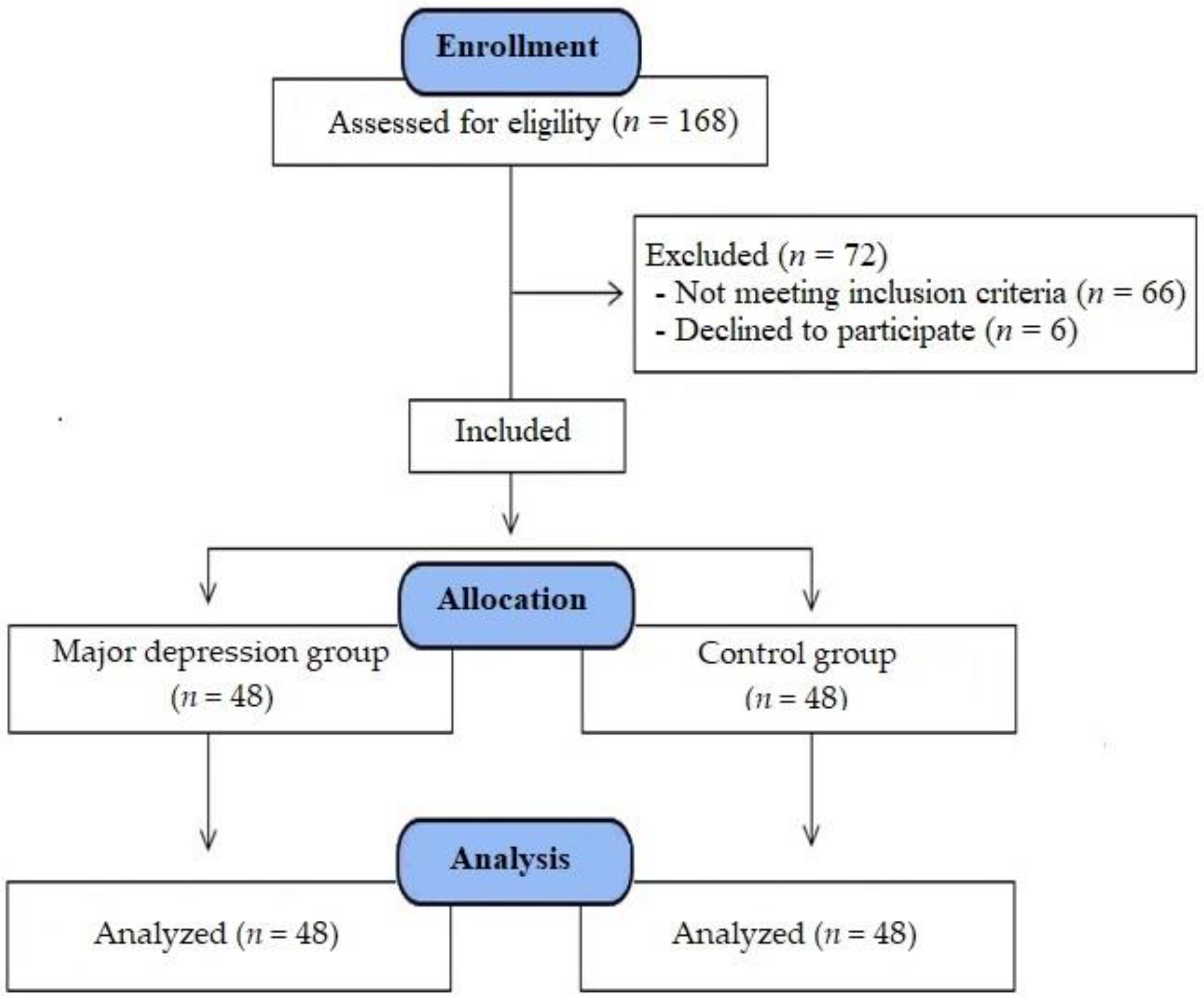
2.1. Study Participants and Design
2.2. Study Plan and Interventions
2.3. Determination of Serum AMH Levels
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratt, L.A.; Brody, D.J. Depression in the U.S. household population, 2009–2012. NCHS Data Brief 2014, 172, 1–8. [Google Scholar]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Segall-Gutierrez, P.; Berman, C.S.; Opper, N.; Dossett, E.; Moore, K.; Martin, C.; Pine, J. The Incidence of Depression by Fertility Status in Overweight and Obese Latina Women. J. Immigr. Minor. Health 2012, 14, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Kit, B.K.; Ogden, C.L.; Flegal, K.M. Prescription Medication Use Among Normal Weight, Overweight, and Obese Adults, United States, 2005–2008. Ann. Epidemiol. 2012, 22, 112–119. [Google Scholar] [CrossRef]
- Murphy, S.E.; Capitão, L.P.; Giles, S.L.C.; Cowen, P.J.; Stringaris, A.; Harmer, C.J. The knowns and unknowns of SSRI treatment in young people with depression and anxiety: Efficacy, predictors, and mechanisms of action. Lancet Psychiatry 2021, 8, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2015, 103, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.W.; La Marca, A.; Lambalk, C.; et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum. Reprod. Updat. 2014, 20, 370–385. [Google Scholar] [CrossRef] [Green Version]
- Weenen, C.; Laven, J.S.; Von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.; Themmen, A.P. Anti-Mullerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Louwers, Y.V.; Laven, J.S.E.; Visser, J.A. Comparison of 3 Different AMH Assays With AMH Levels and Follicle Count in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e3714–e3722. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef]
- Hansen, K.R.; Hodnett, G.M.; Knowlton, N.; Craig, L.B. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil. Steril. 2011, 95, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A.; Ecochard, R.; Thalabard, J.C. Age-Related Changes of the Population of Human Ovarian Follicles: Increase in the Disappearance Rate of Non-Growing and Early-Growing Follicles in Aging Women. Biol. Reprod. 1994, 50, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, A.; Türkçapar, M.; Örsel, S.; Demirergi, N.; Dag, I.; Özbay, M. Reliability and validity of the Turkish version of the Hamilton Depression Rating Scale. Compr. Psychiatry 2001, 42, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.M.; Newhouse, P.A. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Sacher, J. Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology 2019, 236, 3063–3079. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, D.; Dima, D.; Jones, L.; Jones, I.; Craddock, N.; Owen, M.J.; Rietschel, M.; Maier, W.; Korszun, A.; Rice, J.P.; et al. Polygenic risk for circulating reproductive hormone levels and their influence on hippocampal volume and depression susceptibility. Psychoneuroendocrinology 2019, 106, 284–292. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef]
- Claes, S.J. CRH, Stress, and Major Depression: A Psychobiological Interplay. Vitam. Horm. 2004, 69, 117–150. [Google Scholar] [CrossRef]
- Raftogianni, A.; Roth, L.C.; García-González, D.; Bus, T.; Kühne, C.; Monyer, H.; Spergel, D.J.; Deussing, J.M.; Grinevich, V. Deciphering the Contributions of CRH Receptors in the Brain and Pituitary to Stress-Induced Inhibition of the Reproductive Axis. Front. Mol. Neurosci. 2018, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Crawford, N.M.; Hoff, H.S.; Mersereau, J.E. Infertile women who screen positive for depression are less likely to initiate fertility treatments. Hum. Reprod. 2017, 32, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, J.P.; Jiang, L.; Liu, H.; Shu, L.; Zhang, Q. Factors that influence in vitro fertilization treatment outcomes of Chinese men: A cross-sectional study. Appl. Nurs. Res. 2016, 32, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Golenbock, S.W.; Wise, L.A.; Lambert-Messerlian, G.M.; Eklund, E.E.; Harlow, B.L. Association between a history of depression and anti-müllerian hormone among late-reproductive aged women: The Harvard study of moods and cycles. Women's Midlife Heal. 2020, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.T.; Bronstein, A.C. Selective Serotonin Reuptake Inhibitor Exposure. Top. Companion Anim. Med. 2013, 28, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Jing, E.; Straw-Wilson, K. Sexual dysfunction in selective serotonin reuptake inhibitors (SSRIs) and potential solutions: A narrative literature review. Ment. Health Clin. 2016, 6, 191–196. [Google Scholar] [CrossRef]
- Healy, D.; Le Noury, J.; Mangin, D. Post-SSRI sexual dysfunction: Patient experiences of engagement with healthcare professionals. Int. J. Risk Saf. Med. 2019, 30, 167–178. [Google Scholar] [CrossRef]
- Casilla-Lennon, M.M.; Meltzer-Brody, S.; Steiner, A.Z. The effect of antidepressants on fertility. Am. J. Obstet. Gynecol. 2016, 215, 314.e1–314.e5. [Google Scholar] [CrossRef] [Green Version]
- Nillni, Y.I.; Wesselink, A.K.; Gradus, J.L.; Hatch, E.E.; Rothman, K.J.; Mikkelsen, E.M.; Wise, L.A. Depression, anxiety, and psychotropic medication use and fecundability. Am. J. Obstet. Gynecol. 2016, 215, 453.e1–453.e8. [Google Scholar] [CrossRef]
- Serafini, P.; Lobo, D.S.; Grosman, A.; Seibel, D.; Rocha, A.M.; Motta, E.L. Fluoxetine treatment for anxiety in women undergoing in vitro fertilization. Int. J. Gynecol. Obstet. 2009, 105, 136–139. [Google Scholar] [CrossRef]
- Cesta, C.E.; Viktorin, A.; Olsson, H.; Johansson, V.; Sjölander, A.; Bergh, C.; Skalkidou, A.; Nygren, K.-G.; Cnattingius, S.; Iliadou, A.N. Depression, anxiety, and antidepressant treatment in women: Association with in vitro fertilization outcome. Fertil. Steril. 2016, 105, 1594–1602.e3. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Nieto, C.; Lee, J.; Nazem, T.; Gounko, D.; Copperman, A.; Sandler, B. Embryo aneuploidy is not impacted by selective serotonin reuptake inhibitor exposure. Fertil. Steril. 2017, 108, 973–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans-Hoeker, E.A.; Eisenberg, E.; Diamond, M.P.; Legro, R.S.; Alvero, R.; Coutifaris, C.; Casson, P.R.; Christman, G.M.; Hansen, K.R.; Zhang, H.; et al. Major depression, antidepressant use, and male and female fertility. Fertil. Steril. 2018, 109, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, M.; Pierre, A.; Rey, R.A.; Leclerc, A.; Arouche, N.; Hesters, L.; Catteau-Jonard, S.; Frydman, R.; Picard, J.-Y.; Fanchin, R.; et al. Differential Regulation of Ovarian Anti-Müllerian Hormone (AMH) by Estradiol through α- and β-Estrogen Receptors. J. Clin. Endocrinol. Metab. 2012, 97, E1649–E1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Control Group | MDD Group | ||||
|---|---|---|---|---|---|
| Mean ± S.D | Med (IQR) | Mean ± S.D | Med (IQR) | Inter Group p | |
| Age | 27.69 ± 3.38 | 28 (24.25–30) | 28.29 ± 3.65 | 29 (24.25–31.75) | 0.355 (z = −0.926) |
| BMI (kg/m2) | 27.54 ± 3.28 | 27.3 (24.35–30.2) | 28.29 ± 3.32 | 28.25 (25.38–31.28) | 0.312 (z = −1.012) |
| Parity | 1.08 ± 0.96 | 1 (0–2) | 0.98 ± 0.89 | 1 (0–2) | 0.644 (z = −0.463) |
| Menstrual cycle length (days) | 27.54 ± 1.86 | 28 (26–29) | 27.81 ± 1.76 | 28 (26–29) | 0.46 (z = −0.739) |
| Menstrual cycle duration (days) | 4.81 ± 1.82 | 5 (3–6) | 5.83 ± 1.74 | 6 (4–7) | 0.007 * (z = −2.699) |
| Menstrual bleeding (pads/day) | 5.27 ± 1.72 | 5 (4–7) | 6.29 ± 1.91 | 6 (5–8) | 0.005 * (z = −2.786) |
| Control Group | MDD Group | ||||
|---|---|---|---|---|---|
| Mean ± S.D | Med (IQR) | Mean ± S.D | Med (IQR) | Inter Group p | |
| First ET (mm) | 6.25 ± 1.72 | 6 (5–8) | 6.17 ± 1.55 | 6 (5–8) | 0.884 (z = −0.146) |
| Final ET (mm) | 6.21 ± 1.71 | 6 (5–7) | 6.81 ± 1.9 | 7 (5–9) | 0.126 (z = −1.53) |
| Delta ET (mm) | 0.04 ± 1.43 | 0 (−1–1) | −0.65 ± 1.45 | −1 (−1–0.75) | 0.047 * (z = −1.99) |
| Intra Group p | 0.819 (z = −0.228) | 0.005 * (z = −2.799) | |||
| First E2 (ng/L) | 56.54 ± 13.91 | 56 (46.25–69) | 51.83 ± 12.83 | 53.5 (39.5–61.75) | 0.125 (z = −1.536) |
| Final E2 (ng/L) | 54.44 ± 15.62 | 56.5 (40.25–62.75) | 60.13 ± 11.81 | 63 (53–69) | 0.021 * (z = −2.314) |
| Delta E2 (ng/L) | 2.1 ± 13.29 | 2 (−7.75–10) | −8.29 ± 7 | −9 (−14–−4) | 0.0001 * (t = 4.796) |
| Intra Group p | 0.278 (t = 1.097) | 0.0001 * (t = −8.212) | |||
| First FSH (mIU/mL) | 7.21 ± 1.46 | 7.5 (6–8) | 7.79 ± 1.37 | 8 (7–9) | 0.063 (z = −1.862) |
| Final FSH (mIU/mL) | 7.33 ± 1.6 | 8 (6–9) | 8.25 ± 1.33 | 8.5 (7–9) | 0.006 * (z = −2.768) |
| Delta FSH (mIU/mL) | −0.13 ± 2.08 | 0 (−2–1) | −0.46 ± 1.56 | −1 (−1–1) | 0.265 (z = −1.115) |
| Intra Group p | 0.679 (t = −0.416) | 0.05 * (z = −1.954) | |||
| First LH (mIU/mL) | 6.02 ± 1.47 | 6 (5–7) | 5.38 ± 1.52 | 5 (4–6) | 0.045 * (z = −2.001) |
| Final LH (mIU/mL) | 5.88 ± 1.28 | 6 (5–7) | 5.25 ± 1.06 | 5 (4–6) | 0.015 * (z = −2.432) |
| Delta LH (mIU/mL) | 0.15 ± 1.62 | 0 (−1–1) | 0.13 ± 1.28 | 0.5 (−1–1) | 0.803 (z = −0.249) |
| Intra Group p | 0.722 (z = −0.356) | 0.541 (z = −0.611) | |||
| First AMH (ng/mL) | 3.95 ± 1.45 | 4.2 (2.4–5.18) | 3.69 ± 1.53 | 3.7 (2.15–4.88) | 0.344 (z = −0.946) |
| Final AMH (ng/mL) | 3.82 ± 1.27 | 3.6 (2.95–4.88) | 3.15 ± 1.21 | 3.1 (2.13–3.95) | 0.009 * (z = −2.623) |
| Delta AMH (ng/mL) | 0.13 ± 1.3 | 0.15 (−0.68–1.1) | 0.54 ± 1.6 | 0.35 (−0.5–1.45) | 0.172 (t = −1.378) |
| Intra Group p | 0.495 (t = 0.688) | 0.024 * (t = 2.334) | |||
| First TotalAFC | 9.56 ± 1.67 | 10 (8–11) | 9.21 ± 1.64 | 9 (8–10) | 0.293 (z = −1.052) |
| Final TotalAFC | 9.38 ± 1.52 | 9.5 (8–10) | 8.65 ± 1.3 | 9 (8–9.75) | 0.018 * (z = −2.374) |
| Delta AFC | 0.19 ± 1.58 | 0 (−1–1) | 0.56 ± 1.82 | 0 (−1–2) | 0.552 (z = −0.594) |
| Intra Group p | 0.415 (t = 0.822) | 0.042 * (z = −2.034) | |||
| First Average ovarian volume (mm3) | 6.23 ± 1.12 | 6.1 (5.3–7.05) | 6.06 ± 1.09 | 6 (5.1–6.7) | 0.387 (z = −0.865) |
| Final Average ovarian volume (mm3) | 6.14 ± 1.02 | 6.1 (5.3–6.78) | 5.66 ± 0.82 | 5.4 (5.13–6.28) | 0.036 * (z = −2.092) |
| Delta Average ovarian volume (mm3) | 0.09 ± 1.07 | 0 (−0.68–0.88) | 0.39 ± 1.23 | 0.05 (−0.48–1.23) | 0.422 (z = −0.803) |
| Intra Group p | 0.547 (t = 0.607) | 0.125 (z = −1.535) | |||
| ET Delta | E2 Delta | FSH Delta | LH Delta | AMH Delta | AFC Delta | AOV Delta | |||
|---|---|---|---|---|---|---|---|---|---|
| Control group | Age (year) | r | −0.09 | −0.279 | 0.167 | 0.053 | −0.195 | −0.173 | −0.184 |
| p | 0.541 | 0.055 | 0.258 | 0.718 | 0.184 | 0.24 | 0.21 | ||
| BMI (kg/m2) | r | 0.302 * | −0.184 | −0.248 | −0.132 | 0.327 * | 0.300 * | 0.312 * | |
| p | 0.037 | 0.211 | 0.09 | 0.373 | 0.023 | 0.038 | 0.031 | ||
| Parity | r | −0.077 | −0.265 | −0.043 | −0.06 | 0.006 | 0.011 | −0.02 | |
| p | 0.604 | 0.069 | 0.77 | 0.686 | 0.967 | 0.94 | 0.89 | ||
| Menstrual cycle length (days) | r | 0.012 | 0 | −0.157 | 0.07 | 0.206 | 0.102 | 0.158 | |
| p | 0.938 | 0.999 | 0.286 | 0.635 | 0.161 | 0.49 | 0.285 | ||
| Menstrual cycle duration (days) | r | −0.039 | −0.134 | −0.076 | −0.15 | 0.046 | 0.177 | −0.041 | |
| p | 0.794 | 0.362 | 0.608 | 0.309 | 0.757 | 0.23 | 0.784 | ||
| Menstrual bleeding (pads/day) | r | 0.106 | −0.217 | −0.139 | −0.13 | 0.121 | 0.133 | 0.034 | |
| p | 0.473 | 0.138 | 0.346 | 0.379 | 0.411 | 0.369 | 0.819 | ||
| MDD group | Age (year) | r | −0.033 | −0.168 | −0.047 | 0.054 | 0.037 | 0.034 | 0.023 |
| p | 0.826 | 0.255 | 0.751 | 0.714 | 0.801 | 0.817 | 0.877 | ||
| BMI (kg/m2) | r | 0.078 | −0.031 | −0.165 | −0.013 | 0.199 | 0.324 * | 0.166 | |
| p | 0.597 | 0.833 | 0.262 | 0.929 | 0.176 | 0.025 | 0.261 | ||
| Parity | r | 0.058 | −0.201 | −0.021 | 0.161 | 0.042 | −0.004 | 0.032 | |
| p | 0.693 | 0.171 | 0.887 | 0.273 | 0.776 | 0.979 | 0.827 | ||
| Menstrual cycle length (days) | r | 0.053 | −0.009 | 0.338 * | 0.196 | −0.255 | −0.224 | −0.195 | |
| p | 0.72 | 0.951 | 0.019 | 0.183 | 0.08 | 0.125 | 0.184 | ||
| Menstrual cycle duration (days) | r | −0.259 | 0.092 | 0.039 | 0.027 | 0.268 | 0.229 | 0.188 | |
| p | 0.075 | 0.535 | 0.791 | 0.855 | 0.066 | 0.118 | 0.202 | ||
| Menstrual bleeding (pads/day) | r | −0.165 | 0.112 | 0.099 | 0.101 | 0.165 | 0.178 | 0.145 | |
| p | 0.262 | 0.449 | 0.504 | 0.496 | 0.262 | 0.225 | 0.325 | ||
| HAM-D Scores (before Treatment) | HAM-D Scores (after Treatment) | ||||
|---|---|---|---|---|---|
| Mean ± S.D | Med (IQR) | Mean ± S.D | Med (IQR) | p | |
| MDD Group (n = 48) | 28.3 ± 4.1 | 28 (24–32) | 8.4 ± 2.8 | 8 (5–12) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gök, S.; Gök, B.C.; Alataş, E.; Senol, H.; Topak, O.Z. Effects of Selective Serotonin Reuptake Inhibitor Treatment on Ovarian Reserves in Patients with Depression. Medicina 2023, 59, 517. https://doi.org/10.3390/medicina59030517
Gök S, Gök BC, Alataş E, Senol H, Topak OZ. Effects of Selective Serotonin Reuptake Inhibitor Treatment on Ovarian Reserves in Patients with Depression. Medicina. 2023; 59(3):517. https://doi.org/10.3390/medicina59030517
Chicago/Turabian StyleGök, Soner, Berfin Can Gök, Erkan Alataş, Hande Senol, and Osman Zülkif Topak. 2023. "Effects of Selective Serotonin Reuptake Inhibitor Treatment on Ovarian Reserves in Patients with Depression" Medicina 59, no. 3: 517. https://doi.org/10.3390/medicina59030517




