Watchful Waiting in Pediatric Acute Otitis Media: A Real Practice Approach or an Intangible Desideratum?
Abstract
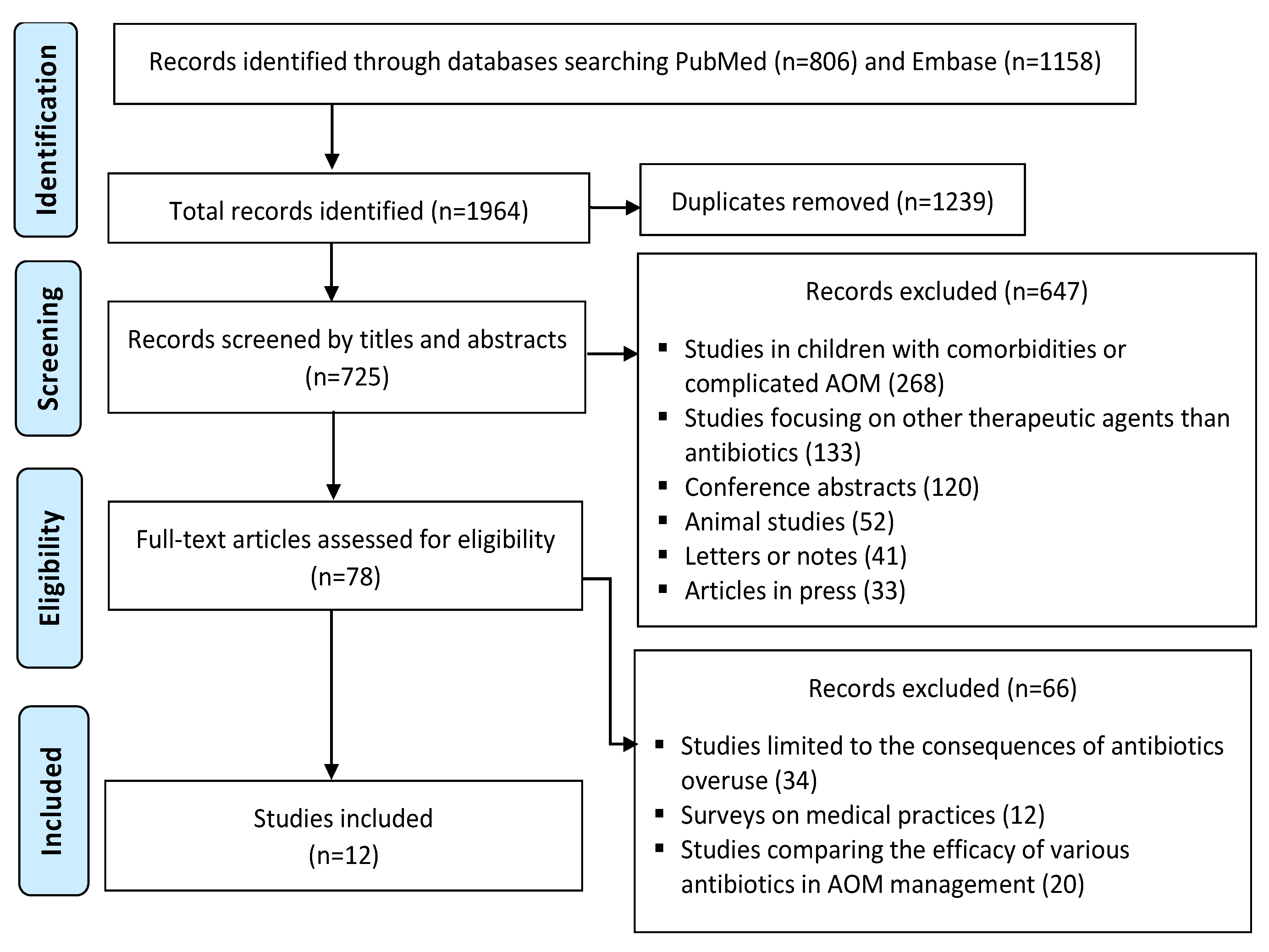
:1. Introduction
2. Materials and Methods
3. Results
3.1. Antibiotic Choice
3.2. Antibiotic Trends
3.3. Watchful-Waiting Approach
3.4. Adherence to Guidelines
- The high rate of cephalosporins use reported by Park et al. in Korea [11] is not justified by the guidelines, as this class of antibiotics is reserved only for children with non-type I hypersensitivity. Furthermore, macrolides are recommended only for children with a history of type I hypersensitivity reaction to penicillin (e.g., urticaria, anaphylaxis). The fact that 64% of the children with AOM receiving antibiotics were 0–23 months old was expected as immediate antibiotherapy is required in all the children <6 months old with AOM.
- Amoxicillin was the most frequent choice of antibiotic by the clinicians in both studies reporting AOM cases from the United States [12,19]. However, in most of the cases (54.1%) reported by Frost et al. [12], using a 10-day regimen of antibiotics exceeded the duration recommended by the guidelines.
- The report from Israel [11] revealed the highest rate of azithromycin use, which is recommended by the local guidelines only in cases of major penicillin allergy. As the cephalosporins do not seem to be recommended at all, 48%–54% of AOM episodes were inappropriately treated, against the guideline recommendations. The results are similar to those reported by the Australian report, as 39% of children received inappropriate antibiotic treatment according to the Australian guidelines, including a 7% rate of ciprofloxacin use.
- The study from Italy [15] reported a lack of adherence to the guidelines in cases of treatment failure, as instead of switching from amoxicillin to amoxicillin/clavulanic acid, the most frequent antibiotic switches were from amoxicillin directly to cephalosporins.
- The most unsatisfying rate of amoxicillin use was recorded in Japan [16], contrary to the recommendations in the guidelines. Moreover, both quinolones and carbapenemes are not recommended by the local guidelines. The use of tosufloxacin in 21.4% of cases was also unexpected.
- In the study by Levy et al. (France), an increasing rate of amoxicillin use was observed after the introduction of the 2011 French guidelines [17].
- The lowest proportion of antibiotic prescriptions in children <2 years old was reported by Csonka et al. in Finland, which is contrary to our expectations, as children under 6 months old are recommended to undergo immediate antibiotherapy according to ten out of eleven guidelines [14].
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Størdal, K.; Wyder, C.; Trobisch, A.; Grossman, Z.; Hadjipanayis, A. Overtesting and overtreatment-statement from the European Academy of Paediatrics (EAP). Eur. J. Pediatr. 2019, 178, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.H.; Rusan, M.; Ovesen, T. Acute otitis media and antibiotics—A systematic review. Dan. Med. J. 2020, 67, A04200272. [Google Scholar]
- Jamal, A.; Alsabea, A.; Tarakmeh, M.; Safar, A. Etiology, Diagnosis, Complications, and Management of Acute Otitis Media in Children. Cureus 2022, 14, e28019. [Google Scholar] [CrossRef] [PubMed]
- Danishyar, A.; Ashurst, J.V. Acute Otitis Media. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470332/ (accessed on 28 February 2023).
- Gavrilovici, C.; Pânzaru, C.V.; Cozma, S.; Mârţu, C.; Lupu, V.V.; Ignat, A.; Miron, I.; Stârcea, M. “Message from a turtle”: Otitis with Salmonella arizonae in children: Case report. Medicine 2017, 96, e8455. [Google Scholar] [CrossRef]
- Darmawan, A.B.; Dewantari, A.K.; Putri, H.F.M.; Wiyatno, A.; Wahyono, D.J.; Safari, D. Identification of the Viral Pathogens in School Children with Acute Otitis Media in Central Java, Indonesia. Glob. Pediatr. Health 2023, 10, 2333794X221149899. [Google Scholar] [CrossRef]
- Kaur, R.; Morris, M.; Pichichero, M.E. Epidemiology of AOM in the Postpneumococcal Conjugate Vaccine Era. Pediatrics 2017, 140, e20170181. [Google Scholar] [CrossRef] [Green Version]
- Venekamp, R.P.; Sanders, S.L.; Glasziou, P.P.; Del Mar, C.B.; Rovers, M.M. Antibiotics for AOM in children. Cochrane Database Syst. Rev. 2015, 2015, CD000219. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.G.; Dewez, J.E.; Nijman, R.G.; Yeung, S. Clinical practice guidelines for AOM in children: A systematic review and appraisal of European national guidelines. BMJ Open 2020, 10, 35343. [Google Scholar] [CrossRef]
- Lieberthal, A.S.; Carroll, A.E.; Chonmaitree, T.; Ganiats, T.G.; Hoberman, A.; Jackson, M.A.; Joffe, M.D.; Miller, D.T.; Rosenfeld, R.M.; Sevilla, X.D.; et al. The diagnosis and management of AOM. Pediatrics 2013, 131, e964–e999. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Choe, S.A.; Shin, J.Y.; Choe, Y.J. Trend of Antibiotic Use in Children with Acute Otitis Media in Korea. J. Korean Med. Sci. 2021, 36, e317. [Google Scholar] [CrossRef]
- Smolinski, N.E.; Antonelli, P.J.; Winterstein, A.G. Watchful Waiting for Acute Otitis Media. Pediatrics 2022, 150, e2021055613. [Google Scholar] [CrossRef] [PubMed]
- Marom, T.; Shefer, G.; Tshori, S.; Mingelgrin, S.; Pitaro, J. Antibiotic prescription policy for acute otitis media: Do we follow the guidelines? J. Antimicrob. Chemother. 2021, 76, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Csonka, P.; Palmu, S.; Heikkilä, P.; Huhtala, H.; Korppi, M. Outpatient Antibiotic Prescribing for 357,390 Children with Otitis Media. Pediatr. Infect. Dis. J. 2022, 41, 947–952. [Google Scholar] [CrossRef]
- Barbieri, E.; di Chiara, C.; Costenaro, P.; Cantarutti, A.; Giaquinto, C.; Hsia, Y.; Doná, D. Antibiotic Prescribing Patterns in Paediatric Primary Care in Italy: Findings from 2012–2018. Antibiotics 2021, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Matsubayashi, K.; Mizuno, K.; Noda, M.; Takeuchi, M.; Kawakami, K. First-line antibiotic prescription patterns for acute otitis media in children: A descriptive study using Japanese claims data (2014–2018). J. Infect. Chemother. 2021, 27, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Pereira, M.; Guedj, R.; Abt-Nord, C.; Gelbert, N.B.; Cohen, R.; Alberti, C.; Gajdos, V.; Angoulvant, F. Impact of 2011 French guidelines on antibiotic prescription for acute otitis media in infants. Med. Mal. Infect. 2014, 44, 102–106. [Google Scholar] [CrossRef]
- Palma, S.; Rosafio, C.; Del Giovane, C.; Patianna, V.D.; Lucaccioni, L.; Genovese, E.; Bertolani, P.; Iughetti, L. The impact of the Italian guidelines on antibiotic prescription practices for acute otitis media in a paediatric emergency setting. Ital. J. Pediatr. 2015, 41, 37. [Google Scholar] [CrossRef] [Green Version]
- Frost, H.M.; Becker, L.F.; Knepper, B.C.; Shihadeh, K.C.; Jenkins, T.C. Antibiotic Prescribing Patterns for Acute Otitis Media for Children 2 Years and Older. J. Pediatr. 2020, 220, 109–115.e1. [Google Scholar] [CrossRef]
- García Ventura, M.; García Vera, C.; Ruiz-Canela Cáceres, J. Abordaje terapéutico de la otitis media aguda en atención primaria de un área urbana. Evaluación de la prescripción diferida de antibióticos [Therapeutic approach to acute otitis media in primary care in an urban area. Delayed antibiotic prescription evaluation]. An. Pediatr. 2021, 23, S1695–S4033. (In Spanish) [Google Scholar] [CrossRef]
- Balasundaram, N.; Phan, D.; Mazzoni, D.; Duong, E.; Sweeny, A.; Del Mar, C.; Keijzers, G. AOM in children presenting to the emergency department: Is it diagnosed and managed appropriately? J. Paediatr. Child Health 2019, 55, 1335–1343. [Google Scholar] [CrossRef]
- Olsen, J.K.; Lykkegaard, J.; Hansen, M.P. Prescription of antibiotics to children with acute otitis media in Danish general practice. BMC Fam. Pract. 2020, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, S.K.; Choi, K.Y.; Park, S.E.; Chun, Y.M.; Kim, K.S.; Park, S.N.; Cho, Y.S.; Kim, Y.J.; Kim, H.J.; et al. Korean clinical practice guidelines: Otitis media in children. J. Korean Med. Sci. 2012, 27, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Shviro-Roseman, N.; Reuveni, H.; Gazala, E.; Leibovitz, E. Adherence to acute otitis media treatment guidelines among primary health care providers in Israel. Braz. J. Infect. Dis. 2014, 18, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Finnish Medical Society Duodecim. MOtitis Media (Acute Otitis Media in Children). The Current Care Guideline. 2017. Available online: www.kaypahoito.fi (accessed on 10 January 2023).
- Marchisio, P.; Bellussi, L.; Di Mauro, G.; Doria, M.; Felisati, G.; Longhi, R.; Novelli, A.; Speciale, A.; Mansi, N.; Principi, N. Acute otitis media: From diagnosis to prevention. Summary of the Italian guideline. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1209–1216. [Google Scholar] [CrossRef]
- Subcommittee of Clinical Practice Guideline for Diagnosis and Management of Acute Otitis Media in Children (Japan Otological Society, Japan Society for Pediatric Otorhinolaryngology, Japan Society for Infectious Diseases in Otolaryngology). Clinical practice guidelines for the diagnosis and management of acute otitis media (AOM) in children in Japan. Auris Nasus Larynx 2012, 39, 1–8. [Google Scholar] [CrossRef]
- Agence Française de Sécurité Sanitaire des Produits de Santé. Recommandations de Bonne Pratique—Antibiothérapie par Voie Générale en Pratique Courante dans les Infections Respiratoires Hautes [Recommendations for Good Practice—Antimicrobials by General Route in Current Practice in Upper Respiratory Tract Infections]. 2011. Available online: http://www.infectiologie.com/UserFiles/File/medias/Recos/2011-infections-respir-hautes-recommandations.pdf (accessed on 10 January 2023).
- Del Castillo Martín, F.; Baquero Artigao, F.; de la Calle Cabrera, T.; López Robles, M.V.; Ruiz-Canela Cáceres, J.; Alfayate Miguélez, S.; Moraga Llop, F.; Cilleruelo Ortega, M.J.; Calvo Rey, C. Consensus document on the aetiology, diagnosis and treatment of acute otitis media. Pediatr. Atension Primaria 2012, 14, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Australian Government Department of Health. Darwin Otitis Guidelines Group Recommendations for Clinical Care Guidelines on the Management of Otitis Media in Aboriginal and Torres Strait Islander Populations. Available online: https://www1.health.gov.au/internet/publications/publishing.nsf/Content/oatsih-otitis-media-toc~Using-the-Recommendations-for-Clinical-Care-Guidelines-on-Otitis-Media (accessed on 28 January 2023).
- Dansk Selskab for Almen Medicin. Luftvejsinfektioner—DSAM Vejledninger. Respiratory Tract Infections—Diagnosis and Treatment. 2014. Available online: https://vejledninger.dsam.dk/luftvejsinfektioner/ (accessed on 10 January 2023).
- Piglansky, L.; Leibovitz, E.; Raiz, S. Bacteriologic and clinical efficacy of high dose amoxicillin for therapy of acute otitis media in children. Pediatr. Infect. Dis. J. 2003, 22, 405–413. [Google Scholar] [CrossRef]
- Gavrilovici, C.; Spoială, E.-L.; Miron, I.-C.; Stârcea, I.M.; Haliţchi, C.O.I.; Zetu, I.N.; Lupu, V.V.; Pânzaru, C. AOM in Children—Challenges of Antibiotic Resistance in the Post-Vaccination Era. Microorganisms 2022, 10, 1598. [Google Scholar] [CrossRef]
- Hum, S.W.; Shaikh, K.J.; Musa, S.S.; Shaikh, N. Adverse Events of Antibiotics Used to Treat Acute Otitis Media in Children: A Systematic Meta-Analysis. J. Pediatr. 2019, 215, 139–143.e7. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M.; Bizune, D.; Gerber, J.S.; Hersh, A.L.; Hicks, L.A.; Tsay, S.V. Amoxicillin Versus Other Antibiotic Agents for the Treatment of Acute Otitis Media in Children. J. Pediatr. 2022, 251, 98–104.e5. [Google Scholar] [CrossRef]
- Committee on Infectious Diseases. The use of systemic fluoroquinolones. Pediatrics 2006, 118, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Noel, G.J.; Blumer, J.L.; Pichichero, M.E.; Hedrick, J.A.; Schwartz, R.H.; Balis, D.A.; Melkote, R.; Bagchi, P.; Arguedas, A. A randomized comparative study of levofloxacin versus amoxicillin/clavulanate for treatment of infants and young children with recurrent or persistent acute otitis media. Pediatr. Infect. Dis. J. 2008, 27, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M.; Sugita, G.; Gunduz, M.; Gunduz, E.; Tamagawa, S. Tosufloxacin for Eradicating Biofilm-Forming Nontypeable Haemophilus influenzae Isolated from Intractable Acute Otitis Media. Jundishapur. J. Microbiol. 2019, 12, e69583. [Google Scholar] [CrossRef]
- Jahagirdar, D.; Loshak, H. Fluoroquinolones for the Treatment of Otitis Media: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542753/ (accessed on 12 January 2023).
- Oliphant, C.M.; Green, G.M. Quinolones: A comprehensive review. Am. Fam. Physician 2002, 65, 455–464. [Google Scholar] [PubMed]
- Wernroth, M.L.; Fall, K.; Svennblad, B.; Ludvigsson, J.F.; Sjölander, A.; Almqvist, C.; Fall, T. Early Childhood Antibiotic Treatment for Otitis Media and Other Respiratory Tract Infections Is Associated with Risk of Type 1 Diabetes: A Nationwide Register-Based Study With Sibling Analysis. Diabetes Care 2020, 43, 991–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautenbach, E.; Gerber, J.; Grundmeier, R.; Hamilton, K.W.; Hicks, L.; Neuhauser, M.M.; Frager, N.; Menon, M.; Kratz, E.; Jaskowiak, A.; et al. Development of an Electronic Algorithm to Identify Inappropriate Antibiotic Prescribing for Pediatric Otitis Media. Open Forum. Infect. Dis. 2020, 7, S176–S177. [Google Scholar] [CrossRef]
- Uhl, B.D.; Boutzoukas, A.; Gallup, N.; Patrick, M.; Stultz, J.; Porter, C.; Watson, J.R. Increasing Adherence to Acute Otitis Media Treatment Duration Guidelines using a Quality Improvement Approach. Pediatr. Qual. Saf. 2021, 6, e501. [Google Scholar] [CrossRef]
- McCormick, D.P.; Chonmaitree, T.; Pittman, C. Nonsevere acute otitis media: A clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics 2005, 115, 1455–1465. [Google Scholar] [CrossRef] [Green Version]
- Di Mario, S.; Gagliotti, C.; Buttazzi, R.; Marchetti, F.; Dodi, I.; Barbieri, L.; Moro, M.L. Reducing antibiotic prescriptions in children is not associated with higher rate of complications. Eur. J. Pediatr. 2021, 180, 1185–1192. [Google Scholar] [CrossRef]
- Gerber, J.S.; Ross, R.K.; Bryan, M.; Localio, A.R.; Szymczak, J.E.; Wasserman, R.; Barkman, D.; Odeniyi, F.; Conaboy, K.; Bell, L.; et al. Association of Broad—vs. Narrow-Spectrum Antibiotics with Treatment Failure, Adverse Events, and Quality of Life in Children with Acute Respiratory Tract Infections. JAMA 2017, 318, 2325–2336. [Google Scholar] [CrossRef] [Green Version]
- Spoială, E.L.; Stanciu, G.D.; Bild, V.; Ababei, D.C.; Gavrilovici, C. From Evidence to Clinical Guidelines in Antibiotic Treatment in Acute Otitis Media in Children. Antibiotics 2021, 10, 52. [Google Scholar] [CrossRef]
- Kurabi, A.; Pak, K.; Ryan, A.F.; Wasserman, S.I. Innate Immunity: Orchestrating Inflammation and Resolution of Otitis Media. Curr. Allergy Asthma Rep. 2016, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.D.; Downing, H.; Francis, N.A.; Young, G.J.; Clement, C.; Harris, S.D.; Ahern, A.; Schofield, B.; Thomas, T.E.; Horwood, J.; et al. Anaesthetic-analgesic ear drops to reduce antibiotic consumption in children with AOM: The CEDAR RCT. Health Technol. Assess. 2019, 23, 1–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NICE Guideline on Otitis Media (Acute): Antimicrobial Prescribing Updated on 11 March 2022. Available online: https://www.nice.org.uk/guidance/ng91/resources/otitis-media-acute-antimicrobial-prescribing-pdf-1837750121413 (accessed on 15 January 2023).
- Foxlee, R.; Johansson, A.; Wejfalk, J.; Dawkins, J.; Dooley, L.; Del Mar, C. Topical analgesia for acute otitis media. Cochrane Database Syst. Rev. 2006, 2006, CD005657. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.govinfo.gov/content/pkg/FR-2015-07-02/html/2015-16360.htm (accessed on 28 February 2023).
- Gavrilovici, C.; Spoială, E.-L.; Ivanov, A.-V.; Mocanu, A.; Ștreangă, V.; Alecsa, M.-S.; Miron, I. Otitis Media and Obesity—An Unusual Relationship in Children. Healthcare 2021, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Calatayud-Sáez, F.M.; Calatayud, B.; Calatayud, A. Recurrent Acute Otitis Media Could Be Related to the Pro-Inflammatory State That Causes an Incorrect Diet. Sinusitis 2022, 6, 36–48. [Google Scholar] [CrossRef]
- Gavrilovici, C.; Oprea, L. Clinical ethics, research ethics and community ethics—The moral triad of nowadays society. Rev. Romana Bioet. 2013, 11, 55–57. [Google Scholar]
| Study, Publication Year, Region | Period | Population Sample | Antibiotic Prescription Rate | Prescribed Antibiotics | Attitude Towards Watchful-Waiting Approach | Observations |
|---|---|---|---|---|---|---|
| Park et al., 2021, Korea [11] | 7 years (2012 to 2018) | 25,212,264 children aged 0–6 years old | Not specified |
| Not specified | Of the children with AOM receiving antibiotics, 64% were 0–23 months old. |
| Smolinski et al., 2022, United States [12] | 15 years (2005 to 2019) | 2,176,617 children aged 1–12 years old | 77.8% |
| Among 22.2% of cases managed with WW ***, only 2.8% cases required antibiotic treatment (days 4 to 7). | Otolaryngologists adopted more frequently the WW approach (odds ratio 5.45, 95% CI 5.21–5.70) compared to pediatricians. |
| Marom et al., 2021, Israel [13] | 8 years (2011 to 2018) | 491,106 children aged 0–10 years old | 71.5% |
| 28.5% of cases managed with WW. | Azithromycin was the most cited antibiotic in cases with treatment failure (6.6%). |
| Csonka et al., 2022, Finland [14] | 7 years (2014 to 2020) | 357,390 children aged 0–18 years old | 44.8% |
| 55.2% of cases managed with WW. | Pediatricians prescribed AMX or AMC to 80% of cases compared to 67% by GPs and 55% by ENT physicians. The lowest proportion (44.1%) of antibiotic prescriptions was in children <2 years old. |
| Barbieri et al., 2021, Italy [15] | 7 years (2012 to 2018) | 157,915 children aged 0–14 years old | Not specified |
| Not specified | The most common antibiotic switches due to treatment failure were from AMC to cephalosporins (16.7%), followed by switches from AMX to AMC (9.6%). |
| Yamaguchi et al., 2021, Japan [16] | 5 years (2014 to 2018) | 109,051 children aged 0–7 years old | Not specified |
| Not specified | Although there was an increasing trend in the use of AMX, the rate was still low. Physicians in hospitals were more likely to prescribe AMX than those in otolaryngology clinics (aOR = 1.71, 95% CI 1.63 to 1.79). |
| Levy et al., 2014, France [17] | 4 years (2009 to 2012) | 14,661 children aged 0.5–2 years old | 85.1% |
| 14.9% of cases managed with WW. | The study highlights the importance of guidelines in decreasing the prescription of broad-spectrum antibiotics as AMX became the most frequently prescribed antibiotic for AOM in 2012, after the introduction of the 2011 French guidelines. |
| Palma et al., 2015, Italy [18] | 7 years (2007 to 2013) | 4573 children aged 0–14 years old | 81% |
| 19% of cases managed with WW. | The introduction of the Italian guidelines had no significant effect on the frequency of the antibiotic prescribing rate (82% versus 81%). Of the antibiotics prescribed, 48% were in children under 2 years old. |
| Frost et al., 2020, Colorado (United States) [19] | 1 year (2018) | 1025 children aged 2–18 years old | 98% |
| 2% of cases managed with WW. | Children aged 2 to 5 years old or admitted to emergency departments received ≥10 days of antibiotics compared with those admitted to pediatric clinics (p < 0.001). |
| García Ventura et al., 2021, Aragon (Spain) [20] | 6 years (2013 to 2018) | 696 children aged 0–14 years old | 81.3% |
| Among 18.7% of cases managed with WW, 53.5% cases required antibiotic treatment. | Children aged 0 to 2 years old were treated more frequently with immediate antibiotics (63%) compared to children aged 6 to 14 years old (36.6%). |
| Balasundaram et al., 2019, Southeast Queensland (Australia) [21] | 1 year (January 2016 to June 2017) | 305 children aged 0–15 years old | 62% |
| 7.5% of cases managed with WW. | According to the Australian guidelines, 39% of children received inappropriate antibiotic treatment. |
| Olsen et al., 2020, Denmark [22] | A 4-week winter period in 2017/2018 | 278 children aged 0–7 years old | 74% |
| 26% of cases managed with WW. | Fever, otorrhea, and poor general condition were associated with immediate antibiotic treatment. |
| Region | Recommendations According to the Local Guidelines | Watchful-Waiting Approach | ||
|---|---|---|---|---|
| First-Line Antibiotic | Second-Line Antibiotic/Treatment Failure | Third Line/Allergy to First Line | ||
| Korea [23] | AMX, 5–7 days for mild cases, 10 days for moderate and severe cases | AMC, 5–7 days for mild cases, 10 days for moderate and severe cases | Ceftriaxone | WW in children >6 months old and reevaluation after 2–3 days in cases of mild AOM in children with no recent history of taking antibiotics and no other accompanying disease. |
| United States [10] | AMX, <2 years of age: 10 days; 2–5 years of age: 7 days; >6 years of age: 5–7 days | AMC | Cefdinir OR ceftriaxone | WW in children >6 months old in the absence of severe signs or symptoms (mild otalgia for less than 48 h, temperature <39 °C). |
| Colorado (United States) [10] | ||||
| Israel [24] | AMX | AMC OR cefuroxime | Azithromycin OR clarithromycin | WW for 1–2 days in non-severe cases in children ≥6 months old who can be followed up by a primary care physician. |
| Finland [25] | AMX or AMC, 5–7 days | Ceftriaxone | Cefaclor or macrolide | WW in nonpurulent AOM and reexamination after 2–3 days if symptoms continue. |
| Italy [26] | AMX, 5–10 days | AMC, 5–10 days | Macrolide | WW in children >2 years old and reevaluation after 2–3 days in cases of mild AOM (unilateral, without otorrhea). |
| Japan [27] | AMX, 5 days | AMC, 5 days | Cefditoren pivoxil | WW for 3 days in mild (based on a severity score) AOM. |
| France [28] | AMX, 8–10 days | AMC OR ceftriaxone, 3 days | Erythromycin-sulfafurazole/ cotrimoxazole/ cefpodoxime | WW in children >2 years old and reevaluation after 2–3 days in cases of mild AOM (no high fever or severe otalgia). |
| Aragon (Spain) [29] | AMX, 5 days | AMC OR ceftriaxone, 7–10 days | Cefuroxime | WW in children >2 years old and reevaluation after 2 days in cases of mild AOM (fever <39 °C, light pain). |
| Southeast Queensland (Australia) [30] | AMX, 7–14 days | High doses of AMX (90 mg/kg) | Cefuroxime | WW in children >6 months old and reevaluation after 2 days in cases of mild AOM (if the child is not immunocompromised or of Aboriginal descent or from the Torres Strait Islands). |
| Denmark [31] | Penicillin V, 7 days | AMC, 7 days | Clarithromycin | WW in children >2 years old and reevaluation after 3 days in the absence of otorrhea or other severe symptoms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoială, E.-L.; Stârcea, I.M.; Ioniuc, I.K.; Cozma, R.S.; Rusu, D.C.; Bozomitu, L.; Lupu, V.V.; Iliescu Haliţchi, C.O.; Roşu, V.E.; Roşu, S.T.; et al. Watchful Waiting in Pediatric Acute Otitis Media: A Real Practice Approach or an Intangible Desideratum? Medicina 2023, 59, 520. https://doi.org/10.3390/medicina59030520
Spoială E-L, Stârcea IM, Ioniuc IK, Cozma RS, Rusu DC, Bozomitu L, Lupu VV, Iliescu Haliţchi CO, Roşu VE, Roşu ST, et al. Watchful Waiting in Pediatric Acute Otitis Media: A Real Practice Approach or an Intangible Desideratum? Medicina. 2023; 59(3):520. https://doi.org/10.3390/medicina59030520
Chicago/Turabian StyleSpoială, Elena-Lia, Iuliana Magdalena Stârcea, Ileana Katerina Ioniuc, Romică Sebastian Cozma, Daniela Carmen Rusu, Laura Bozomitu, Vasile Valeriu Lupu, Codruţa Olimpiada Iliescu Haliţchi, Vasile Eduard Roşu, Solange Tamara Roşu, and et al. 2023. "Watchful Waiting in Pediatric Acute Otitis Media: A Real Practice Approach or an Intangible Desideratum?" Medicina 59, no. 3: 520. https://doi.org/10.3390/medicina59030520






