Evaluating Diagnostic Ultrasound of the Vagus Nerve as a Surrogate Marker for Autonomic Neuropathy in Diabetic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessments
2.3. Ultrasound
2.4. Sympathetic Skin Response and Nerve Conduction Studies
2.5. Controls
2.6. Statistics
3. Results
3.1. Patients
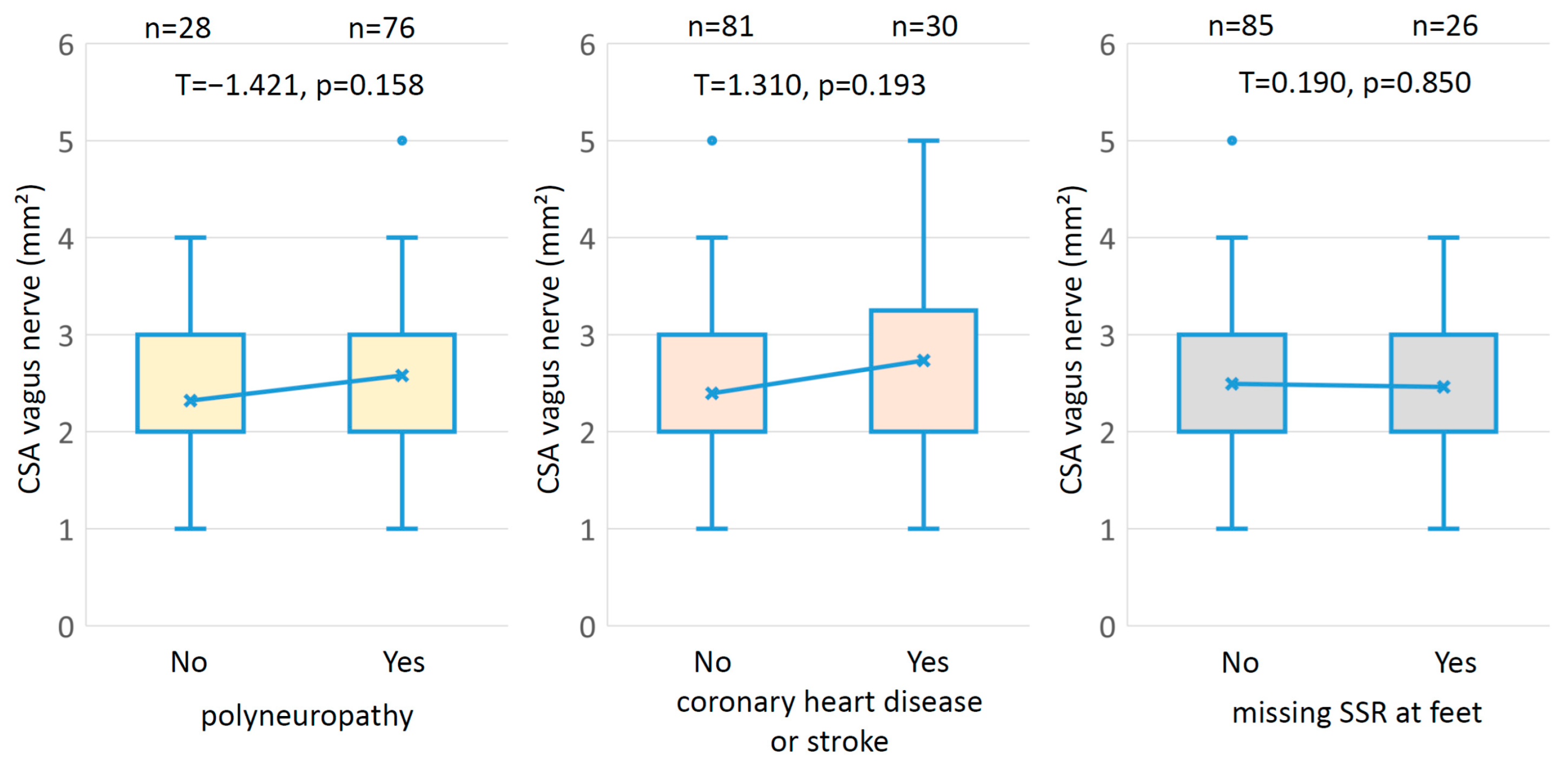
3.2. Interactions
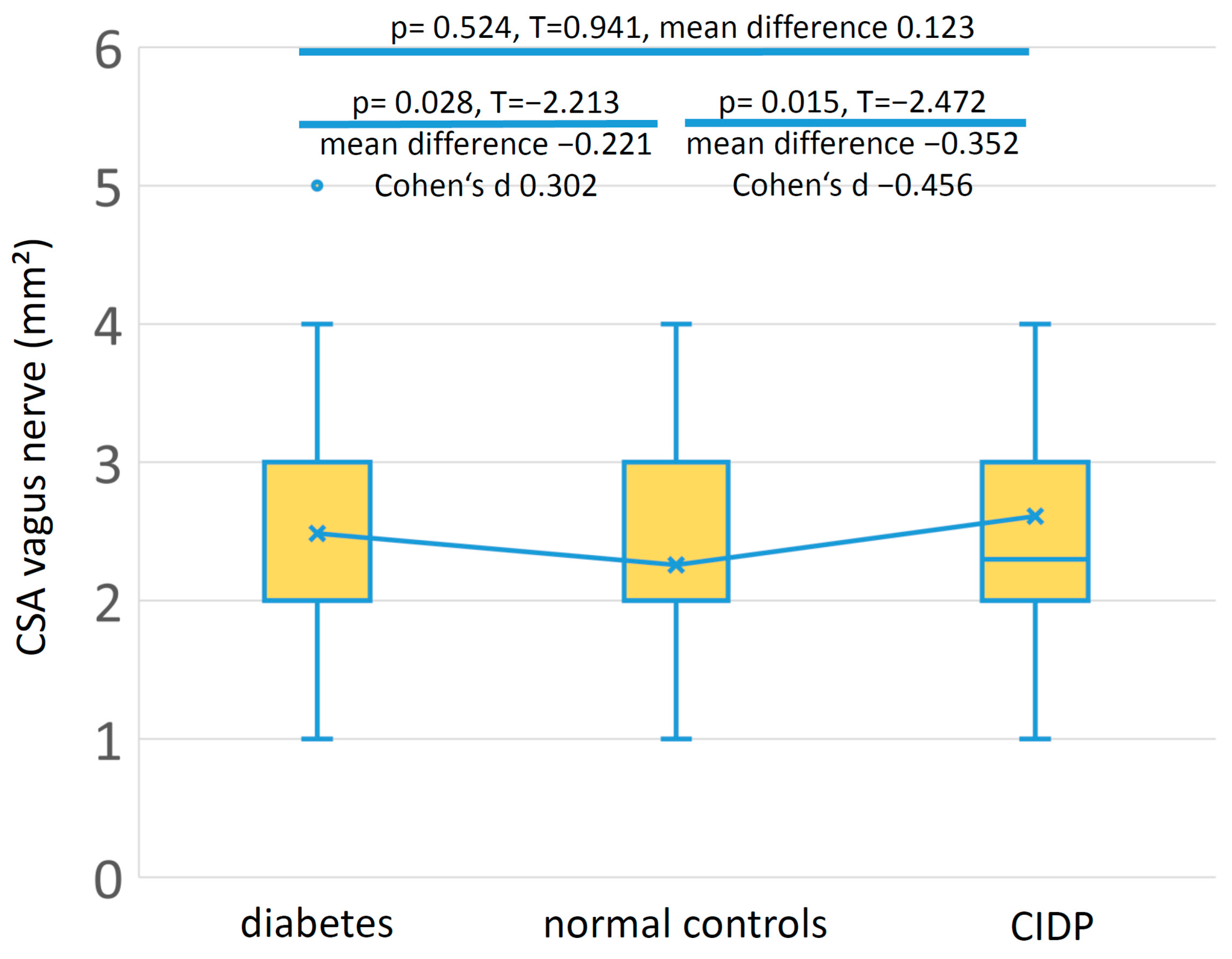
3.3. Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; Mizokami-Stout, K.; Eid, S.A.; Elafros, M.; Callaghan, B.; Feldman, E.L.; Pop-Busui, R. The Conundrum of Diabetic Neuropathies-Past, Present, and Future. J. Diabetes Complicat. 2022, 36, 108334. [Google Scholar] [CrossRef]
- Motataianu, A.; Barcutean, L.; Bajko, Z.; Stoian, A.; Maier, S.; Voidazan, S.; Balasa, R. Autonomic and Somatic Nerve Functions in Type 2 Diabetes Mellitus Patients: Electrophysiological Aspects. Diagnostics 2021, 11, 2005. [Google Scholar] [CrossRef]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Cardiovascular Autonomic Neuropathy in Diabetes: Clinical Impact, Assessment, Diagnosis, and Management. Diabetes Metab. Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef]
- Tawfik, E.A.; Walker, F.O.; Cartwright, M.S.; El-Hilaly, R.A. Diagnostic Ultrasound of the Vagus Nerve in Patients with Diabetes. J. Neuroimaging 2017, 27, 589–593. [Google Scholar] [CrossRef]
- Du, K.; Xu, K.; Chu, X.; Tang, Y.; Lv, H.; Zhang, W.; Wang, Z.; Yuan, Y.; Meng, L. Vagus Nerve Ultrasound in Transthyretin Familial Amyloid Polyneuropathy: A Pilot Study. J. Neuroimaging 2022, 32, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Heiling, B.; Wiedfeld, L.I.E.E.; Müller, N.; Kobler, N.J.; Grimm, A.; Kloos, C.; Axer, H. Electrodiagnostic Testing and Nerve Ultrasound of the Carpal Tunnel in Patients with Type 2 Diabetes. J. Clin. Med. 2022, 11, 3374. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Boulton, A.J.; MacLeod, A.F.; Williams, D.R.; Sonksen, P.H. A Multicentre Study of the Prevalence of Diabetic Peripheral Neuropathy in the United Kingdom Hospital Clinic Population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Keller, J.; Maier, C.; Pannek, J. Diabetic Neuropathy. Exp. Clin. Endocrinol. Diabetes 2021, 129, S70–S81. [Google Scholar] [CrossRef]
- Vetrugno, R.; Liguori, R.; Cortelli, P.; Montagna, P. Sympathetic Skin Response: Basic Mechanisms and Clinical Applications. Clin. Auton. Res. 2003, 13, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Shahani, B.T.; Halperin, J.J.; Boulu, P.; Cohen, J. Sympathetic Skin Response--a Method of Assessing Unmyelinated Axon Dysfunction in Peripheral Neuropathies. J. Neurol. Neurosurg. Psychiatry 1984, 47, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Dettmers, C.; Faust, H.; Fatepour, D.; Tackmann, W. Sympathetic skin response--physiologic principles, normal values and clinical use. Neurol. Psychiatr. 1993, 61, 369–377. [Google Scholar] [CrossRef]
- Grimm, A.; Axer, H.; Heiling, B.; Winter, N. Nerve Ultrasound Normal Values—Readjustment of the Ultrasound Pattern Sum Score UPSS. Clin. Neurophysiol. 2018, 129, 1403–1409. [Google Scholar] [CrossRef]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on Management of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. J. Peripher. Nerv. Syst. 2010, 15, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Ye, Y.; Wang, L.; Song, X.; Cao, J.; Qi, Y.; Xing, Y. Nerve Ultrasound Evaluation of Guillain-Barré Syndrome Subtypes in Northern China. Muscle Nerve 2021, 64, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhang, L.; Ding, Q.; Liu, J.; Zhang, Z.; Cui, L.; Liu, M. Vagus Nerve Ultrasound in Chronic Inflammatory Demyelinating Polyradiculoneuropathy and Charcot-Marie-Tooth Disease Type 1A. J. Neuroimaging 2020, 30, 910–916. [Google Scholar] [CrossRef]
- Abdelnaby, R.; Elsayed, M.; Mohamed, K.A.; Dardeer, K.T.; Sonbol, Y.T.; ELgenidy, A.; Barakat, M.H.; Alwerdani, M.M.; Maier, A. Vagus Nerve Ultrasonography in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Auton. Neurosci. 2021, 234, 102835. [Google Scholar] [CrossRef]
- Sartucci, F.; Bocci, T.; Santin, M.; Bongioanni, P.; Orlandi, G. High-Resolution Ultrasound Changes of the Vagus Nerve in Idiopathic Parkinson’s Disease (IPD): A Possible Additional Index of Disease. Neurol Sci 2021, 42, 5205–5211. [Google Scholar] [CrossRef]
- Fedtke, N.; Witte, O.W.; Prell, T. Ultrasonography of the Vagus Nerve in Parkinson’s Disease. Front. Neurol. 2018, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Decard, B.; Axer, H. Ultrasonography of the Peripheral Nervous System in the Early Stage of Guillain-Barré Syndrome. J. Peripher. Nerv. Syst. 2014, 19, 234–241. [Google Scholar] [CrossRef]
- Thompson, N.; Mastitskaya, S.; Holder, D. Avoiding Off-Target Effects in Electrical Stimulation of the Cervical Vagus Nerve: Neuroanatomical Tracing Techniques to Study Fascicular Anatomy of the Vagus Nerve. J. Neurosci. Methods 2019, 325, 108325. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, R.; Elsayed, M.; Mohamed, K.A.; Dardeer, K.T.; Sonbol, Y.T.; ELgenidy, A.; Barakat, M.H.; NasrEldin, Y.K.; Maier, A. Sonographic Reference Values of Vagus Nerve: A Systematic Review and Meta-Analysis. J. Clin. Neurophysiol. 2022, 39, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Fisse, A.L.; Katsanos, A.H.; Gold, R.; Pitarokoili, K.; Krogias, C. Cross-Sectional Area Reference Values for Peripheral Nerve Ultrasound in Adults: A Systematic Review and Meta-Analysis-Part III: Cervical Nerve Roots and Vagal Nerve. Eur. J. Neurol. 2021, 28, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Dörschner, J.; Pelz, J.O.; Kerner, A.M.; Labuschagne, J.J.; Hammer, N.; Löffler, S. Comparing the Accuracy of Ultrasound-Based Measurements of the Cervical Vagus Nerve. Sci. Rep. 2023, 13, 884. [Google Scholar] [CrossRef]
- Härtig, F.; Ross, M.; Dammeier, N.M.; Fedtke, N.; Heiling, B.; Axer, H.; Décard, B.F.; Auffenberg, E.; Koch, M.; Rattay, T.W.; et al. Nerve Ultrasound Predicts Treatment Response in Chronic Inflammatory Demyelinating Polyradiculoneuropathy-a Prospective Follow-Up. Neurotherapeutics 2018, 15, 439–451. [Google Scholar] [CrossRef]
- Ziemssen, T.; Siepmann, T. The Investigation of the Cardiovascular and Sudomotor Autonomic Nervous System-A Review. Front. Neurol. 2019, 10, 53. [Google Scholar] [CrossRef]
- Lin, X.; Chen, C.; Liu, Y.; Peng, Y.; Chen, Z.; Huang, H.; Xu, L. Peripheral Nerve Conduction And Sympathetic Skin Response Are Reliable Methods to Detect Diabetic Cardiac Autonomic Neuropathy. Front. Endocrinol. 2021, 12, 709114. [Google Scholar] [CrossRef]
- Memiş, Z.; Çevik, N.; Acar, H.; Günaydin, S.; Çokar, Ö. The First Electrophysiological Abnormality in New-Onset DM: Autonomic Tests. Noro Psikiyatr. Ars. 2022, 59, 197–200. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Bots, M.L. Imaging of Atherosclerosis: Carotid Intima–Media Thickness. Eur. Heart J. 2010, 31, 1682–1689. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Polak, J.F.; Kavousi, M.; Mathiesen, E.B.; Völzke, H.; Tuomainen, T.-P.; Sander, D.; Plichart, M.; Catapano, A.L.; Robertson, C.M.; et al. Carotid Intima-Media Thickness Progression to Predict Cardiovascular Events in the General Population (the PROG-IMT Collaborative Project): A Meta-Analysis of Individual Participant Data. Lancet 2012, 379, 2053–2062. [Google Scholar] [CrossRef]
- Tanaka, S.; Kanazawa, I.; Sugimoto, T. Nerve Conduction Velocity Is Negatively Associated with Intima-Media Thickness and Brachial-Ankle Pulse Wave Velocity in Men with Type 2 Diabetes Mellitus. PLoS ONE 2018, 13, e0209503. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Moon, S.; Kim, H.-S.; Lim, D.J.; Cho, J.H.; Kwon, H.S.; Ahn, C.W.; Yoon, K.H.; Kang, M.I.; Cha, B.Y.; et al. Diabetic Peripheral Neuropathy Is Associated with Increased Arterial Stiffness without Changes in Carotid Intima-Media Thickness in Type 2 Diabetes. Diabetes Care 2011, 34, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Yokota, Y.; Tada, J.; Kanno, S. Diabetic Neuropathy Is Closely Associated with Arterial Stiffening and Thickness in Type 2 Diabetes. Diabet. Med. 2007, 24, 1329–1335. [Google Scholar] [CrossRef]
- Jung, C.-H.; Baek, A.-R.; Kim, K.-J.; Kim, B.-Y.; Kim, C.-H.; Kang, S.-K.; Mok, J.-O. Association between Cardiac Autonomic Neuropathy, Diabetic Retinopathy and Carotid Atherosclerosis in Patients with Type 2 Diabetes. Endocrinol. Metab. 2013, 28, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Kotani, K.; Yagyu, H.; Koibuchi, H.; Fujii, Y.; Konno, K.; Yamada, T.; Ishibashi, S.; Taniguchi, N. The Correlation between CVR-R and Carotid Atherosclerosis in Type 2 Diabetes Mellitus Patients with Diabetic Neuropathy. J. Physiol. Anthr. 2010, 29, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Tsiberidou, P. Differential Age-, Gender-, and Side-Dependency of Vagus, Spinal Accessory, and Phrenic Nerve Calibers Detected with Precise Ultrasonography Measures. Muscle Nerve 2019, 59, 486–491. [Google Scholar] [CrossRef]
- Pelz, J.O.; Belau, E.; Henn, P.; Hammer, N.; Classen, J.; Weise, D. Sonographic Evaluation of the Vagus Nerves: Protocol, Reference Values, and Side-to-Side Differences. Muscle Nerve 2018, 57, 766–771. [Google Scholar] [CrossRef]
- Hammer, N.; Glätzner, J.; Feja, C.; Kühne, C.; Meixensberger, J.; Planitzer, U.; Schleifenbaum, S.; Tillmann, B.N.; Winkler, D. Human Vagus Nerve Branching in the Cervical Region. PLoS ONE 2015, 10, e0118006. [Google Scholar] [CrossRef]

| Categorical Variables | N | % | |||
| Gender | Female/Male | 43/68 | 38.7/61.3 | ||
| Polyneuropathy if NDS > 5 or (NDS > 2 and NSS > 4) | Yes/No | 76/28 | 61.3/38.7 | ||
| NCS-based diagnosis of polyneuropathy | Yes/No | 80/31 | 72.1/27.9 | ||
| Known coronary heart disease | Yes/No | 21/90 | 18.1/81.9 | ||
| Stroke in history | Yes/No | 12/99 | 10.8/89.2 | ||
| Metric Variables | Mean | SD | Minimum | Maximum | |
| Age (years) | 66.31 | 10.05 | 42 | 84 | |
| Duration of diabetes (years) | 16.08 | 9.46 | 0.5 | 39.6 | |
| Body mass index (kg/m2) | 32.46 | 6.28 | 19.3 | 51.7 | |
| HbA1c (mmol/mol) | 61.03 | 11.02 | 31.04 | 95.63 | |
| HbA1c (%) | 7.73 | 1.01 | 4.99 | 10.90 | |
| Glomerular filtration rate (ml/min) | 73.77 | 21.44 | 22.04 | 120.00 | |
| CSA vagus nerve (mm2) | 2.49 | 0.88 | 1.0 | 5.0 | |
| SSR hand latency (ms) | 1.45 | 0.35 | 0.43 | 2.69 | |
| SSR hand amplitude (mV) | 1764.43 | 1570.61 | 115.0 | 9415.0 | |
| SSR foot latency (ms) | 1.89 | 0.44 | 0.85 | 2.94 | |
| SSR foot amplitude (mV) | 747.07 | 873.42 | 0 | 5265.0 | |
| CIMT (mm) | 0.97 | 0.24 | 0.6 | 2.0 | |
| NSS (0–10 points) | 3.83 | 3.15 | 0 | 9 | |
| NDS (0–10 points) | 6.60 | 2.61 | 0 | 10 | |
| Variable | SRR Hand Latency | SRR Hand Amplitude | SRR Foot Latency | SRR Foot Amplitude | CIMT | Age | Duration of Diabetes | BMI | HBA1c | GFR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA vagus nerve (mm2) | correlation | 0.035 | 0.018 | 0.037 | −0.161 | 0.097 | 0.060 | 0.163 | 0.155 | 0.062 | −0.175 |
| p | 0.715 | 0.847 | 0.740 | 0.092 | 0.314 | 0.530 | 0.091 | 0.110 | 0.524 | 0.073 | |
| SRR hand latency (ms) | correlation | −0.171 | −0.050 | 0.173 | 0.048 | 0.190 * | 0.175 | −0.096 | −0.023 | 0.057 | |
| p | 0.073 | 0.650 | 0.069 | 0.618 | 0.046 | 0.069 | 0.325 | 0.817 | 0.561 | ||
| SRR hand amplitude (mV) | correlation | 0.147 | 0.223 * | 0.049 | −0.160 | −0.147 | 0.212 * | −0.024 | 0.038 | ||
| p | 0.178 | 0.018 | 0.611 | 0.096 | 0.129 | 0.028 | 0.807 | 0.701 | |||
| SRR foot latency (ms) | correlation | 0.044 | 0.075 | 0.156 | 0.073 | 0.076 | 0.119 | −0.102 | |||
| p | 0.690 | 0.497 | 0.155 | 0.507 | 0.493 | 0.285 | 0.363 | ||||
| SRR foot amplitude (mV) | correlation | −0.173 | 0.036 | −0.078 | −0.017 | −0.137 | 0.033 | ||||
| p | 0.072 | 0.706 | 0.421 | 0.862 | 0.160 | 0.740 | |||||
| CIMT (mm) | correlation | 0.224 * | 0.033 | −0.058 | 0.141 | −0.118 | |||||
| p | 0.020 | 0.738 | 0.559 | 0.151 | 0.234 | ||||||
| Age (years) | correlation | 0.440 ** | −0.115 | 0.204 * | −0.636 ** | ||||||
| p | 0.000 | 0.242 | 0.035 | 0.000 | |||||||
| Duration of diabetes (years) | correlation | −0.104 | 0.122 | −0.432 ** | |||||||
| p | 0.285 | 0.212 | 0.000 | ||||||||
| Body mass index (kg/m2) | correlation | 0.180 | −0.153 | ||||||||
| p | 0.064 | 0.120 | |||||||||
| HbA1c (mmol/mol) | correlation | −0.163 | |||||||||
| p | 0.096 |
| Group | N | Age Years (SD) | Gender Female:Male | Vagus Nerve CSA mm2 (SD) |
|---|---|---|---|---|
| Diabetes mellitus | 111 | 66.3 (SD 10.05) | 43:68 | 2.49 (SD 0.88) |
| Healthy controls | 104 | 52.49 (SD 17.89) | 46:58 | 2.26 (SD 0.59) |
| CIDP | 41 | 65.44 (SD 12.91) | 9:32 | 2.61 (SD 1.11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiling, B.; Karl, A.; Fedtke, N.; Müller, N.; Kloos, C.; Grimm, A.; Axer, H. Evaluating Diagnostic Ultrasound of the Vagus Nerve as a Surrogate Marker for Autonomic Neuropathy in Diabetic Patients. Medicina 2023, 59, 525. https://doi.org/10.3390/medicina59030525
Heiling B, Karl A, Fedtke N, Müller N, Kloos C, Grimm A, Axer H. Evaluating Diagnostic Ultrasound of the Vagus Nerve as a Surrogate Marker for Autonomic Neuropathy in Diabetic Patients. Medicina. 2023; 59(3):525. https://doi.org/10.3390/medicina59030525
Chicago/Turabian StyleHeiling, Bianka, Adriana Karl, Nadin Fedtke, Nicolle Müller, Christof Kloos, Alexander Grimm, and Hubertus Axer. 2023. "Evaluating Diagnostic Ultrasound of the Vagus Nerve as a Surrogate Marker for Autonomic Neuropathy in Diabetic Patients" Medicina 59, no. 3: 525. https://doi.org/10.3390/medicina59030525
APA StyleHeiling, B., Karl, A., Fedtke, N., Müller, N., Kloos, C., Grimm, A., & Axer, H. (2023). Evaluating Diagnostic Ultrasound of the Vagus Nerve as a Surrogate Marker for Autonomic Neuropathy in Diabetic Patients. Medicina, 59(3), 525. https://doi.org/10.3390/medicina59030525







