Maternal Periodontal Status as a Factor Influencing Obstetrical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis and Graph Editing
3. Results
3.1. The Main Gestational Age at Dental Examination, Gestational and Maternal Age at Delivery and Birth Weight at Delivery
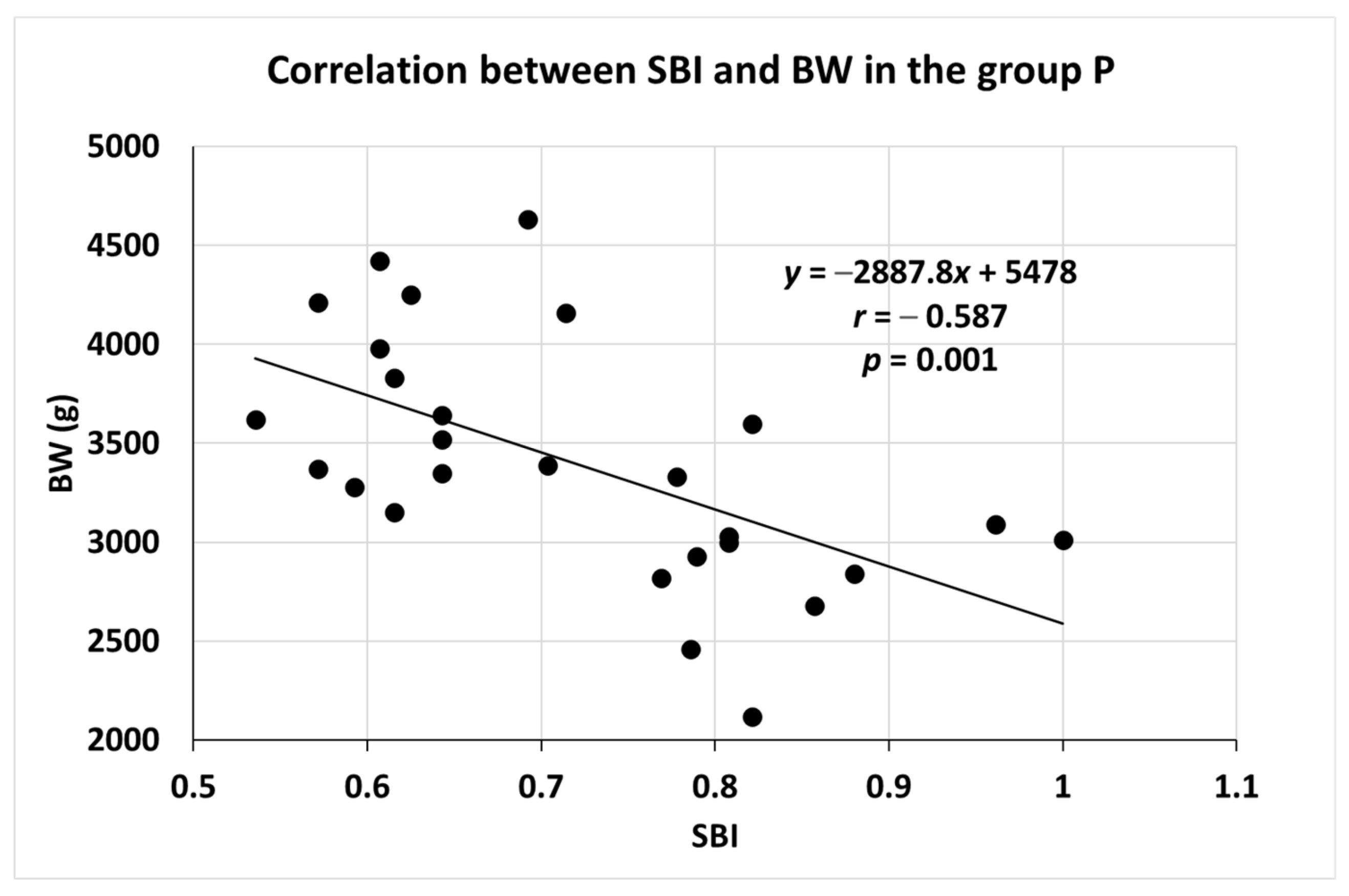
3.2. Correlation between SBI and BW in Group P
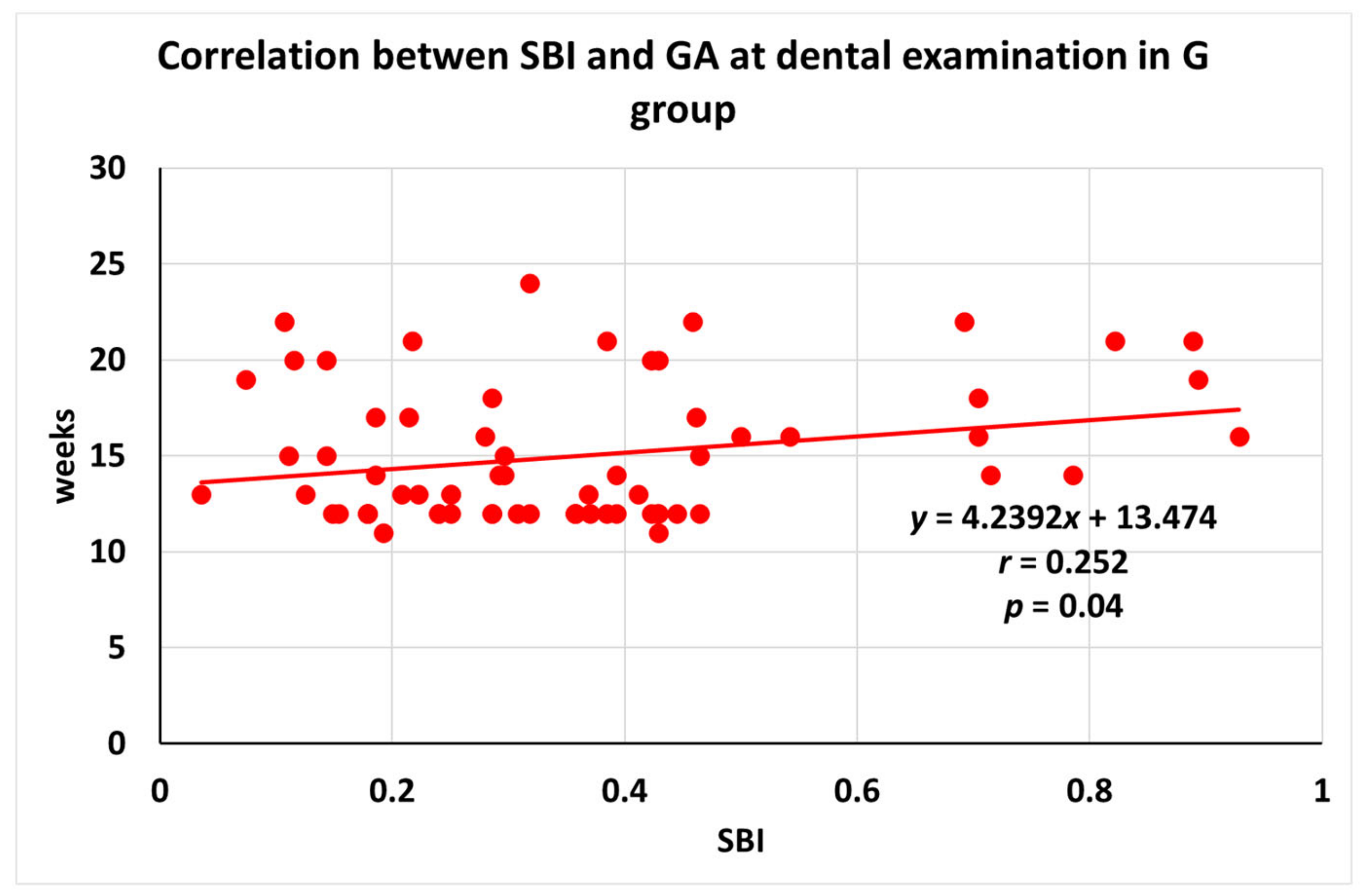
3.3. Correlation between SBI and GA at Dental Examination in Different Dental Status Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daskalakis, G.; Arabin, B.; Antsaklis, A. CaberoRoura, Preterm Labor: Up to Date. Biomed. Res. Int. 2019, 2019, 4870938. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.; Cousens, S.; Mathers, C.; Black, R. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.; Merialdi, M.; Requejo, J.; Rubens, C.; Menon, R.; Van Look, P. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef]
- Offenbacher, S.; Katz, V.; Fertik, G.; Collins, J.; Boyd, D.; Maynor, G.; McKaig, R.; Beck, J. Periodontal Infection as a Possible Risk Factor for Preterm Low Birth Weight. J. Periodontol. 1996, 67 (Suppl. S10), 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.; Radnai, M.; Gorzo, I.; Urban, E.; Orvos, H.; Eller, J.; Pal, A. Prevention of preterm delivery with periodontal treatment. Fetal Diagn. Ther. 2009, 25, 230–233. [Google Scholar] [CrossRef]
- Novak, T.; Nemeth, G.; Kozinszky, Z.; Urban, E.; Gorzo, I.; Radnai, M. Could Poor Periodontal Status be a Warning Sign for Worse Pregnancy Outcome? Oral Health Prev. Dent. 2020, 18, 165–170. [Google Scholar]
- Kinane, D.; Stathopoulou, P.; Papapanou, P. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.; Michalowicz, B.; Johnson, N. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [PubMed]
- Carrillo-de-Albornoz, A.; Figuero, E.; Herrera, D.; Cuesta, P.; Bascones-Martinez, A. Gingival changes during pregnancy: III. Impact of clinical, microbiological, immunological and socio-demographic factors on gingival inflammation. J. Clin. Periodontol. 2012, 39, 272–283. [Google Scholar] [CrossRef]
- Offenbacher, S.; Lin, D.; Strauss, R.; McKaig, R.; Irving, J.; Barros, S.; Moss, K.; Barrow, D.; Hefti, A.; Beck, J.D. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: A pilot study. J. Periodontol. 2006, 77, 2011–2024. [Google Scholar] [CrossRef] [PubMed]
- Yenen, Z.; Atacag, T. Oral care in pregnancy. J. Turk. Ger. Gynecol. Assoc. 2019, 20, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Ekuni, D.; Irie, K.; Furuta, M.; Tomofuji, T.; Morita, M.; Watanabe, T. Relationship between periodontal inflammation and fetal growth in pregnant women: A cross-sectional study. Arch. Gynecol. Obstet. 2013, 287, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Butera, A.; Alovisi, M. Customized minimally invasive protocols for the clinical and microbiological management of the oral microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef]
- Radnai, M.; Pal, A.; Novak, T.; Urban, E.; Eller, J.; Gorzo, I. Benefits of periodontal therapy when preterm birth threatens. J. Dent. Res. 2009, 88, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Kornman, K.; Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84 (Suppl. S4), S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Ream, M.; Lehwald, L. Neurologic Consequences of Preterm Birth. Curr. Neurol. Neurosci. Rep. 2018, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Kim, S.; Lee, H.; Kim, H.; Lee, K.; Han, S.; Oh, M. Association between dental caries and adverse pregnancy outcomes. Sci. Rep. 2020, 10, 5309. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Smith, V.; McCormick, M.; Barfield, W. The association between maternal oral health experiences and risk of preterm birth in 10 states, Pregnancy Risk Assessment Monitoring System, 2004–2006. Matern. Child Health J. 2012, 16, 1688–1695. [Google Scholar] [CrossRef]
- Ressler-Maerlender, J.; Krishna, R.; Robison, V. Oral health during pregnancy: Current research. J. Women’s Health 2005, 14, 880–882. [Google Scholar] [CrossRef]
- AlRatroot, S.; Alotaibi, G.; AlBishi, F.; Khan, S.; Nazir, M.A. Dental Anxiety amongst Pregnant Women: Relationship with Dental Attendance and Sociodemographic Factors. Int. Dent. J. 2022, 72, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.; Aimagambetova, G.; Terzic, S.; Radunovic, M.; Bapayeva, G.; Lagana, A. Periodontal Pathogens and Preterm Birth: Current Knowledge and Further Interventions. Pathogens 2021, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Mainas, G.; Ide, M.; Rizzo, M.; Magan-Fernandez, A.; Mesa, F.; Nibali, L. Managing the Systemic Impact of Periodontitis. Medicina 2022, 58, 621. [Google Scholar] [CrossRef] [PubMed]
| Healthy (H) n = 17 | Gingivitis (G) n = 67 | Periodontitis (P) n = 27 | ANOVA | |
|---|---|---|---|---|
| Gestational age at dental examination (w) | 16.24 ± 3.492 | 15.00 ± 3.516 | 14.00 ± 3.150 | p = 0.111 |
| Gestational age at delivery (w) | 38.53 ± 1.772 | 39.07 ± 1.454 | 38.67 ± 1.414 | p = 0.253 |
| Maternal age at delivery (y) | 31.25 ± 4.337 | 30.91 ± 5.197 | 29.53 ± 6.309 | p = 0.464 |
| Birth weight (g) | 3518.82 ± 548.212 | 3402.24 ± 541.443 | 3396.67 ± 611.826 | p = 0.726 |
| Group | N | Correlation Coefficient | p Value |
|---|---|---|---|
| Healthy | 17 | r = −0.391 | p = 0.120 |
| Gingivitis | 67 | r = 0.252 | p = 0.04 |
| Periodontitis | 27 | r = 0.127 | p = 0.527 |
| Total | 111 | r = −0.035 | p = 0.714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Völgyesi, P.; Radnai, M.; Németh, G.; Boda, K.; Bernad, E.; Novák, T. Maternal Periodontal Status as a Factor Influencing Obstetrical Outcomes. Medicina 2023, 59, 621. https://doi.org/10.3390/medicina59030621
Völgyesi P, Radnai M, Németh G, Boda K, Bernad E, Novák T. Maternal Periodontal Status as a Factor Influencing Obstetrical Outcomes. Medicina. 2023; 59(3):621. https://doi.org/10.3390/medicina59030621
Chicago/Turabian StyleVölgyesi, Petra, Márta Radnai, Gábor Németh, Krisztina Boda, Elena Bernad, and Tibor Novák. 2023. "Maternal Periodontal Status as a Factor Influencing Obstetrical Outcomes" Medicina 59, no. 3: 621. https://doi.org/10.3390/medicina59030621
APA StyleVölgyesi, P., Radnai, M., Németh, G., Boda, K., Bernad, E., & Novák, T. (2023). Maternal Periodontal Status as a Factor Influencing Obstetrical Outcomes. Medicina, 59(3), 621. https://doi.org/10.3390/medicina59030621








