Whole Exome Sequencing of a Patient with a Milder Phenotype of Xeroderma Pigmentosum Group C
Abstract
1. Introduction
2. Methods
2.1. Subjects and DNA Extraction
2.2. Exome Sequencing and Bioinformatics Pipeline
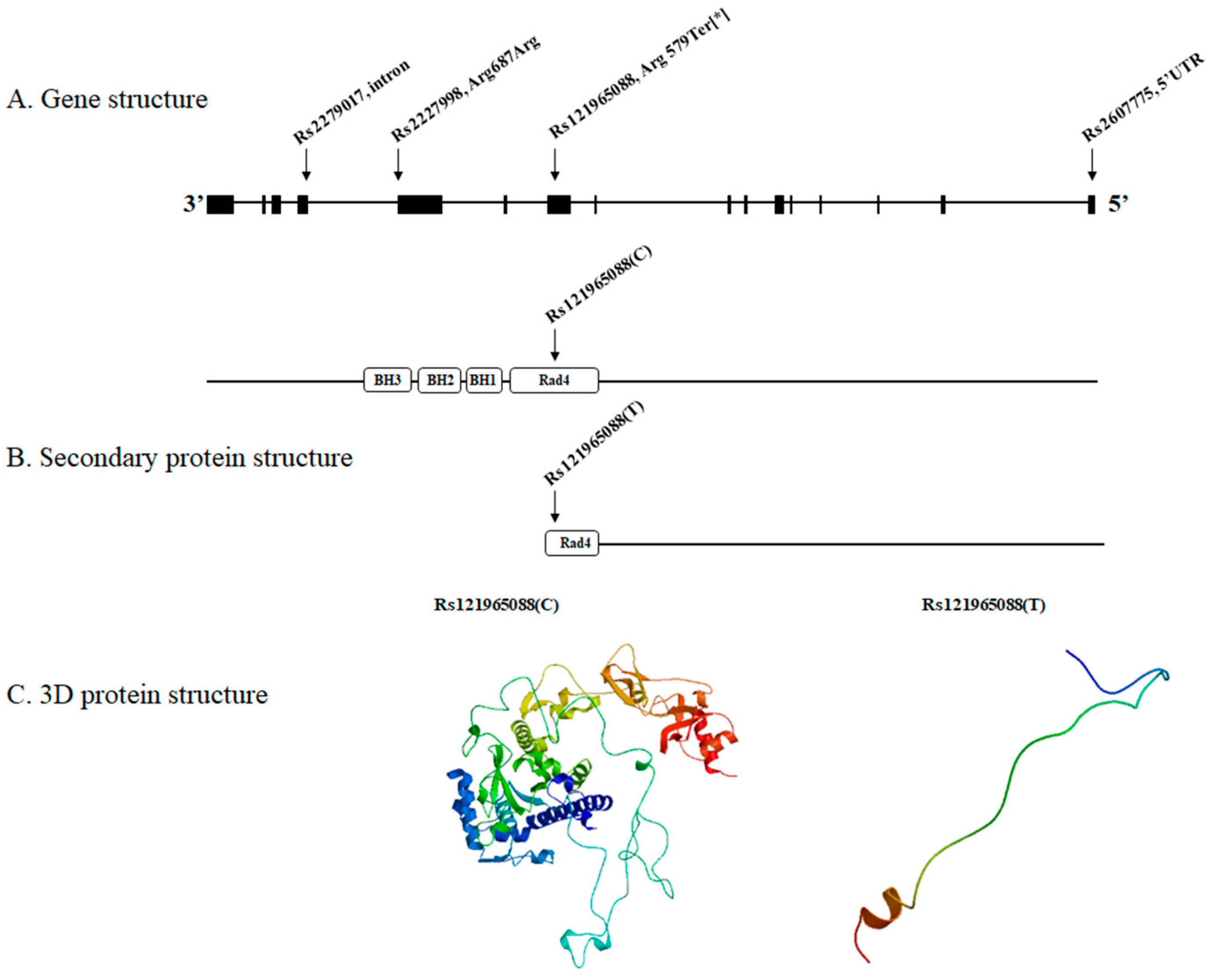
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Xeroderma pigmentosum: An updated review. Drugs Context 2022, 11, 2022-2-5. [Google Scholar] [CrossRef]
- Fassihi, H. Spotlight on ‘xeroderma pigmentosum’. Photochem. Photobiol. Sci. 2013, 12, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Abeti, R.; Zeitlberger, A.; Peelo, C.; Fassihi, H.; Sarkany, R.P.E.; Lehmann, A.R.; Giunti, P. Xeroderma pigmentosum: Overview of pharmacology and novel therapeutic strategies for neurological symptoms. Br. J. Pharmacol. 2019, 176, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Halkud, R.; Shenoy, A.M.; Naik, S.M.; Chavan, P.; Sidappa, K.T.; Biswas, S. Xeroderma pigmentosum: Clinicopathological review of the multiple oculocutaneous malignancies and complications. Indian J. Surg. Oncol. 2014, 5, 120–124. [Google Scholar] [CrossRef]
- Black, J.O. Xeroderma Pigmentosum. Head Neck Pathol. 2016, 10, 139–144. [Google Scholar] [CrossRef]
- Piccione, M.; Fortina, A.B.; Ferri, G.; Andolina, G.; Beretta, L.; Cividini, A.; De Marni, E.; Caroppo, F.; Citernesi, U.; Di Liddo, R. Xeroderma Pigmentosum: General Aspects and Management. J. Pers. Med. 2021, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Brambullo, T.; Colonna, M.R.; Vindigni, V.; Piaserico, S.; Masciopinto, G.; Galeano, M.; Costa, A.L.; Bassetto, F. Xeroderma Pigmentosum: A Genetic Condition Skin Cancer Correlated—A Systematic Review. BioMed Res. Int. 2022, 2022, 8549532. [Google Scholar] [CrossRef]
- Hossain, M.; Hasan, A.; Shawan, M.M.A.K.; Banik, S.; Jahan, I. Current Therapeutic Strategies of Xeroderma Pigmentosum. Indian J. Dermatol. 2021, 66, 660–667. [Google Scholar] [CrossRef]
- Lehmann, A.R.; McGibbon, D.; Stefanini, M. Xeroderma pigmentosum. Orphanet. J. Rare Dis. 2011, 6, 70. [Google Scholar] [CrossRef]
- DiGiovanna, J.J.; Kraemer, K.H. Shining a light on xeroderma pigmentosum. J. Investig. Dermatol. 2012, 132 Pt 2, 785–796. [Google Scholar] [CrossRef]
- DiGiovanna, J.J.; Rünger, T.M.; Kraemer, K.H. Hereditary Disorders of Genome Instability and DNA Repair. In Fitzpatrick’s Dermatology, 9th ed.; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw Hill: New York, NY, USA, 2019. [Google Scholar]
- Masaki, T.; Nakano, E.; Okamura, K.; Ono, R.; Sugasawa, K.; Lee, M.-H.; Suzuki, T.; Nishigori, C. A case of xeroderma pigmentosum complementation group C with diverse clinical features. Br. J. Dermatol. 2018, 178, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Gozukara, E.M.; Khan, S.; Metin, A.; Emmert, S.; Busch, D.B.; Shahlavi, T.; Coleman, D.M.; Miller, M.; Chinsomboon, N.; Stefanini, M.; et al. A Stop Codon in Xeroderma Pigmentosum Group C Families in Turkey and Italy: Molecular Genetic Evidence for a Common Ancestor. J. Investig. Dermatol. 2001, 117, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lucero, R.; Horowitz, D. Xeroderma Pigmentosum; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Costanzo, M.; VanderSluis, B.; Koch, E.N.; Baryshnikova, A.; Pons, C.; Tan, G.; Wang, W.; Usaj, M.; Hanchard, J.; Lee, S.D.; et al. A global genetic interaction network maps a wiring diagram of cellular function. Science 2016, 353, aaf1420. [Google Scholar] [CrossRef]
- Boucher, B.; Jenna, S. Genetic interaction networks: Better understand to better predict. Front. Genet. 2013, 4, 290. [Google Scholar] [CrossRef]
- Shell, S.M.; Zou, Y. Other proteins interacting with XP proteins. Adv. Exp. Med. Biol. 2008, 637, 103–112. [Google Scholar]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Xue, P.; Gao, L.; Xiao, S.; Zhang, G.; Xiao, M.; Zhang, Q.; Zheng, X.; Cai, Y.; Jin, C.; Yang, J.; et al. Genetic polymorphisms in xrcc1, cd3eap, ppp1r13l, xpb, xpc, and xpf and the risk of chronic benzene poisoning in a chinese occupational population. PLoS ONE 2015, 10, e0144458. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Gil, J.; Gaj, P.; Misiak, B.; Ostrowski, J.; Karpinski, P.; Jarczynska, A.; Kielan, W.; Sasiadek, M.M. Cyp1a1 ile462val polymorphism and colorectal cancer risk in polish patients. Med. Oncol. 2014, 31, 72. [Google Scholar] [CrossRef]
- Rondelli, C.M.; El-Zein, R.A.; Wickliffe, J.K.; Etzel, C.J.; Abdel-Rahman, S.Z. A comprehensive haplotype analysis of the xpc genomic sequence reveals a cluster of genetic variants associated with sensitivity to tobacco-smoke mutagens. Toxicol. Sci. 2010, 115, 41–50. [Google Scholar] [CrossRef]
- Joshi, A.D.; Corral, R.; Siegmund, K.D.; Haile, R.W.; Le Marchand, L.; Martinez, M.E.; Ahnen, D.J.; Sandler, R.S.; Lance, P.; Stern, M.C. Red meat and poultry intake, polymorphisms in the nucleotide excision repair and mismatch repair pathways and colorectal cancer risk. Carcinogenesis 2009, 30, 472–479. [Google Scholar] [CrossRef]
- Richards, C.S.; Bale, S.; Bellissimo, D.B.; Das, S.; Grody, W.W.; Hegde, M.R.; Lyon, E.; Ward, B.E.; Molecular Subcommittee of the ACMG Laboratory Quality Assurance Committee. ACMG recommendations for standards for interpretation and reporting of sequence variations. Genet. Med. 2008, 10, 294–300. [Google Scholar] [CrossRef]
- Bodian, D.L.; McCutcheon, J.N.; Kothiyal, P.; Huddleston, K.C.; Iyer, R.K.; Vockley, J.G.; Niederhuber, J.E. Germline variation in cancer-susceptibility genes in a healthy, ancestrally diverse cohort: Implications for individual genome sequencing. PLoS ONE 2014, 9, e94554. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.A.; Weiss, N.S.; Fish, S.; Fan, W.; Loomis, M.M.; Sakoda, L.C.; Rossing, M.A.; Zhao, L.P.; Chen, C. Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.R.; Shields, P.G.; Ambrosone, C.B.; Nie, J.; Marian, C.; Krishnan, S.S.; Goerlitz, D.S.; Modali, R.; Seddon, M.; Lehman, T.; et al. Single-nucleotide polymorphisms in DNA repair genes and association with breast cancer risk in the web study. Carcinogenesis 2011, 32, 1223–1230. [Google Scholar] [CrossRef]
- Chavanne, F.; Broughton, B.C.; Pietra, D.; Nardo, T.; Browitt, A.; Lehmann, A.R.; Stefanini, M. Mutations in the xpc gene in families with xeroderma pigmentosum and consequences at the cell, protein, and transcript levels. Cancer Res. 2000, 60, 1974–1982. [Google Scholar] [PubMed]
- Li, Y.K.; Xu, Q.; Sun, L.P.; Gong, Y.H.; Jing, J.J.; Xing, C.Z.; Yuan, Y. Nucleotide excision repair pathway gene polymorphisms are associated with risk and prognosis of colorectal cancer. World J. Gastroenterol. 2020, 26, 307–323. [Google Scholar] [CrossRef]
- Liang, X.H.; Yan, D.; Zhao, J.X.; Ding, W.; Xu, X.J.; Wang, X.Y. Interaction of polymorphisms in xeroderma pigmentosum group c with cigarette smoking and pancreatic cancer risk. Oncol. Lett. 2018, 16, 5631–5638. [Google Scholar] [CrossRef]
- Hua, R.X.; Zhuo, Z.J.; Shen, G.P.; Zhu, J.; Zhang, S.D.; Xue, W.Q.; Li, X.Z.; Zhang, P.F.; He, J.; Jia, W.H. Polymorphisms in the xpc gene and gastric cancer susceptibility in a southern chinese population. Onco. Targets Ther. 2016, 9, 5513–5519. [Google Scholar] [PubMed]
- Perez-Mayoral, J.; Pacheco-Torres, A.L.; Morales, L.; Acosta-Rodriguez, H.; Matta, J.L.; Dutil, J. Genetic polymorphisms in rad23b and xpc modulate DNA repair capacity and breast cancer risk in puerto rican women. Mol. Carcinog. 2013, 52 (Suppl. 1), E127–E138. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xu, L.; Yang, X.; Hu, Z.; Yuan, J.; Wang, F.; Shao, M.; Yuan, W.; Qian, J.; Ma, H.; et al. Sequence variations in DNA repair gene xpc is associated with lung cancer risk in a chinese population: A case-control study. BMC Cancer 2007, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Sakuma, J.; Takeuchi, I.; Yasukochi, Y.; Kato, K.; Oguri, M.; Fujimaki, T.; Horibe, H.; Muramatsu, M.; Sawabe, M.; et al. Identification of egflam, spatc1l and rnase13 as novel susceptibility loci for aortic aneurysm in japanese individuals by exome-wide association studies. Int. J. Mol. Med. 2017, 39, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Q.; Yang, H.-W.; Sun, L.-P.; Yuan, Y. The association of six polymorphisms of five genes involved in three steps of nucleotide excision repair pathways with hepatocellular cancer risk. Oncotarget 2016, 7, 20357–20367. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Xu, Q.; Tu, H.; He, C.; Xing, C.; Yuan, Y. Association of nucleotide excision repair pathway gene polymorphisms with gastric cancer and atrophic gastritis risks. Oncotarget 2016, 7, 6972–6983. [Google Scholar] [CrossRef]
- Hu, X.; Qin, W.; Li, S.; He, M.; Wang, Y.; Guan, S.; Zhao, H.; Yao, W.; Wei, M.; Liu, M.; et al. Polymorphisms in DNA repair pathway genes and ABCG2 gene in advanced colorectal cancer: Correlation with tumor characteristics and clinical outcome in oxaliplatin-based chemotherapy. Cancer Manag. Res. 2018, 11, 285–297. [Google Scholar] [CrossRef]
- Vineis, P.; Manuguerra, M.; Kavvoura, F.K.; Guarrera, S.; Allione, A.; Rosa, F.; Di Gregorio, A.; Polidoro, S.; Saletta, F.; Ioannidis, J.P.A.; et al. A Field Synopsis on Low-Penetrance Variants in DNA Repair Genes and Cancer Susceptibility. Gynecol. Oncol. 2009, 101, 24–36. [Google Scholar] [CrossRef]
- Costanzo, M.; Kuzmin, E.; van Leeuwen, J.; Mair, B.; Moffat, J.; Boone, C.; Andrews, B. Global Genetic Networks and the Genotype-to-Phenotype Relationship. Cell 2019, 177, 85–100. [Google Scholar] [CrossRef]
- Lehmann, J.; Seebode, C.; Martens, M.C.; Emmert, S. Xeroderma Pigmentosum—Facts and Perspectives. Anticancer Res. 2018, 38, 1159–1164. [Google Scholar]
- Nussinov, R.; Tsai, C.-J.; Jang, H. Protein ensembles link genotype to phenotype. PLoS Comput. Biol. 2019, 15, e1006648. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Guo, Y.; Ni, C.; Liang, J.; Cheng, R.; Li, M.; Yao, Z. Genotype-phenotype correlation of xeroderma pigmentosum in a Chinese Han population. Br. J. Dermatol. 2015, 172, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.H.Y.; Lesage, G.; Bader, G.D.; Ding, H.; Xu, H.; Xin, X.; Young, J.; Berriz, G.F.; Brost, R.L.; Chang, M.; et al. Global Mapping of the Yeast Genetic Interaction Network. Science 2004, 303, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, K.H.; Patronas, N.J.; Schiffmann, R.; Brooks, B.P.; Tamura, D.; DiGiovanna, J.J. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience 2007, 145, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Pigliucci, M. Genotype-phenotype mapping and the end of the ‘genes as blueprint’ metaphor. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikova, A.; Costanzo, M.; Myers, C.L.; Andrews, B.; Boone, C. Genetic Interaction Networks: Toward an Understanding of Heritability. Annu. Rev. Genom. Hum. Genet. 2013, 14, 111–133. [Google Scholar] [CrossRef]
| Gene | rs ID | Chromosome Number | Patient | Brother | Father | Mother | Protein Residue | Effect on Amino Acid | Previous Study |
|---|---|---|---|---|---|---|---|---|---|
| XPC | rs2279017 | Chr 3 | 1/1 | 0/1 | 0/1 | 0/1 | Intron | No effect | [20,21,22,23,24,25] |
| XPC | rs2227998 | Chr 3 | 1/1 | 0/1 | 0/1 | 0/1 | Arg687Arg | Synonymous | [21,26,27,28] |
| XPC | rs121965088 | Chr 3 | 1/1 | 0/1 | 0/1 | 0/1 | Arg579Ter | Non-synonymous | [14,29] |
| XPC | rs2607775 | Chr 3 | 1/1 | 0/1 | 0/1 | 0/1 | 5′UTR | No effect | [21,30,31,32,33,34] |
| OR2T35 | rs72452004 | Chr 1 | 1/1 | 0/1 | 0/1 | 0/1 | Cys203Ter | Non-synonymous | |
| AFF3 | rs202089462 | Chr 2 | 1/1 | 0/1 | 0/1 | 0/1 | Thr619Ser | Non-synonymous | |
| TARP | rs138027161 | Chr 7 | 1/1 | 0/1 | 0/1 | 0/1 | Arg100Gly | Non-synonymous | |
| ANXA7 | rs3750575 | Chr 10 | 1/1 | 0/1 | 0/1 | 0/1 | Arg419Gln | Non-synonymous | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.-I.; Nishigori, C.; Ahn, J.J.; Ryu, J.Y.; Lee, J.; Lee, M.-H.; Kim, S.K.; Jeong, K.-H. Whole Exome Sequencing of a Patient with a Milder Phenotype of Xeroderma Pigmentosum Group C. Medicina 2023, 59, 699. https://doi.org/10.3390/medicina59040699
Seo J-I, Nishigori C, Ahn JJ, Ryu JY, Lee J, Lee M-H, Kim SK, Jeong K-H. Whole Exome Sequencing of a Patient with a Milder Phenotype of Xeroderma Pigmentosum Group C. Medicina. 2023; 59(4):699. https://doi.org/10.3390/medicina59040699
Chicago/Turabian StyleSeo, Ji-In, Chikako Nishigori, Jung Jin Ahn, Jae Young Ryu, Junglok Lee, Mu-Hyoung Lee, Su Kang Kim, and Ki-Heon Jeong. 2023. "Whole Exome Sequencing of a Patient with a Milder Phenotype of Xeroderma Pigmentosum Group C" Medicina 59, no. 4: 699. https://doi.org/10.3390/medicina59040699
APA StyleSeo, J.-I., Nishigori, C., Ahn, J. J., Ryu, J. Y., Lee, J., Lee, M.-H., Kim, S. K., & Jeong, K.-H. (2023). Whole Exome Sequencing of a Patient with a Milder Phenotype of Xeroderma Pigmentosum Group C. Medicina, 59(4), 699. https://doi.org/10.3390/medicina59040699






