The Temporary Mental Nerve Paresthesia as an Outcome of Dentigerous Cyst Removal during Preparation for Dental Implant Placement: A Case Report
Abstract
:1. Introduction
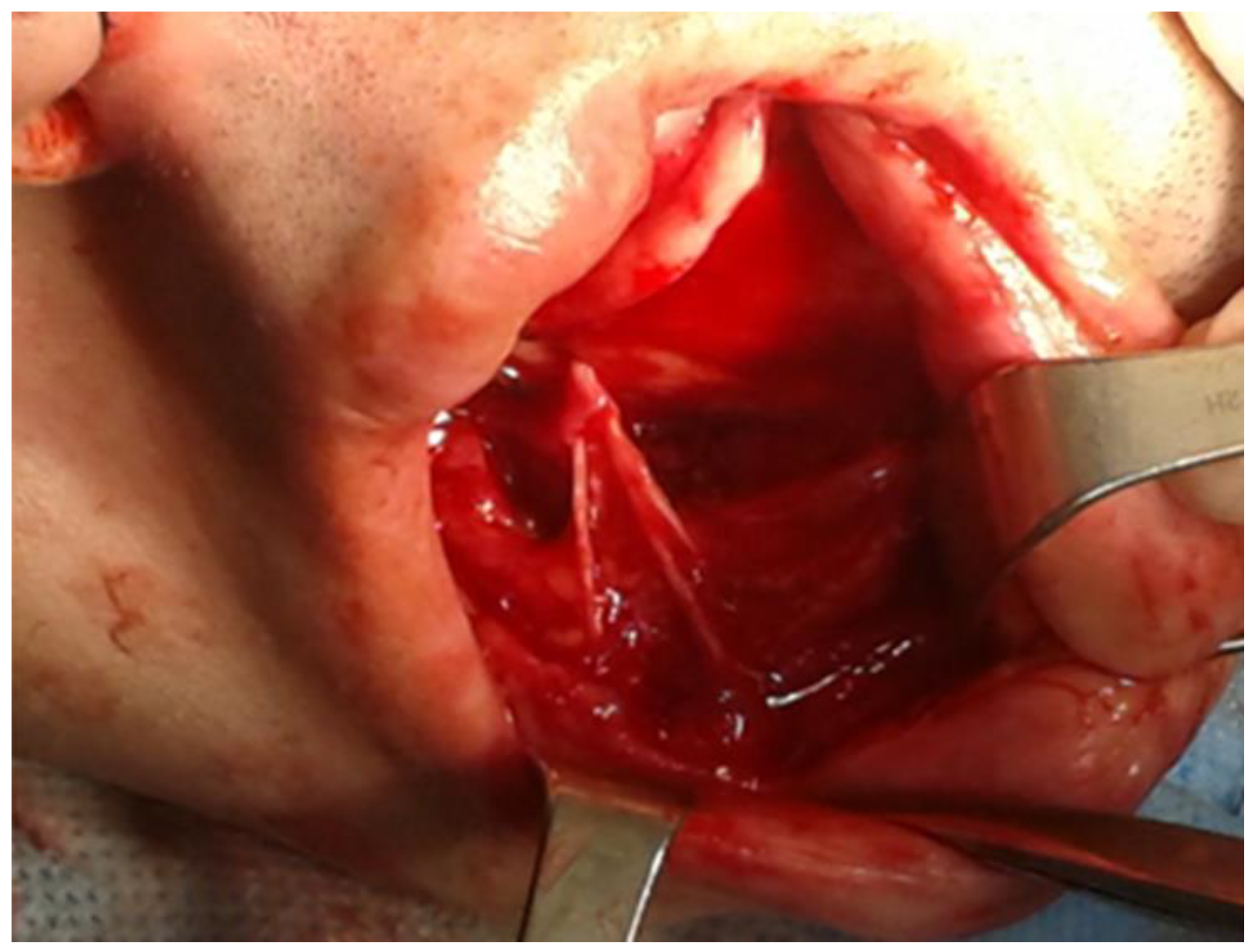
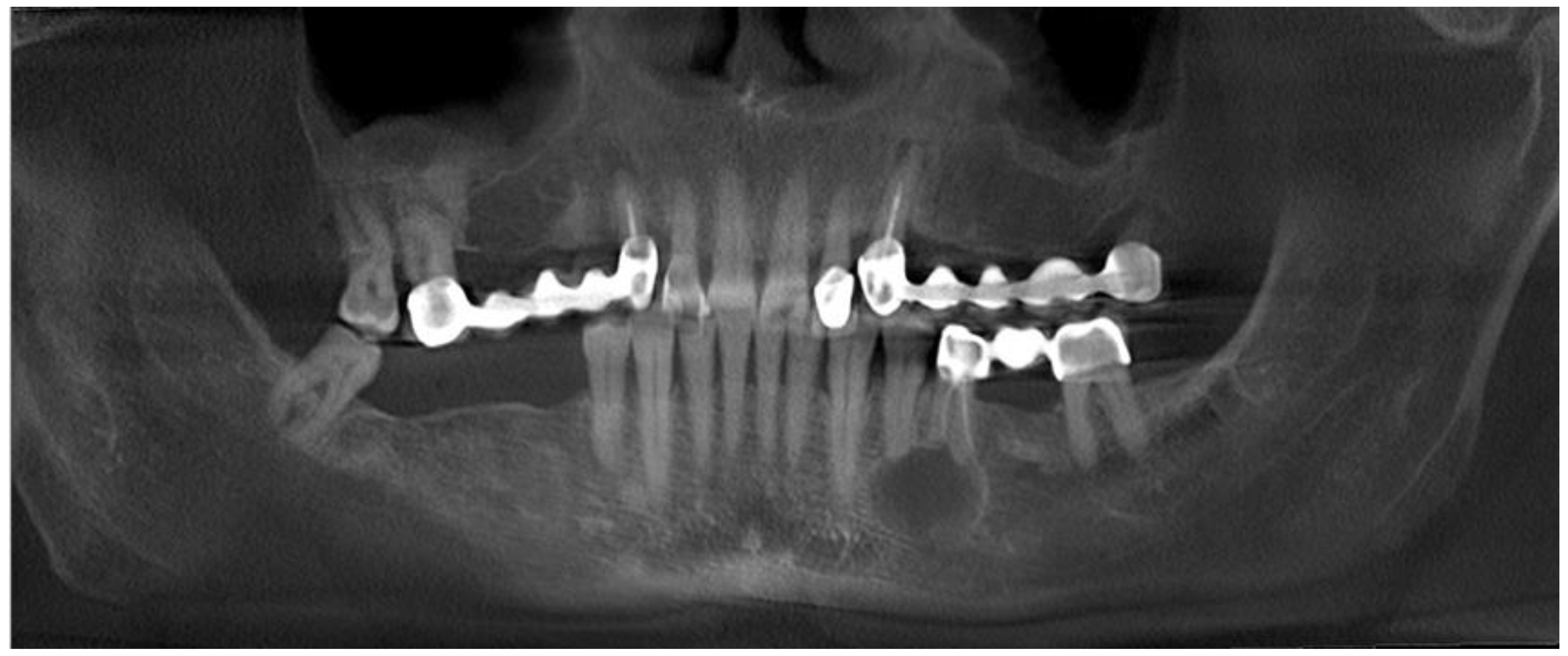
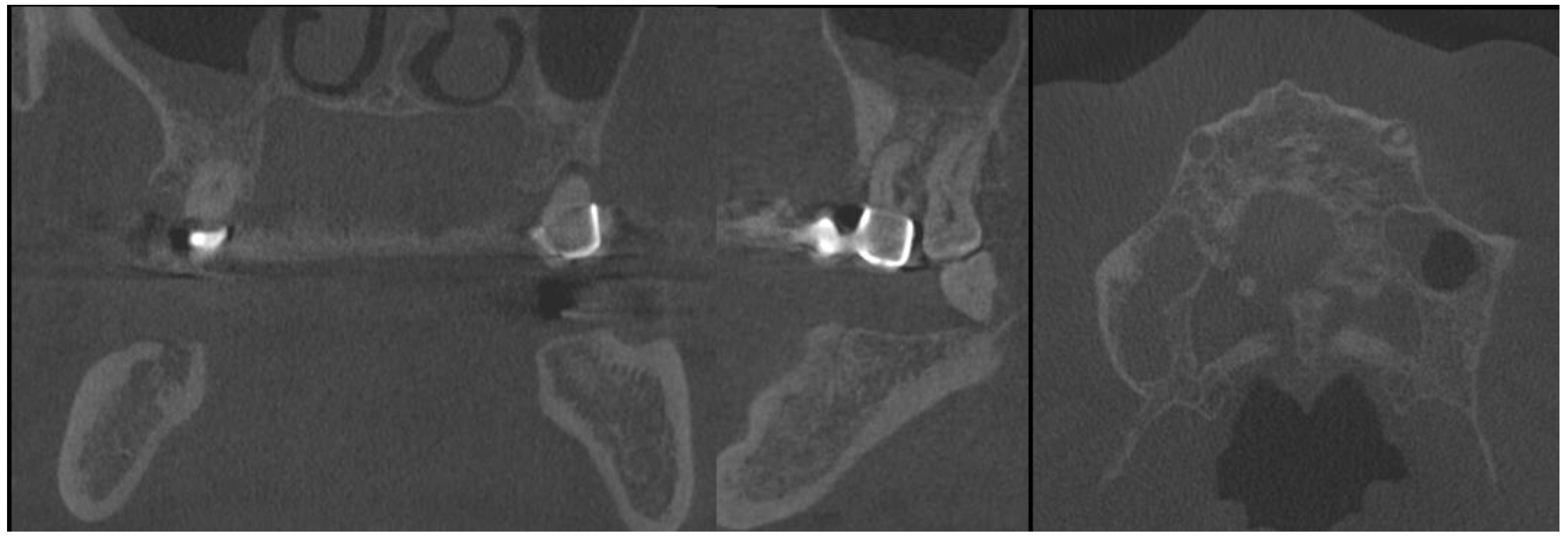
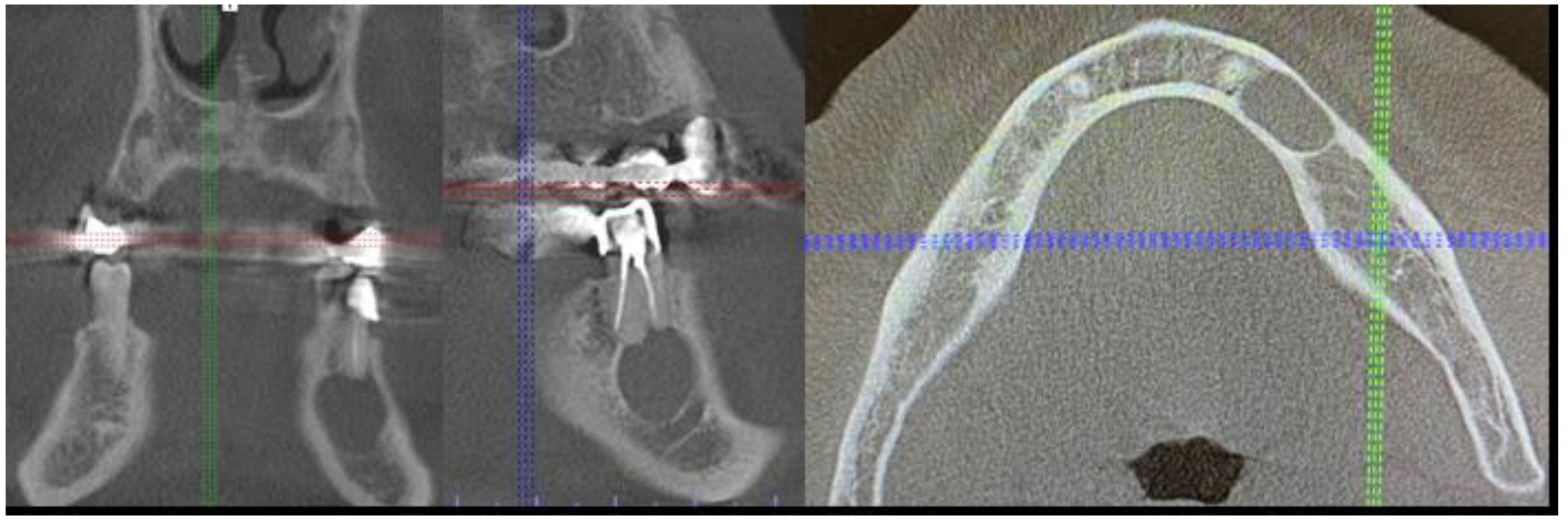
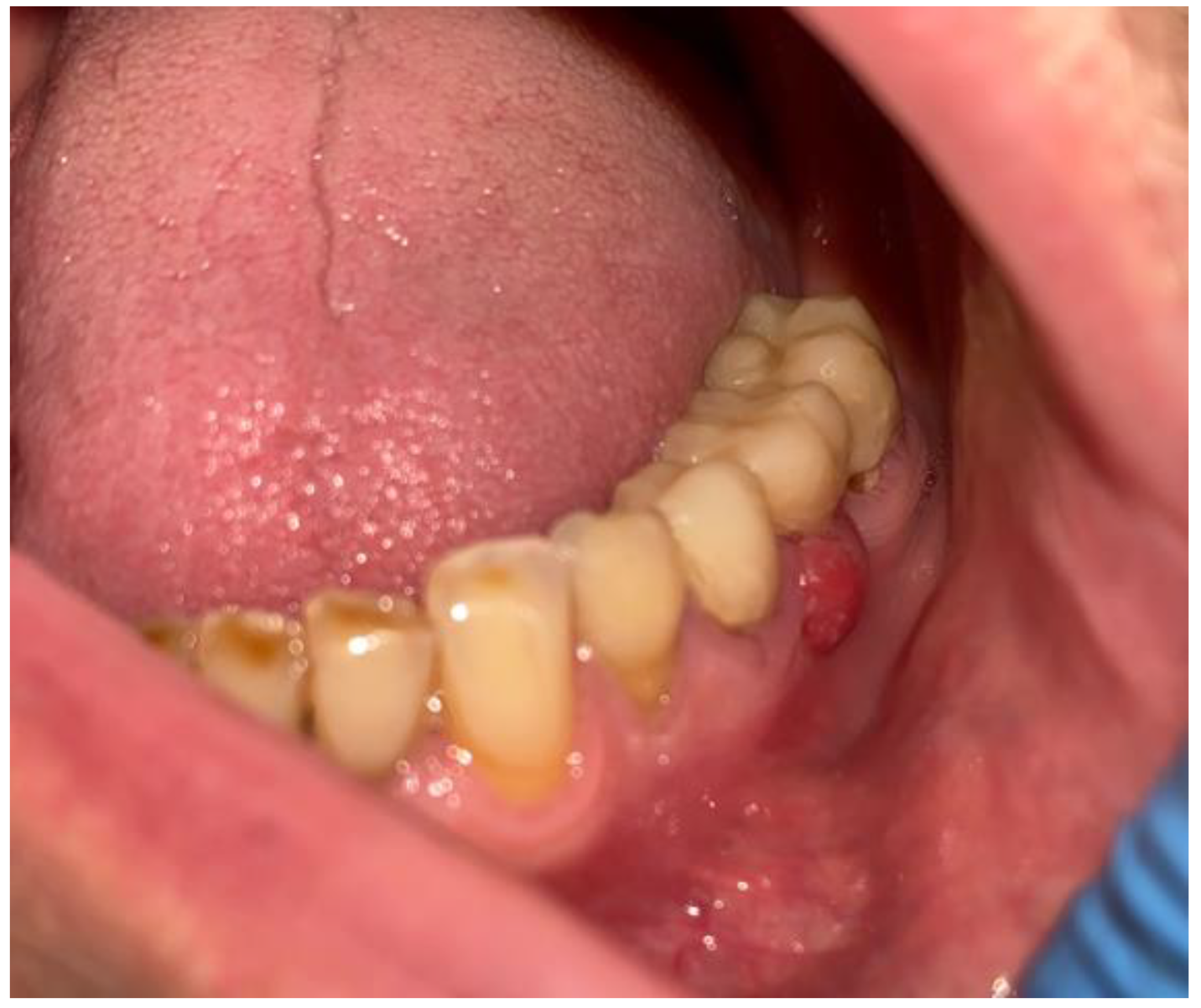
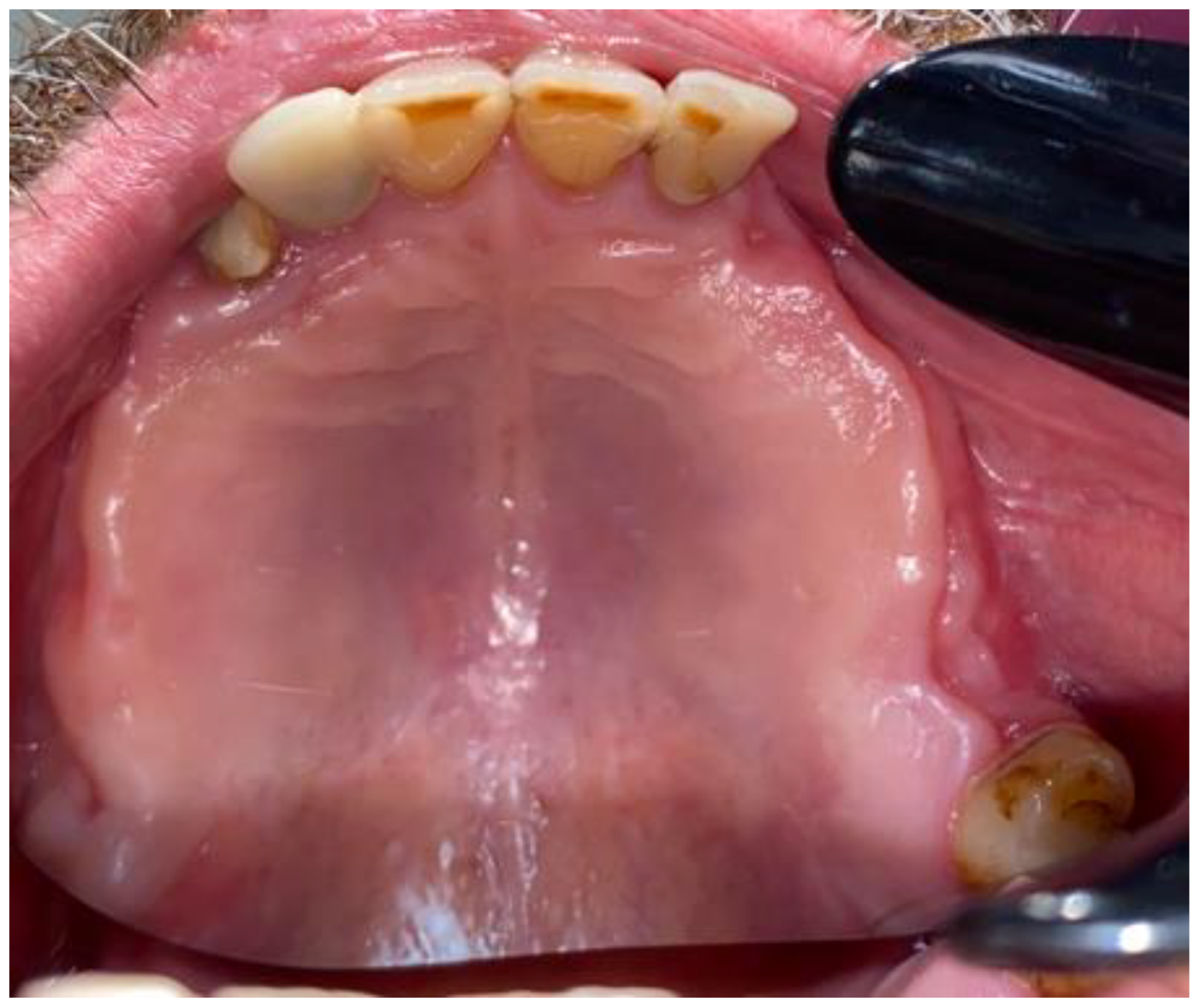
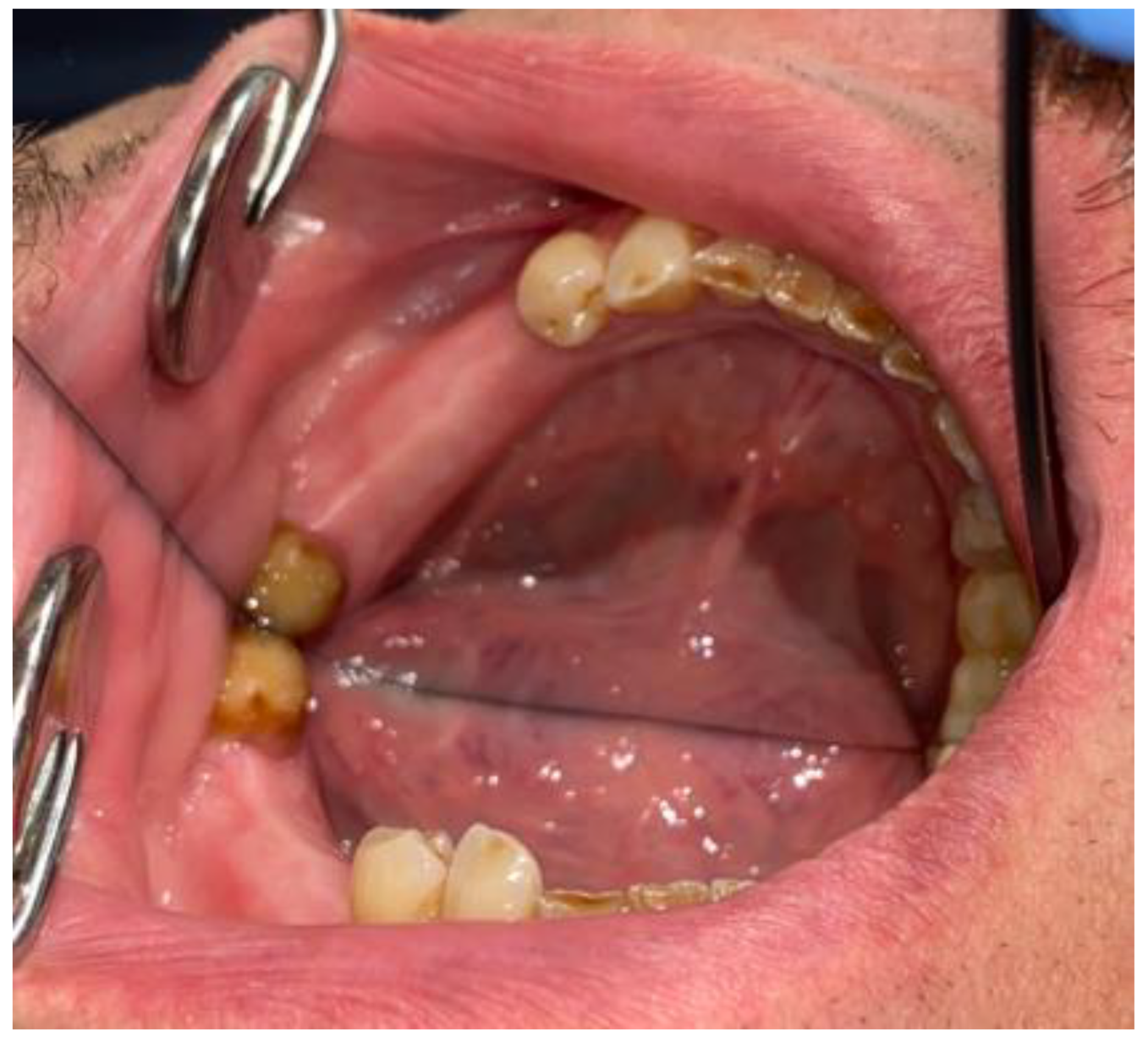
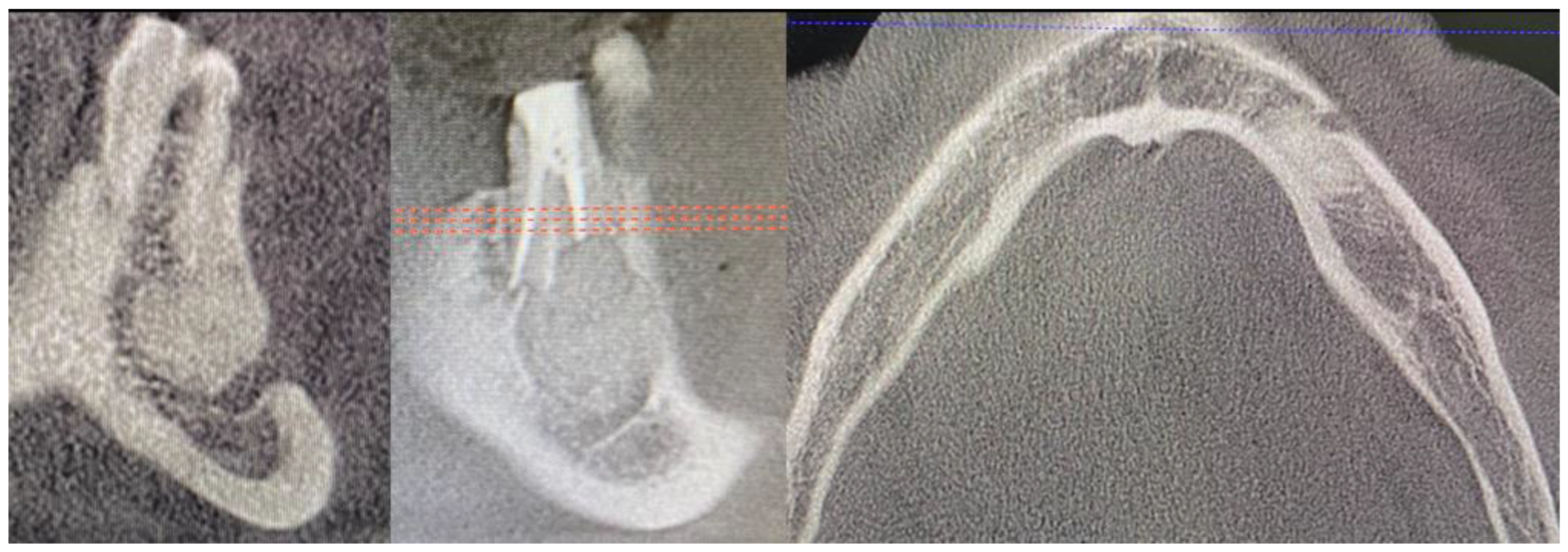
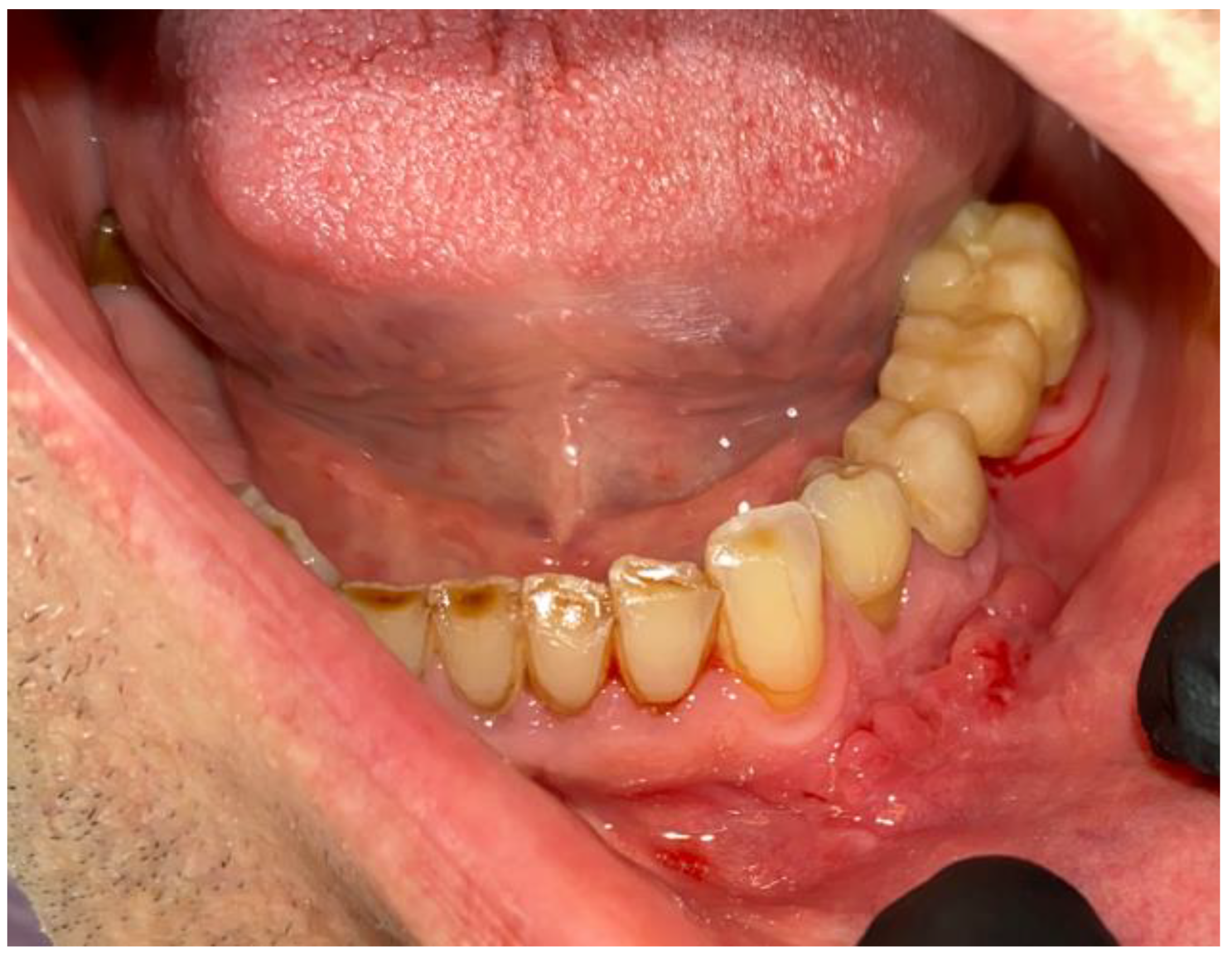
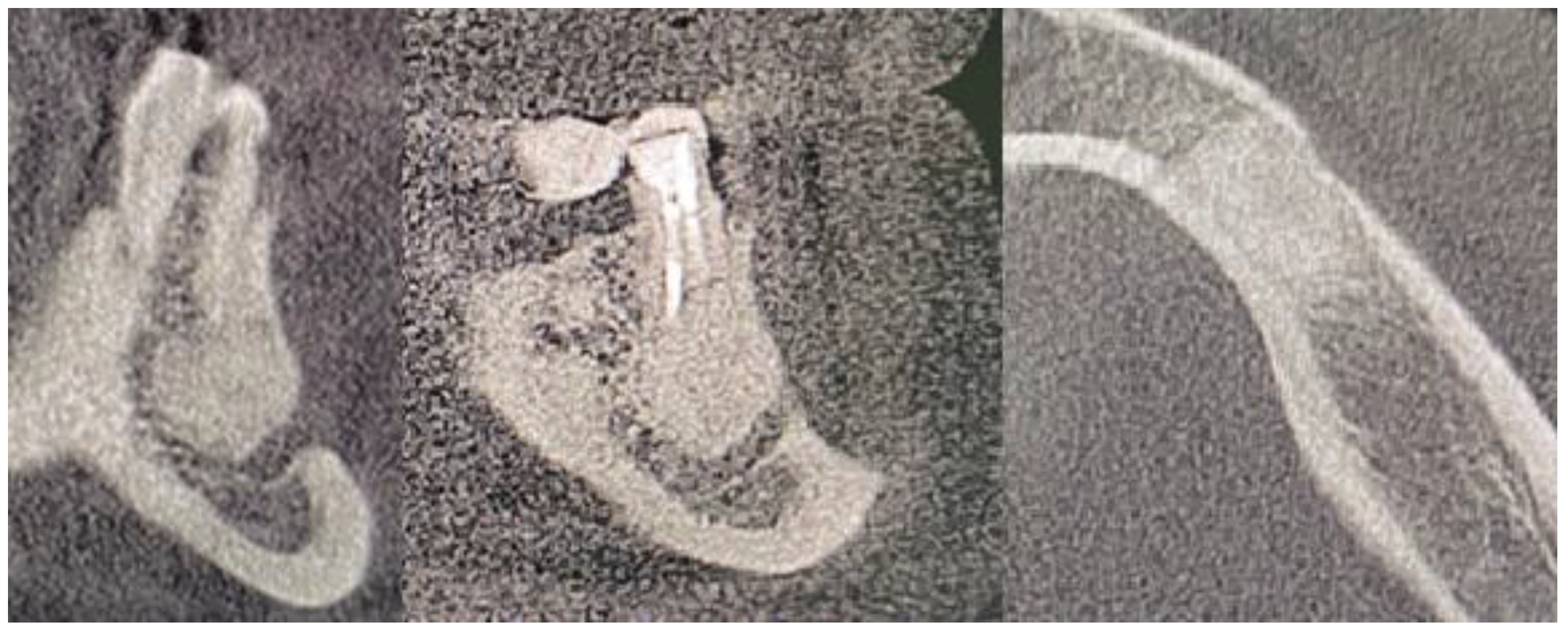
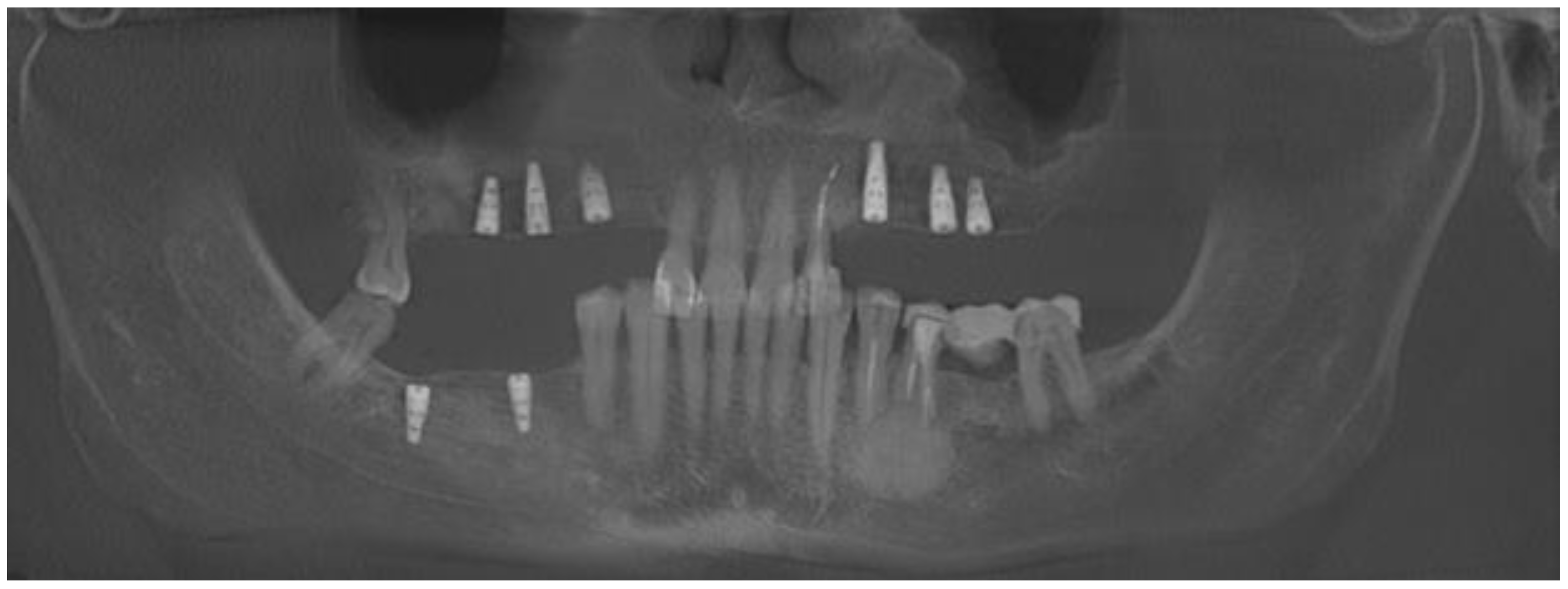
2. Case Description
3. Discussion
- A close CBCT evaluation before surgery;
- Elimination of all possible inflammation sources in the oral cavity and pre-surgical hygienization;
- Good local anesthetic injection into the operation can improve tissue preparation;
- Local anesthetic injection in the nerve should be avoided;
- Appropriate visibility to the operated field with retractors and periosteal elevators;
- Surgical field irrigation with saline for improved visibility;
- Identification of the nerve in the subperiosteal plane grants more nerve safety;
- Traction of the adjacent tissues is safer than only nerve traction;
- MN compression should be limited when retractors are positioned on the bone and not on the MN fibers;
- When using high-speed drills, good irrigation and visibility should be used;
- When suturing the wound, any nerve fibers should be identified to avoid their possible damage;
- Early intraoral vitamin B or other agents should be used;
- Close patient monitoring after the surgery to avoid any hematoma or purulent formation in the operating area.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alsaad, K.; Lee, T.C.; McCartan, B. An anatomical study of the cutaneous branches of the mental nerve. Int. J. Oral Maxillofac. Surg. 2003, 32, 325–333. [Google Scholar] [CrossRef]
- Won, S.Y.; Yang, H.M.; Woo, H.S.; Chang, K.Y.; Youn, K.H.; Kim, H.J.; Hu, K.S. Neuroanastomosis and the innervation territory of the mental nerve. Clin. Anat. 2014, 27, 598–602. [Google Scholar] [CrossRef]
- Bernardi, S.; Angelone, A.M.; Macchiarelli, G. Anatomy in dentistry: From the beginnings to contemporary reality. Clin. Anat. 2022, 35, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.S.; Yun, H.S.; Hur, M.S.; Kwon, H.J.; Abe, S.; Kim, H.J. Branching patterns and intraosseous course of the mental nerve. J. Oral Maxillofac. Surg. 2007, 65, 2288–2294. [Google Scholar] [CrossRef]
- Iwanaga, J.; Haikata, Y.; Nakamura, K.; Kusukawa, J.; Watanabe, K.; Tubbs, R.S. An anatomical and histological study of mental nerve branches to the inferior labial glands. Surg. Radiol. Anat. 2021, 43, 1801–1804. [Google Scholar] [CrossRef]
- Kieser, J.; Kuzmanovic, D.; Payne, A.; Dennison, J.; Herbison, P. Patterns of emergence of the human mental nerve. Arch. Oral Biol. 2002, 47, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Sen, O.G.; Kaplan, V. Temporary Mental Nerve Paresthesia Originating from Periapical Infection. Case Rep. Dent. 2015, 2015, 457645. [Google Scholar] [CrossRef] [Green Version]
- Aljarbou, F.; Riyahi, A.M.; Altamimi, A.; Alabdulsalam, A.; Jabhan, N.; Aldosimani, M.; Alamri, H.M. Anatomy of the accessory mental foramen in a Saudi subpopulation: A multicenter CBCT study. Saudi Dent. J. 2021, 33, 1012–1017. [Google Scholar] [CrossRef]
- Patel, N.; Langaliya, A.; Kanodia, S.; Kumbhar, A.; Buch, A.; Shah, A.; Bhatt, H.; Panchal, D.; Shah, S.; Shah, J. Mental Nerve Paraesthesia: A Report of Two Cases Associated with Endodontic Etiology. Case Rep. Dent. 2021, 2021, 1747519. [Google Scholar] [CrossRef]
- Kumar, U.; Kaur, C.K.; Vashisht, R.; Rattan, V. Paresthesia diagnosed using cone-beam computed tomography: A case report. J. Dent. Anesth. Pain Med. 2020, 20, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.J.; Chae, S.; Oh, M.Y.; Kwon, H.; Park, W.S. Transoral Endoscopic Thyroidectomy Vestibular Approach (TOETVA): Surgical Outcomes and Learning Curve. J. Clin. Med. 2021, 10, 863. [Google Scholar] [CrossRef]
- Chhabra, A.; Ahlawat, S.; Belzberg, A.; Andreseik, G. Peripheral nerve injury grading simplified on MR neurography: As referenced to Seddon and Sunderland classifications. Indian J. Radiol. Imaging 2014, 24, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.S.; Del Grande, F.; Thawait, G.K.; Andreisek, G.; Carrino, J.A.; Chhabra, A. Spectrum of high-resolution MRI findings in diabetic neuropathy. Am. J. Roentgenol. 2012, 199, 407–412. [Google Scholar] [CrossRef]
- Chhabra, A.; Thakkar, R.S.; Andreisek, G.; Chalian, M.; Belzberg, A.J.; Blakeley, J. Anatomic MR imaging and functional diffusion tensor imaging of peripheral nerve tumors and tumorlike conditions. Am. J. Neuroradiol. 2013, 34, 802–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon and Sunderland Classification of Nerve Injury. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557746/figure/article-25766.image.f1/ (accessed on 9 February 2023).
- Do, T.A.; Le, H.S.; Shen, Y.W.; Huang, H.L.; Fuh, L.J. Risk Factors related to Late Failure of Dental Implant-A Systematic Review of Recent Studies. Int. J. Environ. Res. Public Health 2020, 17, 3931. [Google Scholar] [CrossRef] [PubMed]
- Varvara, G.; Feragalli, B.; Turkyilmaz, I.; D’Alonzo, A.; Rinaldi, F.; Bianchi, S.; Piattelli, M.; Macchiarelli, G.; Bernardi, S. Prevalence and Characteristics of Accessory Mandibular Canals: A Cone-Beam Computed Tomography Study in a European Adult Population. Diagnostics 2022, 12, 1885. [Google Scholar] [CrossRef]
- Fatani, B.; Almutairi, E.S.; Almalky, H.A.; Mubarki, M.I.; Al-Safadi, A. A Comparison of Knowledge and Skills Related to Up-to-Date Implant Techniques Among Prosthodontists, Periodontists, and Oral Surgeons: A Cross-Sectional Study. Cureus 2022, 14, 30370. [Google Scholar] [CrossRef]
- The CARE Guidelines (for CAse REports) Were Developed. Available online: https://www.care-statement.org/checklist (accessed on 16 March 2023).
- Yao, L.; Xu, X.; Ren, M.; Liu, D.; Ni, Z.; Lin, F. Inflammatory dentigerous cyst of mandibular first premolar associated with endodontically treated primary first molar: A rare case report. Eur. J. Paediatr. Dent. 2015, 16, 201–204. [Google Scholar] [PubMed]
- Golob Deeb, J.; Deeb, G.R.; Schafer, D.R. Odontogenic Keratocyst Is Frequently Misdiagnosed for a Lateral Periodontal Cyst in Premolar and Anterior Tooth-Bearing Areas. J. Endod. 2022, 48, 337–344. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, J.G.; Chen, X.; Hou, L.Z.; Zhong, Q.; Ma, H.Z.; Zhang, Y.; Shen, X.X. Protection of nerve function during transoral endoscopic thyroidectomy by vestibular approach. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 55, 893–898. [Google Scholar]
- Chhikara, D.; Singh, V.; Bhagol, A.; Dahiya, A. Mental nerve shielding from possible injury during mandibular surgical procedures: Technical note. Int. J. Oral Maxillofac. Surg. 2023. [Google Scholar] [CrossRef]
- Ruan, J.; Yang, X.; Zhao, J.G.; Tao, L.; Ning, X.J.; He, F.; Zhou, C.Y.; Zhou, C.; Karcz, W.K. Axillary channel-assisted TOETVA: An effective way to prevent mental nerve from iatrogenic injury? J. Minim. Access. Surg. 2022, 18, 450–458. [Google Scholar]
- Kajjari, S.; Gowtham, A.; Meharwade, P.; Uppin, C.; Hugar, S.M.; Badakar, C. Infected Radicular Cyst of Deciduous Second Molar Mimicking Dentigerous Cyst of Second Premolar in a Young Child: A Rare Entity. Int. J. Clin. Pediatr. Dent. 2021, 14, 434–437. [Google Scholar] [PubMed]
- Hyomoto, M.; Kawakami, M.; Inoue, M.; Kirita, T. Clinical conditions for eruption of maxillary canines and mandibular premolars associated with dentigerous cysts. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 515–520. [Google Scholar] [CrossRef]
- Vinereanu, A.; Bratu, A.; Didilescu, A.; Munteanu, A. Management of large inflammatory dentigerous cysts adapted to the general condition of the patient: Two case reports. Exp. Ther. Med. 2021, 22, 750. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Yamada, S.I.; Ueda, N.; Soutome, S.; Funahara, M.; Akashi, M.; Furuno, S.; Miyamoto, H.; Hayashida, S.; Amano, R.; et al. Treatment modalities and risk factors associated with refractory neurosensory disturbances of the inferior alveolar nerve following oral surgery: A multicentre retrospective study. Int. J. Oral Maxillofac. Surg. 2018, 47, 794–801. [Google Scholar] [CrossRef] [Green Version]
- Traina, G. The neurobiology of acetyl-L-carnitine. Front. Biosci. 2016, 21, 1314–1329. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.S.; Benton, J.A.; Yates, J.M. Risk of inferior alveolar nerve injury with coronectomy vs surgical extraction of mandibular third molars-A comparison of two techniques and review of the literature. J. Oral Rehabil. 2018, 45, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, S.H.; Tabeie, F.; Bemanali, M.; Tabrizi, R. Effect of Low-Level Laser and Light-Emitting Diode on Inferior Alveolar Nerve Recovery After Sagittal Split Osteotomy of the Mandible: A Randomized Clinical Trial Study. J. Craniofac. Surg. 2017, 28, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.; Gooris, P.J.; Bergsma, J.E.; van Hooft, E.; van Merkesteyn, J.P. Influence of BSSO surgical technique on postoperative inferior alveolar nerve hypoesthesia: A systematic review of the literature. J. Cranio-Maxillofac. Surg. 2014, 42, 976–982. [Google Scholar] [CrossRef]
- Degerliyurt, K.; Akar, V.; Denizci, S.; Yucel, E. Bone lid technique with piezosurgery to preserve inferior alveolar nerve. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 1–5. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelke, K.; Janeczek, M.; Pasicka, E.; Żak, K.; Łukaszewski, M.; Jadach, R.; Dobrzyński, M. The Temporary Mental Nerve Paresthesia as an Outcome of Dentigerous Cyst Removal during Preparation for Dental Implant Placement: A Case Report. Medicina 2023, 59, 711. https://doi.org/10.3390/medicina59040711
Nelke K, Janeczek M, Pasicka E, Żak K, Łukaszewski M, Jadach R, Dobrzyński M. The Temporary Mental Nerve Paresthesia as an Outcome of Dentigerous Cyst Removal during Preparation for Dental Implant Placement: A Case Report. Medicina. 2023; 59(4):711. https://doi.org/10.3390/medicina59040711
Chicago/Turabian StyleNelke, Kamil, Maciej Janeczek, Edyta Pasicka, Krzysztof Żak, Marceli Łukaszewski, Radosław Jadach, and Maciej Dobrzyński. 2023. "The Temporary Mental Nerve Paresthesia as an Outcome of Dentigerous Cyst Removal during Preparation for Dental Implant Placement: A Case Report" Medicina 59, no. 4: 711. https://doi.org/10.3390/medicina59040711
APA StyleNelke, K., Janeczek, M., Pasicka, E., Żak, K., Łukaszewski, M., Jadach, R., & Dobrzyński, M. (2023). The Temporary Mental Nerve Paresthesia as an Outcome of Dentigerous Cyst Removal during Preparation for Dental Implant Placement: A Case Report. Medicina, 59(4), 711. https://doi.org/10.3390/medicina59040711