Research Progress on the Pharmacodynamic Mechanisms of Sini Powder against Depression from the Perspective of the Central Nervous System
Abstract
1. Introduction
2. Sini Powder against Depression
2.1. Advantages of Sini Powder against Depression
2.2. Therapeutic Effects of Sini Powder Herbs against Depression
3. Pharmacodynamic Mechanisms of Active Components of SNP
3.1. Screening of Active Components Crossing the BBB
3.2. Active Components of Zhishi
3.3. Active Components of Baishao
3.4. Active Components of Gancao
4. Pharmacodynamic Pathways of Active Components of SNP
4.1. Acquisition of Potential Targets against Depression
4.2. Enrichment Analyses
5. Discussion and Limitations
6. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekinci, G.N.; Sanlier, N. The relationship between nutrition and depression in the life process: A mini-review. Exp. Gerontol. 2023, 172, 112072. [Google Scholar] [CrossRef]
- Gujral, S.; Aizenstein, H.; Reynolds, C.F., 3rd; Butters, M.A.; Erickson, K.I. Exercise effects on depression: Possible neural mechanisms. Gen. Hosp. Psychiatry 2017, 49, 2–10. [Google Scholar] [CrossRef]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity—Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef]
- Price, R.B.; Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol. Psychiatry 2019, 25, 530–543. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2022, 401, 141–153. [Google Scholar] [CrossRef]
- Reyad, A.A.; Plaha, K.; Girgis, E.; Mishriky, R. Fluoxetine in the Management of Major Depressive Disorder in Children and Adolescents: A Meta-Analysis of Randomized Controlled Trials. Hosp. Pharm. 2020, 56, 525–531. [Google Scholar] [CrossRef]
- Solmi, M.; Fornaro, M.; Ostinelli, E.G.; Zangani, C.; Croatto, G.; Monaco, F.; Krinitski, D.; Fusar-Poli, P.; Correll, C.U. Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: A large scale systematic meta-review of 78 adverse effects. World Psychiatry 2020, 19, 214–232. [Google Scholar] [CrossRef]
- Oliva, V.; Lippi, M.; Paci, R.; Del Fabro, L.; Delvecchio, G.; Brambilla, P.; De Ronchi, D.; Fanelli, G.; Serretti, A. Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110266. [Google Scholar] [CrossRef]
- Boaden, K.; Tomlinson, A.; Cortese, S.; Cipriani, A. Antidepressants in Children and Adolescents: Meta-Review of Efficacy, Tolerability and Suicidality in Acute Treatment. Front. Psychiatry 2020, 11, 717. [Google Scholar] [CrossRef]
- Li, W.; Ali, T.; He, K.; Liu, Z.; Shah, F.A.; Ren, Q.; Liu, Y.; Jiang, A.; Li, S. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain. Behav. Immun. 2021, 92, 10–24. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Casciaro, M.; Vicario, C.M.; Gangemi, S.; Martino, G. Neuro-Inflammaging and Psychopathological Distress. Biomedicines 2022, 10, 2133. [Google Scholar] [CrossRef]
- Lu, A.-P.; Jia, H.-W.; Xiao, C.; Lu, Q.-P. Theory of traditional Chinese medicine and therapeutic method of diseases. World J. Gastroenterol. 2004, 10, 1854–1856. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, Y.; Wang, L.; Yu, Y.; Li, J.; Huang, Y.; Xu, W.; Sun, G.; Dai, W.; Zhao, T.; et al. Antidepressant Active Components of Bupleurum chinense DC-Paeonia lactiflora Pall Herb Pair: Pharmacological Mechanisms. Bio. Med. Res. Int. 2022, 2022, 1024693. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, D.-D.; Tang, T.; Lin, X.-P.; Yang, Z.-Y.; Yang, S.; Xia, Z.-A.; Wang, Y.; Zheng, P.; Zhang, C.-H. Nine traditional Chinese herbal formulas for the treatment of depression: An ethnopharmacology, phytochemistry, and pharmacology review. Neuropsychiatr Dis. Treat 2016, 12, 2387–2402. [Google Scholar] [CrossRef]
- Zhao, H.; Shan, Y.; Ma, Z.; Yu, M.; Gong, B. A network pharmacology approach to explore active compounds and pharmacological mechanisms of epimedium for treatment of premature ovarian insufficiency. Drug Des. Dev. Ther. 2019, 13, 2997–3007. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, V.K.; Purohit, R. Computational targeting of allosteric site of MEK1 by quinoline-based molecules. Cell Biochem. Funct. 2022, 40, 481–490. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Tang, X.-D.; Ma, J.; Mao, M.; Wu, F.-Z.; Bai, S.-J.; Liu, Y.-Y.; Han, C.-X.; Li, X.-X.; et al. Sini powder (四逆散) decoction alleviates mood disorder of insomnia by regulating cation-chloride cotransporters in hippocampus. Chin. J. Integr. Med. 2015. [Google Scholar] [CrossRef]
- He, X.; Zhang, R.; Li, Z.; Yao, Z.; Xie, X.; Bai, R.; Li, L.; Zhang, X.; Zhang, S.; Shen, Y.; et al. Sini powder with paroxetine ameliorates major depressive disorder by modulating circadian rhythm: A randomized, double-blind, placebo-controlled trial. J. Pineal Res. 2022, 73, e12832. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, S.T.; Ji, B.; Liu, F. Clinical Observation on Sini Powder Combined with Gan Mai Dazao Decoction in the Treatment of Type 2 Diabetes Mellitus Complicated with Depression and Anxiety. J. Guangzhou Univ. Tradit. Chin. Med. 2022, 39, 763–769. [Google Scholar] [CrossRef]
- Huang, X.Y. Observation on curative effect of Sini powder on functional dyspepsia with depression. Inn. Mong. J. Tradit. Chin. Med. 2022, 41, 39–40. [Google Scholar] [CrossRef]
- Liang, D.S. Efficacy on Affective Disorder and Dyskinesia Treated with Modified Sini San and Music Therapy in the Patients of Post-Stroke Depression. World J. Integr. West. Tradit. Med. 2016, 11, 1053–1056. [Google Scholar] [CrossRef]
- Wei, S.-S.; Yang, H.-J.; Huang, J.-W.; Lu, X.-P.; Peng, L.-F.; Wang, Q.-G. Traditional herbal formula Sini Powder extract produces antidepressant-like effects through stress-related mechanisms in rats. Chin. J. Nat. Med. 2016, 14, 590–598. [Google Scholar] [CrossRef]
- Pan, J.; Lei, X.; Wang, J.; Huang, S.; Wang, Y.; Zhang, Y.; Chen, W.; Li, D.; Zheng, J.; Cui, H.; et al. Effects of Kaixinjieyu, a Chinese herbal medicine preparation, on neurovascular unit dysfunction in rats with vascular depression. BMC Complement. Altern. Med. 2015, 15, 291. [Google Scholar] [CrossRef]
- Mu, J.; Cheng, F.; Wang, Q.; Wang, X.; Zhu, W.; Ma, C.; Yin, X.; Ren, B.; Lian, Y.; Du, X.; et al. Sini Powder Ameliorates the Inflammatory Response in Rats with Stress-Induced Non-Alcoholic Fatty Liver Disease by Inhibiting the Nuclear Factor Kappa-B / Pyrin Domain-Containing Protein 3 Pathway. J. Tradit. Chin. Med. 2020, 40, 253–266. [Google Scholar]
- Yan, X.; Zhang, Z.; Xu, F.; Li, Y.; Zhu, T.; Ma, C.; Liu, A. Effect of Sini San Freeze-dried powder on sleep-waking cycle in insomnia rats. J. Tradit. Chin. Med. 2014, 34, 572–575. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Qin, X.-M.; Tian, J.-S.; Gao, X.-X.; Du, G.-H.; Zhou, Y.-Z. Integrated network pharmacology and metabolomics to dissect the combination mechanisms of Bupleurum chinense DC-Paeonia lactiflora Pall herb pair for treating depression. J. Ethnopharmacol. 2020, 264, 113281. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Qin, X.; Du, G.; Zhou, Y. Integration of Non-Targeted Metabolomics and Targeted Quantitative Analysis to Elucidate the Synergistic Antidepressant Effect of Bupleurum chinense DC-Paeonia lactiflora Pall Herb Pair by Regulating Purine Metabolism. Front. Pharmacol. 2022, 13, 900459. [Google Scholar] [CrossRef]
- Li, Q.-F.; Lu, W.-T.; Zhang, Q.; Zhao, Y.-D.; Wu, C.-Y.; Zhou, H.-F. Proprietary Medicines Containing Bupleurum chinense DC. (Chaihu) for Depression: Network Meta-Analysis and Network Pharmacology Prediction. Front. Pharmacol. 2022, 13, 773537. [Google Scholar] [CrossRef]
- Xue, B.; Zhao, Q.; Chen, D.; Wang, X.; Li, L.; Li, J.; Tian, J. Network Pharmacology Combined with Molecular Docking and Experimental Verification Reveals the Bioactive Components and Potential Targets of Danlong Dingchuan Decoction against Asthma. Evid. -Based Complement. Altern. Med. 2022, 2022, 7895271. [Google Scholar] [CrossRef]
- Press, B. Permeability for Intestinal Absorption: Caco-2 Assay and Related Issues. Curr. Drug Metab. 2008, 9, 893–900. [Google Scholar] [CrossRef]
- Ge, P.-Y.; Qi, Y.-Y.; Qu, S.-Y.; Zhao, X.; Ni, S.-J.; Yao, Z.-Y.; Guo, R.; Yang, N.-Y.; Zhang, Q.-C.; Zhu, H.-X. Potential Mechanism of S. baicalensis on Lipid Metabolism Explored via Network Pharmacology and Untargeted Lipidomics. Drug Des. Dev. Ther. 2021, 15, 1915–1930. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, Y.; Tan, C.; Sundararajan, B.; Li, Z.; Wang, D.; Zhou, Z. Comparative effects of five polymethoxyflavones purified from Citrus tangerina on inflammation and cancer. Front. Nutr. 2022, 9, 963662. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, R.; Wang, R.; Dai, J.; Chen, H.; Wang, J.; Li, X. Sinensetin attenuates IL-1β-induced cartilage damage and ameliorates osteoarthritis by regulating SERPINA3. Food Funct. 2022, 13, 9973–9987. [Google Scholar] [CrossRef]
- Zhi, Z.; Tang, X.; Wang, Y.; Chen, R.; Ji, H. Sinensetin Attenuates Amyloid Beta25-35-Induced Oxidative Stress, Inflammation, and Apoptosis in SH-SY5Y Cells Through the TLR4/NF-κB Signaling Pathway. Neurochem. Res. 2021, 46, 3012–3024. [Google Scholar] [CrossRef]
- Xu, Y.; Hang, W.-L.; Zhou, X.-M.; Wu, Q. Exploring the Mechanism Whereby Sinensetin Delays the Progression of Pulmonary Fibrosis Based on Network Pharmacology and Pulmonary Fibrosis Models. Front. Pharmacol. 2021, 12, 693061. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Shen, J.; Huang, L.; Men, S.; Wang, T. Sinensetin attenuates oxygen–glucose deprivation/reperfusion-induced neurotoxicity by MAPK pathway in human cerebral microvascular endothelial cells. J. Appl. Toxicol. 2021, 42, 683–693. [Google Scholar] [CrossRef]
- Kang, S.-I.; Shin, H.-S.; Kim, S.-J. Sinensetin Enhances Adipogenesis and Lipolysis by Increasing Cyclic Adenosine Monophosphate Levels in 3T3-L1 Adipocytes. Biol. Pharm. Bull. 2015, 38, 552–558. [Google Scholar] [CrossRef]
- Huang, Q.; Bai, F.; Nie, J.; Lu, S.; Lu, C.; Zhu, X.; Zhuo, L.; Lin, X. Didymin ameliorates hepatic injury through inhibition of MAPK and NF-κB pathways by up-regulating RKIP expression. Int. Immunopharmacol. 2017, 42, 130–138. [Google Scholar] [CrossRef]
- Zhang, Y.; RuXian, G. Didymin, a natural flavonoid, relieves the progression of myocardial infarction via inhibiting the NLR family pyrin domain containing 3 inflammasome. Pharm. Biol. 2022, 60, 2319–2327. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Liu, X. Didymin Alleviates Cerebral Ischemia-Reperfusion Injury by Activating the PPAR Signaling Pathway. Yonsei Med. J. 2022, 63, 956–965. [Google Scholar] [CrossRef]
- Ali, Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Didymin, a dietary citrus flavonoid exhibits anti-diabetic complications and promotes glucose uptake through the activation of PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Chem. Interact. 2019, 305, 180–194. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I. Tetramethoxyluteolin for the Treatment of Neurodegenerative Diseases. Curr. Top. Med. Chem. 2019, 18, 1872–1882. [Google Scholar] [CrossRef]
- Qin, Y.; Song, D.; Liao, S.; Chen, J.; Xu, M.; Su, Y.; Lian, H.; Peng, H.; Wei, L.; Chen, K.; et al. Isosinensetin alleviates estrogen deficiency-induced osteoporosis via suppressing ROS-mediated NF-κB/MAPK signaling pathways. Biomed. Pharmacother. 2023, 160, 114347. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, J.; Cheng, Y.; Dong, X.; Zhang, M.; Pei, C. Palbinone alleviates diabetic retinopathy in STZ-induced rats by inhibiting NLRP3 inflammatory activity. J. Biochem. Mol. Toxicol. 2020, 34, e22489. [Google Scholar] [CrossRef]
- Bi, Y.; Han, X.; Lai, Y.; Fu, Y.; Li, K.; Zhang, W.; Wang, Q.; Jiang, X.; Zhou, Y.; Liang, H.; et al. Systems pharmacological study based on UHPLC-Q-Orbitrap-HRMS, network pharmacology and experimental validation to explore the potential mechanisms of Danggui-Shaoyao-San against atherosclerosis. J. Ethnopharmacol. 2021, 278, 114278. [Google Scholar] [CrossRef]
- Hu, P.-F.; Chen, W.-P.; Bao, J.-P.; Wu, L.-D. Paeoniflorin inhibits IL-1β-induced chondrocyte apoptosis by regulating the Bax/Bcl-2/caspase-3 signaling pathway. Mol. Med. Rep. 2018, 17, 6194–6200. [Google Scholar] [CrossRef]
- Lu, R.; Zhou, J.; Liu, B.; Liang, N.; He, Y.; Bai, L.; Zhang, P.; Zhong, Y.; Zhou, Y.; Zhou, J. Paeoniflorin ameliorates Adriamycin-induced nephrotic syndrome through the PPARγ/ANGPTL4 pathway in vivo and vitro. Biomed. Pharmacother. 2017, 96, 137–147. [Google Scholar] [CrossRef]
- Zhan, Y.; Wen, Y.; Zhang, L.-L.; Shen, X.-L.; Chen, X.-H.; Wu, X.-H.; Tang, X.-G. Paeoniflorin Improved Constipation in the Loperamide-Induced Rat Model via TGR5/TRPA1 Signaling-Mediated 5-Hydroxytryptamine Secretion. Evid. -Based Complement. Altern. Med. 2021, 2021, 6076293. [Google Scholar] [CrossRef]
- Qiu, Z.-K.; He, J.-L.; Liu, X.; Zeng, J.; Xiao, W.; Fan, Q.-H.; Chai, X.-M.; Ye, W.-H.; Chen, J.-S. Anxiolytic-like effects of paeoniflorin in an animal model of post traumatic stress disorder. Metab. Brain Dis. 2018, 33, 1175–1185. [Google Scholar] [CrossRef]
- Li, Y.C.; Zheng, X.X.; Xia, S.Z.; Li, Y.; Deng, H.H.; Wang, X.; Chen, Y.W.; Yue, Y.S.; He, J.; Cao, Y.J. Paeoniflorin ameliorates depressive-like behavior in prenatally stressed offspring by restoring the HPA axis- and glucocorticoid receptor- associated dysfunction. J. Affect. Disord. 2020, 274, 471–481. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Zhu, Y.; He, C.; Fei, W.; Yue, N.; Wang, C.; Wang, L. Study of Antidepressant-Like Effects of Albiflorin and Paeoniflorin Through Metabolomics From the Perspective of Cancer-Related Depression. Front. Neurol. 2022, 13, 828612. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, X.; Zhu, Y.; Zhao, Y.; Wang, J.; Li, R.; Chen, C.; Wei, S.; Jiao, W.; Zhang, Y.; et al. Paeoniflorin ameliorates ANIT-induced cholestasis by activating Nrf2 through an PI3K/Akt-dependent pathway in rats. Phytother. Res. 2015, 29, 1768–1775. [Google Scholar] [CrossRef]
- Ha, D.T.; Phuong, T.T.; Oh, J.; Bae, K.; Thuan, N.D.; Na, M. Palbinone from Paeonia suffruticosa Protects Hepatic Cells via Up-regulation of Heme Oxygenase-1. Phytother. Res. 2013, 28, 308–311. [Google Scholar] [CrossRef]
- Liu, X.; Chen, K.; Zhuang, Y.; Huang, Y.; Sui, Y.; Zhang, Y.; Lv, L.; Zhang, G. Paeoniflorin improves pressure overload-induced cardiac remodeling by modulating the MAPK signaling pathway in spontaneously hypertensive rats. Biomed. Pharmacother. 2019, 111, 695–704. [Google Scholar] [CrossRef]
- Yu, M.; Wu, X.; Wang, J.; He, M.; Han, H.; Hu, S.; Xu, J.; Yang, M.; Tan, Q.; Wang, Y.; et al. Paeoniflorin attenuates monocrotaline-induced pulmonary arterial hypertension in rats by suppressing TAK1-MAPK/NF-κB pathways. Int. J. Med. Sci. 2022, 19, 681–694. [Google Scholar] [CrossRef]
- Bai, Y.; He, Z.; Duan, W.; Gu, H.; Wu, K.; Yuan, W.; Liu, W.; Huang, H.; Li, Y. Sodium formononetin-3’-sulphonate alleviates cerebral ischemia–reperfusion injury in rats via suppressing endoplasmic reticulum stress-mediated apoptosis. BMC Neurosci. 2022, 23, 74. [Google Scholar] [CrossRef]
- Jia, C.; Hu, F.; Lu, D.; Jin, H.; Lu, H.; Xue, E.; Wu, D. Formononetin inhibits IL-1β-induced inflammation in human chondrocytes and slows the progression of osteoarthritis in rat model via the regulation of PTEN/AKT/NF-κB pathway. Int. Immunopharmacol. 2022, 113, 109309. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Huang, L.-H.; Cao, Y.-X. Antihypertensive effect of formononetin through regulating the expressions of eNOS, 5-HT2A/1B receptors and α1-adrenoceptors in spontaneously rat arteries. Eur. J. Pharmacol. 2013, 699, 241–249. [Google Scholar] [CrossRef]
- Li, T.; Zhong, Y.; Tang, T.; Luo, J.; Cui, H.; Fan, R.; Wang, Y.; Wang, D. Formononetin induces vasorelaxation in rat thoracic aorta via regulation of the PI3K/PTEN/Akt signaling pathway. Drug Des. Dev. Ther. 2018, 12, 3675–3684. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhang, L.; Xia, Z.-Y.; Chen, J.-Y.; Fang, Y.; Ding, Y.-Q. PTEN in prefrontal cortex is essential in regulating depression-like behaviors in mice. Transl. Psychiatry 2021, 11, 185. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, T.; Zhang, H.; Li, X.; Shi, S.; Tian, X.; Huang, Z.; Zhang, R.; Liu, Z.; Cheng, Y. Formononetin improves cardiac function and depressive behaviours in myocardial infarction with depression by targeting GSK-3β to regulate macrophage/microglial polarization. Phytomedicine 2023, 109, 154602. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ko, R.; Goshima, H.; Koike, A.; Shibano, M.; Fujimori, K. Formononetin attenuates H2O2-induced cell death through decreasing ROS level by PI3K/Akt-Nrf2-activated antioxidant gene expression and suppressing MAPK-regulated apoptosis in neuronal SH-SY5Y cells. Neurotoxicology 2021, 85, 186–200. [Google Scholar] [CrossRef]
- Li, P.; Yu, C.; Zeng, F.-S.; Fu, X.; Yuan, X.-J.; Wang, Q.; Fan, C.; Sun, B.-L.; Sun, Q.-S. Licochalcone A Attenuates Chronic Neuropathic Pain in Rats by Inhibiting Microglia Activation and Inflammation. Neurochem. Res. 2021, 46, 1112–1118. [Google Scholar] [CrossRef]
- Chu, X.; Jiang, L.; Wei, M.; Yang, X.; Guan, M.; Xie, X.; Wei, J.; Liu, D.; Wang, D. Attenuation of allergic airway inflammation in a murine model of asthma by Licochalcone A. Immunopharmacol. Immunotoxicol. 2013, 35, 653–661. [Google Scholar] [CrossRef]
- Yang, G.; Lee, H.E.; Yeon, S.H.; Kang, H.C.; Cho, Y.-Y.; Zouboulis, C.C.; Han, S.-H.; Lee, J.-H.; Lee, J.Y. Licochalcone A attenuates acne symptoms mediated by suppression of NLRP3 inflammasome. Phytother. Res. 2018, 32, 2551–2559. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, M.; Jin, L.; Yang, B.; Bai, B.; Mutsinze, R.N.; Zuo, W.; Chattipakorn, N.; Huh, J.Y.; Liang, G.; et al. Licochalcone A protects against LPS-induced inflammation and acute lung injury by directly binding with myeloid differentiation factor 2 (MD2). Br. J. Pharmacol. 2023, 180, 1114–1131. [Google Scholar] [CrossRef]
- Herbrechter, R.; Ziemba, P.M.; Hoffmann, K.M.; Hatt, H.; Werner, M.; Gisselmann, G. Identification of Glycyrrhiza as the rikkunshito constituent with the highest antagonistic potential on heterologously expressed 5-HT3A receptors due to the action of flavonoids. Front. Pharmacol. 2015, 6, 130. [Google Scholar] [CrossRef]
- Huang, W.-C.; Su, H.-H.; Fang, L.-W.; Wu, S.-J.; Liou, C.-J. Licochalcone A Inhibits Cellular Motility by Suppressing E-cadherin and MAPK Signaling in Breast Cancer. Cells 2019, 8, 218. [Google Scholar] [CrossRef]
- Guo, W.; Liu, B.; Yin, Y.; Kan, X.; Gong, Q.; Li, Y.; Cao, Y.; Wang, J.; Xu, D.; Ma, H.; et al. Licochalcone A Protects the Blood–Milk Barrier Integrity and Relieves the Inflammatory Response in LPS-Induced Mastitis. Front. Immunol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Tian, M.; Li, N.; Liu, R.; Li, K.; Du, J.; Zou, D.; Ma, Y. The protective effect of licochalcone A against inflammation injury of primary dairy cow claw dermal cells induced by lipopolysaccharide. Sci. Rep. 2022, 12, 1593. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P. Vestitol drives LPS-activated macrophages into M2 phenotype through modulation of NF-κB pathway. Int. Immunopharmacol. 2020, 82, 106329. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Wei, H.; Liang, Z. Maackiain Protects the Kidneys of Type 2 Diabetic Rats via Modulating the Nrf2/HO-1 and TLR4/NF-κB/Caspase-3 Pathways. Drug Des. Dev. Ther. 2021, 15, 4339–4358. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, J.; Li, H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-κB and NLRP3 inflammasome pathways. Biomed. Pharmacother. 2018, 106, 976–982. [Google Scholar] [CrossRef]
- Tao, W.; Dong, Y.; Su, Q.; Wang, H.; Chen, Y.; Xue, W.; Chen, C.; Xia, B.; Duan, J.; Chen, G. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 2016, 308, 177–186. [Google Scholar] [CrossRef]
- Su, Q.; Tao, W.; Huang, H.; Du, Y.; Chu, X.; Chen, G. Protective effect of liquiritigenin on depressive-like behavior in mice after lipopolysaccharide administration. Psychiatry Res. 2016, 240, 131–136. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, Y.; Zheng, B.; Li, L.; Chu, X.; Zhao, Y.; Wu, Y.; Zhang, J.; Han, X.; Wu, Z.; et al. Liquiritigenin Protects against Arsenic Trioxide-Induced Liver Injury by Inhibiting Oxidative Stress and Enhancing MTOR-Mediated Autophagy. Biomed. Pharmacother. 2021, 143, 112167. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Zhang, J.; Zhou, Q.; Liu, X.; Zhou, G. Medicarpin Improves Depressive-Like Behaviors in a Chronic Unpredictable Mild Stress-Induced Mouse Model of Depression by Upregulating Liver X Receptor β Expression in the Amygdala. Neurotox. Res. 2022, 40, 1937–1947. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Yan, F.; Jin, Y.; Liu, X.; Wang, T. Medicarpin Protects Cerebral Microvascular Endothelial Cells Against Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury via the PI3K/Akt/FoxO Pathway: A Study of Network Pharmacology Analysis and Experimental Validation. Neurochem. Res. 2021, 47, 347–357. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhiza glabra roots and their PPAR-γ ligand-binding activity. Bioorganic Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef]
- Dogra, A.; Gupta, D.; Bag, S.; Ahmed, I.; Bhatt, S.; Nehra, E.; Dhiman, S.; Kumar, A.; Singh, G.; Abdullah, S.T.; et al. Glabridin ameliorates methotrexate-induced liver injury via attenuation of oxidative stress, inflammation, and apoptosis. Life Sci. 2021, 278, 119583. [Google Scholar] [CrossRef]
- Gao, H.; Peng, X.; Zhan, L.; Lin, J.; Zhang, Y.; Huan, Y.; Zhao, G. The role of Glabridin in antifungal and anti-inflammation effects in Aspergillus fumigatus keratitis. Exp. Eye Res. 2021, 214, 108883. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Zhong, B.; Liao, Q.; Wang, X.; Xie, Y.; He, X. Review on the Diverse Biological Effects of Glabridin. Drug Des. Dev. Ther. 2023, 17, 15–37. [Google Scholar] [CrossRef]
- Chung, C.-L.; Chen, J.-H.; Huang, W.-C.; Sheu, J.-R.; Hsia, C.-W.; Jayakumar, T.; Hsia, C.-H.; Chiou, K.-R.; Hou, S.-M. Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice. Int. J. Mol. Sci. 2022, 23, 11372. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Gu, J.; Xu, W.; Yuan, N.; Sun, J.; Li, H. Glabridin inhibits liver fibrosis and hepatic stellate cells activation through suppression of inflammation and oxidative stress by activating PPARγ in carbon tetrachloride-treated mice. Int. Immunopharmacol. 2022, 113, 109433. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, K.; Hu, S.; Lan, G.; Gan, X.; Gao, S.; Deng, Y.; Hu, J.; Li, L.; Hu, B.; et al. Integrated Transcriptome and Metabolome Analysis Reveals the Regulatory Mechanisms of FASN in Geese Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 14717. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Hsiang, C.-Y.; Ho, S.-C.; Ho, T.-Y.; Lee, K.-T. Novel serotonin-boosting effect of incense smoke from Kynam agarwood in mice: The involvement of multiple neuroactive pathways. J. Ethnopharmacol. 2021, 275, 114069. [Google Scholar] [CrossRef]
- Yao, W.; Cao, Q.; Luo, S.; He, L.; Yang, C.; Chen, J.; Qi, Q.; Hashimoto, K.; Zhang, J.-C. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol. Psychiatry 2021, 27, 1618–1629. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, D.; Zhou, Q.; Wei, G.; Song, J.; Liang, Y.; Du, G. Baicalein alleviates depression-like behavior in rotenone-induced Parkinson’s disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed. Pharmacother. 2021, 140, 111556. [Google Scholar] [CrossRef]
- Bazovkina, D.; Naumenko, V.; Bazhenova, E.; Kondaurova, E. Effect of Central Administration of Brain-Derived Neurotrophic Factor (BDNF) on Behavior and Brain Monoamine Metabolism in New Recombinant Mouse Lines Differing by 5-HT1A Receptor Functionality. Int. J. Mol. Sci. 2021, 22, 11987. [Google Scholar] [CrossRef]
- Gold, P.W. The PPARg System in Major Depression: Pathophysiologic and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 9248. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, Y.-J.; Wang, H.; Song, L.; Huang, C.; Zhu, Q.; Wu, F.; Zhang, W. Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br. J. Pharmacol. 2016, 174, 177–194. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Gao, S.-F.; Bao, A.-M. Corticotropin-Releasing Hormone, Glutamate, and γ-Aminobutyric Acid in Depression. Neuroscientist 2010, 17, 124–144. [Google Scholar] [CrossRef]
- Choi, K.W.; Na, E.J.; Fava, M.; Mischoulon, D.; Cho, H.; Jeon, H.J. Increased adrenocorticotropic hormone (ACTH) levels predict severity of depression after six months of follow-up in outpatients with major depressive disorder. Psychiatry Res. 2018, 270, 246–252. [Google Scholar] [CrossRef]
- Song, J.; Zhou, N.; Ma, W.; Gu, X.; Chen, B.; Zeng, Y.; Yang, L.; Zhou, M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019, 10, 2947–2957. [Google Scholar] [CrossRef]
- Xie, X.; Shen, Q.; Ma, L.; Chen, Y.; Zhao, B.; Fu, Z. Chronic corticosterone-induced depression mediates premature aging in rats. J. Affect. Disord. 2018, 229, 254–261. [Google Scholar] [CrossRef]
- Amigo, J.; Garro-Martinez, E.; Casado, R.V.; Compan, V.; Pilar-Cuéllar, F.; Pazos, A.; Díaz, A.; Castro, E. 5-HT4 Receptors Are Not Involved in the Effects of Fluoxetine in the Corticosterone Model of Depression. ACS Chem. Neurosci. 2021, 12, 2036–2044. [Google Scholar] [CrossRef]
- Gold, P.W.; Licinio, J.; Wong, M.-L.; Chrousos, G.P. Corticotropin Releasing Hormone in the Pathophysiology of Melancholic and Atypical Depression and in the Mechanism of Action of Antidepressant Drugs. Ann. New York Acad. Sci. 1995, 771, 716–729. [Google Scholar] [CrossRef]
- Pitchot, W.; Herrera, C.; Ansseau, M. HPA Axis Dysfunction in Major Depression: Relationship to 5-HT1A Receptor Activity. Neuropsychobiology 2001, 44, 74–77. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, J.; Cao, Z.; Zhao, J.; Zhang, J.; Lu, Y.; Chu, L.; Zhang, S.; Chen, Y.; Pei, L. Baicalin ameliorates chronic unpredictable mild stress-induced depression through the BDNF/ERK/CREB signaling pathway. Behav. Brain Res. 2021, 414, 113463. [Google Scholar] [CrossRef]
- Wang, J.Q.; Mao, L. The ERK Pathway: Molecular Mechanisms and Treatment of Depression. Mol. Neurobiol. 2019, 56, 6197–6205. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Nie, Y.; Wei, W.; Xiong, W. proBDNF expression induces apoptosis and inhibits synaptic regeneration by regulating the RhoA-JNK pathway in an in vitro post-stroke depression model. Transl. Psychiatry 2021, 11, 578. [Google Scholar] [CrossRef]
- El Rawas, R.; Amaral, I.M.; Hofer, A. Is p38 MAPK Associated to Drugs of Abuse-Induced Abnormal Behaviors? Int. J. Mol. Sci. 2020, 21, 4833. [Google Scholar] [CrossRef]
- Lucassen, P.; Heine, V.; Muller, M.; van der Beek, E.; Wiegant, V.; De Kloet, E.R.; Joels, M.; Fuchs, E.; Swaab, D.; Czeh, B. Stress, Depression and Hippocampal Apoptosis. CNS Neurol. Disord. Drug Targets 2006, 5, 531–546. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef]
- Patrício, P.; Mateus-Pinheiro, A.; Machado-Santos, A.R.; Alves, N.D.; Correia, J.S.; Morais, M.; Bessa, J.M.; Rodrigues, A.J.; Sousa, N.; Pinto, L. Cell Cycle Regulation of Hippocampal Progenitor Cells in Experimental Models of Depression and after Treatment with Fluoxetine. Int. J. Mol. Sci. 2021, 22, 11798. [Google Scholar] [CrossRef]
- Fronza, M.G.; Baldinotti, R.; Fetter, J.; Rosa, S.G.; Sacramento, M.; Nogueira, C.W.; Alves, D.; Praticò, D.; Savegnago, L. Beneficial effects of QTC-4-MeOBnE in an LPS-induced mouse model of depression and cognitive impairments: The role of blood-brain barrier permeability, NF-κB signaling, and microglial activation. Brain. Behav. Immun. 2021, 99, 177–191. [Google Scholar] [CrossRef]
- Cao, P.; Chen, C.; Liu, A.; Shan, Q.; Zhu, X.; Jia, C.; Peng, X.; Zhang, M.; Farzinpour, Z.; Zhou, W.; et al. Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron 2021, 109, 2573–2589. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Wu, Z.; Yu, X.; Yin, Y.; Qian, S.; Wang, Z.; Huang, J.; Wang, W.; Liu, T.; et al. Quercitrin Rapidly Alleviated Depression-like Behaviors in Lipopolysaccharide-Treated Mice: The Involvement of PI3K/AKT/NF-κB Signaling Suppression and CREB/BDNF Signaling Restoration in the Hippocampus. ACS Chem. Neurosci. 2021, 12, 3387–3396. [Google Scholar] [CrossRef]
- Guo, L.-T.; Wang, S.-Q.; Su, J.; Xu, L.-X.; Ji, Z.-Y.; Zhang, R.-Y.; Zhao, Q.-W.; Ma, Z.-Q.; Deng, X.-Y.; Ma, S.-P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflamm. 2019, 16, 95. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Shen, C.-L.; Wang, X.-Y.; Xiong, W.-F.; Shang, X.; Tang, L.-Y.; Zhang, H.-X.; Wan, Y.-H.; Wu, Y.-B.; Fei, J.; et al. Increased Anxiety-like Behaviors in Adgra1−/− Male But Not Female Mice are Attributable to Elevated Neuron Dendrite Density, Upregulated PSD95 Expression, and Abnormal Activation of the PI3K/AKT/GSK-3β and MEK/ERK Pathways. Neuroscience 2022, 503, 131–145. [Google Scholar] [CrossRef]
- Cao, Q.; Zou, L.; Fan, Z.; Yan, Y.; Qi, C.; Wu, B.; Song, B. Ozone causes depressive-like response through PI3K/Akt/GSK3β pathway modulating synaptic plasticity in young rats. Ecotoxicol. Environ. Saf. 2022, 246, 114171. [Google Scholar] [CrossRef]
- Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7682. [Google Scholar] [CrossRef]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef]
| Herb | MOL ID | Active Component |
|---|---|---|
| CH | MOL004644 | Sainfuran |
| MOL013187 | Cubebin | |
| ZS | MOL001803 | Sinensetin |
| MOL001941 | Ammidin | |
| MOL005849 | Didymin | |
| MOL007879 | Tetramethoxyluteolin | |
| MOL013277 | Isosinensetin | |
| MOL013279 | 5,7,4’-Trimethylapigenin | |
| MOL013430 | Prangenin | |
| MOL013435 | Poncimarin | |
| MOL013436 | Isoponcimarin | |
| MOL013437 | 6-Methoxy aurapten | |
| BS | MOL001919 | Palbinone |
| MOL001925 | Paeoniflorin | |
| GC | MOL000392 | Formononetin |
| MOL000497 | Licochalcone a | |
| MOL000500 | Vestitol | |
| MOL001484 | Maackiain | |
| MOL001792 | Liquiritigenin | |
| MOL002565 | Medicarpin | |
| MOL003896 | 7-Methoxy-2-methyl isoflavone | |
| MOL004815 | Kanzonol B | |
| MOL004833 | Phaseolinisoflavan | |
| MOL004835 | Glypallichalcone | |
| MOL004838 | Kanzonol U | |
| MOL004891 | Shinpterocarpin | |
| MOL004908 | Glabridin | |
| MOL004910 | Glabranin | |
| MOL004911 | Glabrene | |
| MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one | |
| MOL004945 | Isobavachin | |
| MOL004957 | Isoformononetin | |
| MOL004959 | 1-Methoxyphaseollidin | |
| MOL004966 | 3’-Hydroxy-4’-O-Methylglabridin | |
| MOL004974 | 3’-Methoxyglabridin | |
| MOL004978 | 4’-Methoxyglabridin | |
| MOL004980 | Inflacoumarin A | |
| MOL004988 | Kanzonol F | |
| MOL004991 | 7-Acetoxy-2-methylisoflavone |
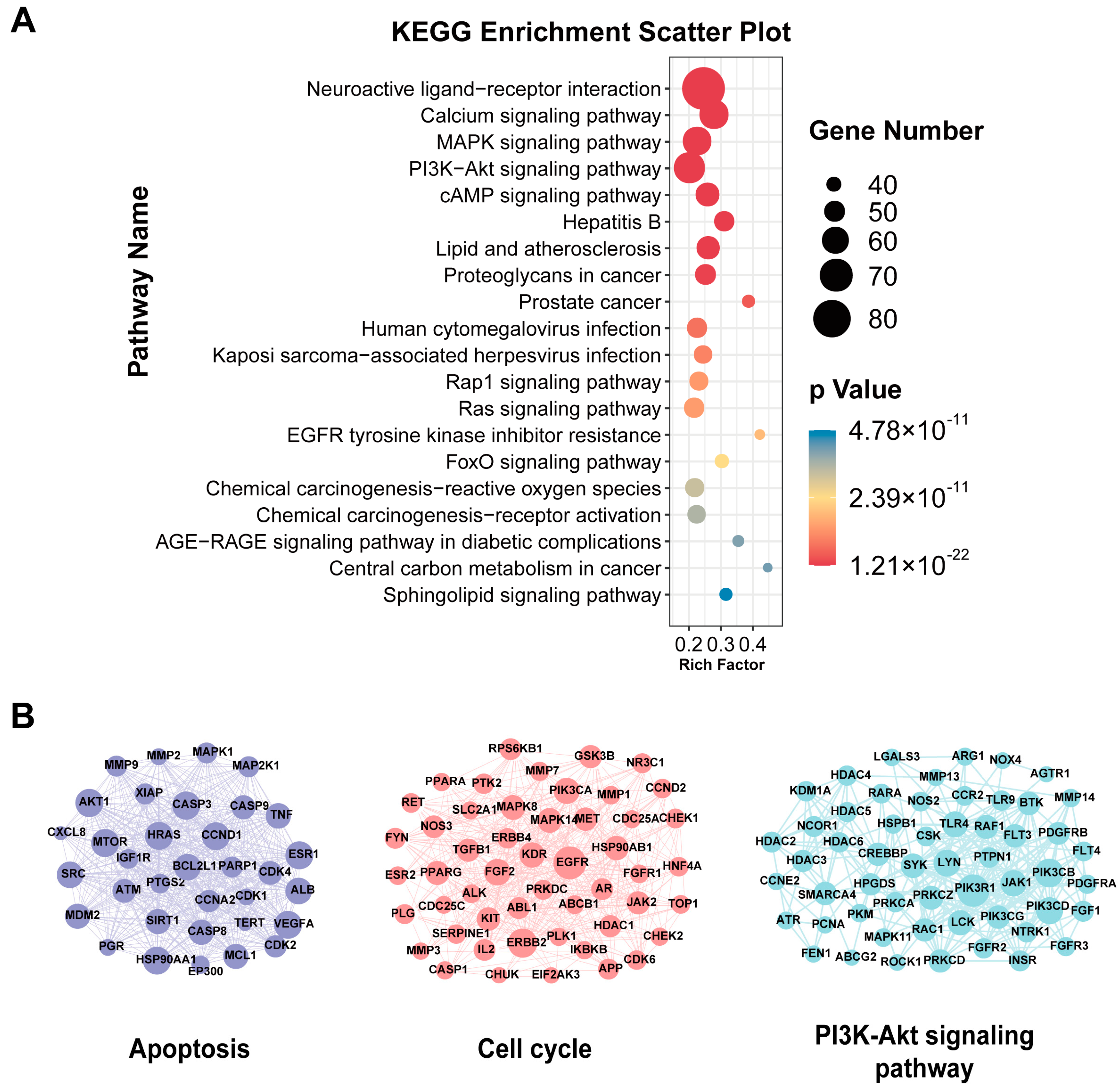
| Cluster | Pathway Description | Number of Targets | Number of Edges | Score |
|---|---|---|---|---|
| 1 | Apoptosis | 34 | 502 | 30.424 |
| 2 | Cell cycle | 51 | 415 | 16.600 |
| 3 | PI3K-Akt signaling pathway | 55 | 283 | 10.481 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Yu, M.; Dong, Z. Research Progress on the Pharmacodynamic Mechanisms of Sini Powder against Depression from the Perspective of the Central Nervous System. Medicina 2023, 59, 741. https://doi.org/10.3390/medicina59040741
Shen Z, Yu M, Dong Z. Research Progress on the Pharmacodynamic Mechanisms of Sini Powder against Depression from the Perspective of the Central Nervous System. Medicina. 2023; 59(4):741. https://doi.org/10.3390/medicina59040741
Chicago/Turabian StyleShen, Zhongqi, Meng Yu, and Zhenfei Dong. 2023. "Research Progress on the Pharmacodynamic Mechanisms of Sini Powder against Depression from the Perspective of the Central Nervous System" Medicina 59, no. 4: 741. https://doi.org/10.3390/medicina59040741
APA StyleShen, Z., Yu, M., & Dong, Z. (2023). Research Progress on the Pharmacodynamic Mechanisms of Sini Powder against Depression from the Perspective of the Central Nervous System. Medicina, 59(4), 741. https://doi.org/10.3390/medicina59040741







