Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Harms or Unexpected Benefits?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
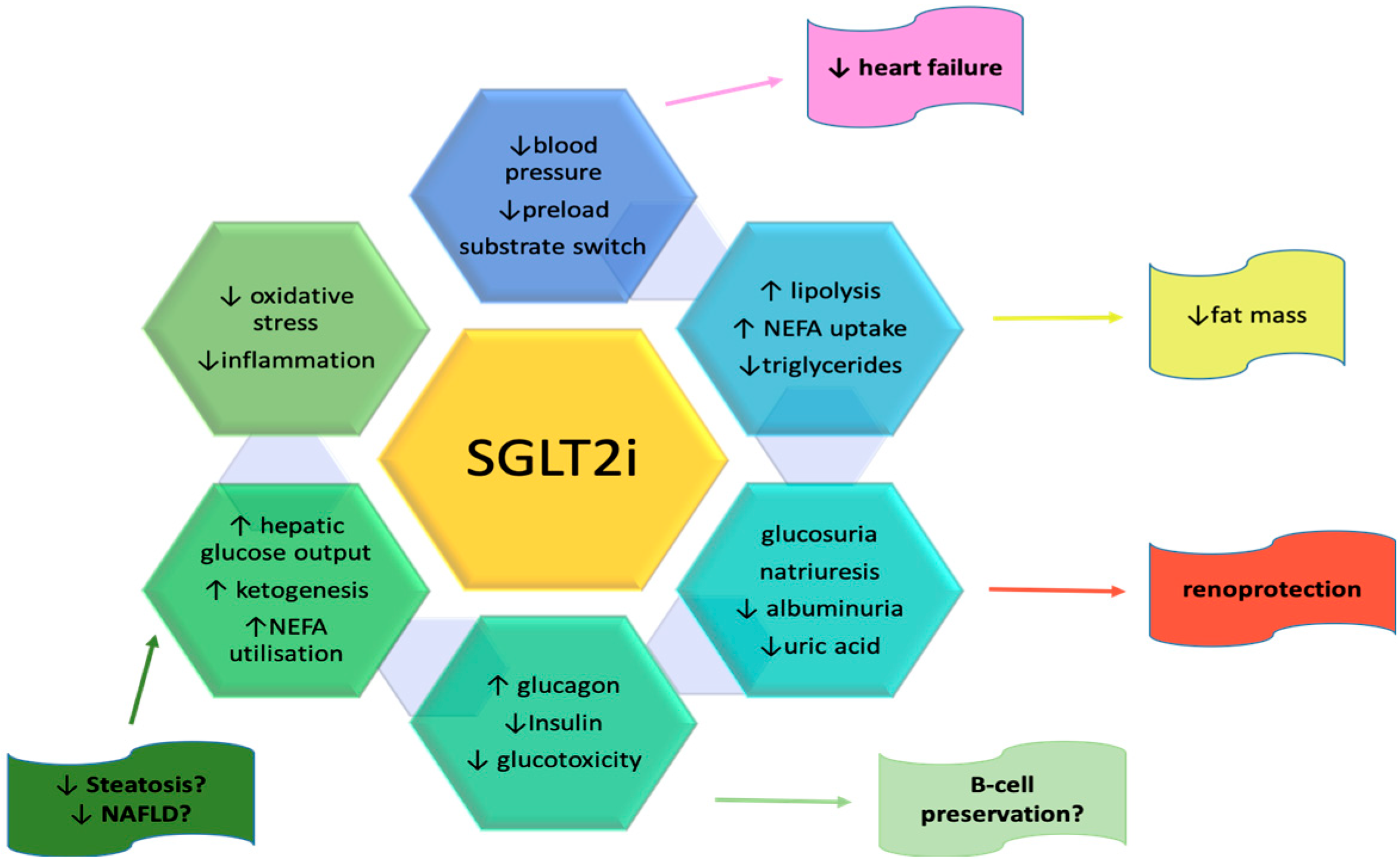
3.1. Benefits and Risks of SGLT2 Inhibitors on the Renal System
3.2. Benefits and Risks of SGLT2 Inhibitors on the Hepatic System
3.3. Benefits and Risks of SGLT2 Inhibitors on the Pancreas
3.4. Benefits and Risks of SGLT2 Inhibitors on the Central Nervous System
3.5. Benefits and Risks of SGLT2 Inhibitors on the Pulmonary System
3.6. Benefits and Risks of SGLT2 Inhibitors on the Cardiovascular System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidson, J.A.; Kuritzky, L. Sodium glucose co-transporter 2 inhibitors and their mechanism for improving glycemia in patients with type 2 diabetes. Postgrad. Med. 2014, 126, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium-glucose cotransporter inhibitors and oxidative stress: An update. J. Cell. Physiol. 2018, 234, 3231–3237. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Platt, K.A.; Cunard, R.; Schroth, J.; Whaley, J.; Thomson, S.C.; Koepsell, H.; Rieg, T. SGLT2 Mediates Glucose Reabsorption in the Early Proximal Tubule. J. Am. Soc. Nephrol. 2011, 22, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 4901. [Google Scholar] [PubMed]
- Martínez, M.S.; Manzano, A.; Olivar, L.C.; Nava, M.; Salazar, J.; D’Marco, L.; Ortiz, R.; Chacín, M.; Guerrero-Wyss, M.; Cabrera de Bravo, M.; et al. The Role of the α Cell in the Pathogenesis of Diabetes: A World beyond the Mirror. Int. J. Mol. Sci. 2021, 22, 9504. [Google Scholar] [CrossRef]
- Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.-T.; Kałużna-Oleksy, M.; Lesiak, M.; Straburzyńska-Migaj, E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review. J. Clin. Med. 2022, 11, 1470. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Swedberg, K.; Carlsson, J.; McMurray, J.J.V.; Michelson, E.L.; Olofsson, B.; Pfeffer, M.A.; Yusuf, S. The Hemoglobin A1c Level as a Progressive Risk Factor for Cardiovascular Death, Hospitalization for Heart Failure, or Death in Patients with Chronic Heart Failure: An Analysis of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Arch. Intern. Med. 2008, 168, 1699–1704. [Google Scholar]
- Lippi, G.; Sanchis-Gomar, F. Global epidemiology and future trends of heart failure. AME Med. J. 2020, 5, 15. [Google Scholar] [CrossRef]
- Cho, Y.K.; Kang, Y.M.; Lee, S.E.; Lee, J.; Park, J.-Y.; Lee, W.J.; Kim, Y.-J.; Jung, C.H. Efficacy and safety of combination therapy with SGLT2 and DPP4 inhibitors in treating type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2018, 44, 393–401. [Google Scholar] [CrossRef]
- Winiarska, A.; Knysak, M.; Nabrdalik, K.; Gumprecht, J.; Stompór, T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int. J. Mol. Sci. 2021, 22, 10822. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef] [Green Version]
- Saffo, S.; Taddei, T. SGLT2 inhibitors and cirrhosis: A unique perspective on the comanagement of diabetes mellitus and ascites: SGLT2 Inhibitors and Cirrhosis. Clin. Liver Dis. 2018, 11, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Hakoshima, M.; Adachi, H.; Katsuyama, H. Multi-Organ Protective Effects of Sodium Glucose Cotransporter 2 Inhibitors. Int. J. Mol. Sci. 2021, 22, 4416. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Liberti, M.; Andreucci, M.; Conte, G.; Minutolo, R.; Andreucci, M.; Conte, G.; Minutolo, R.; Provenzano, M.; et al. SGLT2 Inhibitors: Nephroprotective Efficacy and Side Effects. Medicina 2019, 55, 268. [Google Scholar] [CrossRef] [Green Version]
- Ujjawal, A.; Schreiber, B.; Verma, A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: What is the evidence? Ther. Adv. Endocrinol. 2022, 13, 204201882210900. [Google Scholar] [CrossRef]
- Lin, H.-J.; Chuang, H.-N.; Jhan, P.-P.; Ye, H.-Y.; Lee, I.-T.; Hsiao, T.-H.; Liu, P.-Y. Pyuria Is Associated with Dysbiosis of the Urinary Microbiota in Type 2 Diabetes Patients Receiving Sodium–Glucose Cotransporter 2 Inhibitors. Microbiol. Res. 2023, 14, 34–41. [Google Scholar] [CrossRef]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Hansen, L.; Griffen, S.C.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 864–876. [Google Scholar] [CrossRef]
- Dwinata, M.; Putera, D.; Hasan, I.; Raharjo, M. SGLT2 inhibitors for improving hepatic fibrosis and steatosis in non-alcoholic fatty liver disease complicated with type 2 diabetes mellitus: A systematic review. Clin. Exp. Hepatol. 2020, 6, 339–346. [Google Scholar] [CrossRef]
- Kinoshita, T.; Shimoda, M.; Sanada, J.; Fushimi, Y.; Hirata, Y.; Irie, S.; Obata, A.; Kimura, T.; Hirukawa, H.; Kohara, K.; et al. There is a Close Association Between the Recovery of Liver Injury and Glycemic Control after SGLT2 Inhibitor Treatment in Japanese Subjects with Type 2 Diabetes: A Retrospective Clinical Study. Diabetes Ther. 2018, 9, 1569–1580. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Repetto, E.; Guja, C.; Hardy, E.; Han, J.; Jabbour, S.A.; Ferrannini, E. Exenatide and dapagliflozin combination improves markers of liver steatosis and fibrosis in patients with type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Tan, Y.; Hao, Z.; Xu, W.; Zhou, X.; Yu, J. Effects of SGLT2 inhibitors on hepatic fibrosis and steatosis: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1144838. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Friedman, S.L.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Lazo-Del Vallin, S.; Diago, M.; Adams, L.A. Serum biomarkers can predict a change in liver fibrosis 1 year after lifestyle intervention for biopsy-proven NASH. Liver Int. 2017, 37, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Latva-Rasku, A.; Honka, M.-J.; Kullberg, J.; Mononen, N.; Lehtimäki, T.; Saltevo, J.; Kirjavainen, A.K.; Saunavaara, V.; Iozzo, P.; Johansson, L.; et al. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care 2019, 42, 931–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaribeygi, H.; Maleki, M.; Butler, A.E.; Jamialahamdi, T.; Sahebkar, A. Sodium-glucose cotransporter 2 inhibitors and mitochondrial functions: State of the art. EXCLI J. 2023, 22, 53–66. [Google Scholar]
- Saponaro, C.; Pattou, F.; Bonner, C. SGLT2 inhibition and glucagon secretion in humans. Diabetes Metab. 2018, 44, 383–385. [Google Scholar] [CrossRef]
- Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Queniat, G.; Moerman, E.; Thévenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; Malaisse, W.J.; et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 2015, 21, 512–517. [Google Scholar] [CrossRef]
- Kern, M.; Klöting, N.; Mark, M.; Mayoux, E.; Klein, T.; Blüher, M. The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice as monotherapy and in combination with linagliptin. Metabolism 2016, 65, 114–123. [Google Scholar] [CrossRef]
- Tharmaraja, T.; Ho, J.S.Y.; Sia, C.-H.; Lim, N.-A.; Chong, Y.F.; Lim, A.Y.L.; Rathakrishnan, R.R.; Yeo, L.L.L.; Sharma, V.K.; Tan, B.Y.Q. Sodium-glucose cotransporter 2 inhibitors and neurological disorders: A scoping review. Ther. Adv. Chronic Dis. 2022, 13, 204062232210869. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J. Pharmacol. Sci. 2016, 130, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Poppe, R.; Karbach, U.; Gambaryan, S.; Lutzenburg, M.; Kraemer, M.; Witte, O.W.; Koepsell, H. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J. Neurochem. 1997, 69, 84–94. [Google Scholar] [CrossRef]
- Nguyen, T.; Wen, S.; Gong, M.; Yuan, X.; Xu, D.; Wang, C.; Jin, J.; Zhou, L. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting sglt-2 in mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2781–2799. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 diabetes and its role implications in alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Sa-nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharmacol. 2017, 333, 43–50. [Google Scholar] [CrossRef]
- Hierro-bujalance, C.; Infante-garcia, C.; del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimer’s Res. Ther. 2020, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, M.A.; Yusuf, D.; Christy, J.; Solmaz, V.; Erdogan, A.; Taskiran, E.; Erbas, O. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. 2018, 18, 81. [Google Scholar] [CrossRef] [Green Version]
- Hamed, S.A. Atherosclerosis in epilepsy: Its causes and implications. Epilepsy Behav. 2014, 41, 290–296. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Al Hamed, F.A.; Elewa, H. Potential Therapeutic Effects of Sodium Glucose-linked Cotransporter 2 Inhibitors in Stroke. Clin. Ther. 2020, 42, e242–e249. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Empagliflozin Ameliorates Type 2 Diabetes-Induced Ultrastructural Remodeling of the Neurovascular Unit and Neuroglia in the Female db/db Mouse. Brain Sci. 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bdel-Latif, R.G.; Rifaai, R.A.; Amin, E.F. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch. Pharm. Res. 2020, 43, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.-G.; Qiu, M.; Duan, X.-Y. Association Between SGLT2is and Cardiovascular and Respiratory Diseases: A Meta-Analysis of Large Trials. Front. Pharmacol. 2021, 12, 724405. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ju, F.; Du, L.; Liu, T.; Zuo, Y.; Abbott, G.W.; Hu, Z. Empagliflozin Protects against Pulmonary Ischemia/Reperfusion Injury via an ERK1/2-Dependent Mechanism. J. Pharmacol. Exp. Ther. 2021, 230–241. [Google Scholar] [CrossRef]
- Qiu, M.; Ding, L.-L.; Zhan, Z.-L.; Liu, S.-Y. Use of SGLT2 inhibitors and occurrence of noninfectious respiratory disorders: A meta-analysis of large randomized trials of SGLT2 inhibitors. Endocrine 2021, 73, 31–36. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wang, S.; Yue, Y.; Liu, Q.; Huang, S.; Peng, H.; Zhang, Y.; Zeng, W.; Wu, Z. Dapagliflozin has No Protective Effect on Experimental Pulmonary Arterial Hypertension and Pulmonary Trunk Banding Rat Models. Front. Pharmacol. 2021, 12, 756226. [Google Scholar] [CrossRef]
- Jeong, H.E.; Park, S.; Noh, Y.; Bea, S.; Filion, K.B.; Yu, O.H.Y.; Jang, S.H.; Cho, Y.M.; Yon, D.K.; Shin, J.-Y. Association of adverse respiratory events with sodium-glucose cotransporter 2 inhibitors versus dipeptidyl peptidase 4 inhibitors among patients with type 2 diabetes in South Korea: A nationwide cohort study. BMC Med. 2023, 21, 47. [Google Scholar] [CrossRef]
- Au, P.C.M.; Tan, K.C.B.; Lam, D.C.L.; Cheung, B.M.Y.; Wong, I.C.K.; Kwok, W.C.; Sing, C.-W.; Cheung, C.-L. Association of Sodium-Glucose Cotransporter 2 Inhibitor vs Dipeptidyl Peptidase-4 Inhibitor Use with Risk of Incident Obstructive Airway Disease and Exacerbation Events Among Patients with Type 2 Diabetes in Hong Kong. JAMA Netw. Open 2023, 6, e2251177. [Google Scholar] [CrossRef]
- Wu, M.-Z.; Chandramouli, C.; Wong, P.-F.; Chan, Y.-H.; Li, H.-L.; Yu, S.-Y.; Tse, Y.-K.; Ren, Q.-W.; Yu, S.-Y.; Tse, H.-F.; et al. Risk of sepsis and pneumonia in patients initiated on SGLT2 inhibitors and DPP-4 inhibitors. Diabetes Metab. 2022, 48, 101367. [Google Scholar] [CrossRef]
- Zou, C.-Y.; Liu, X.-K.; Sang, Y.-Q.; Wang, B.; Liang, J. Effects of SGLT2 inhibitors on cardiovascular outcomes and mortality in type 2 diabetes: A meta-analysis. Medicine 2019, 98, e18245. [Google Scholar] [CrossRef]
- Giugliano, D.; Longo, M.; Scappaticcio, L.; Bellastella, G.; Maiorino, M.I.; Esposito, K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: A meta-analysis of 11 CVOTs. Cardiovasc. Diabetol. 2021, 20, 236. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Zinman, B.; Wanner, C.; Ferrari, R.; Fitchett, D.; Hantel, S.; Espadero, R.-M.; Woerle, H.-J.; Broedl, U.C.; Johansen, O.E. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diabetes Vasc. Dis. Res. 2015, 12, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z.I. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Bilgin, S.; Kurtkulagi, O.; Duman, T.T.; Tel, B.M.A.; Kahveci, G.; Kiran, M.; Erge, E.; Aktas, G. Sodium glucose co-transporter-2 inhibitor, Empagliflozin, is associated with significant reduction in weight, body mass index, fasting glucose, and A1c levels in Type 2 diabetic patients with established coronary heart disease: The SUPER GATE study. Ir. J. Med. Sci. 2022, 191, 1647–1652. [Google Scholar] [CrossRef]
- Ali, A.; Bain, S.; Hicks, D.; Newland Jones, P.; Patel, D.C.; Evans, M.; Fernando, K.; James, J.; Milne, N.; Viljoen, A.; et al. SGLT2 Inhibitors: Cardiovascular Benefits Beyond HbA1c—Translating Evidence into Practice. Diabetes Ther. 2019, 10, 1595–1622. [Google Scholar] [CrossRef] [Green Version]
- Scisciola, L.; Taktaz, F.; Fontanella, R.A.; Pesapane, A.; Surina; Cataldo, V.; Ghosh, P.; Franzese, M.; Puocci, A.; Paolisso, P.; et al. Targeting high glucose-induced epigenetic modifications at cardiac level: The role of SGLT2 and SGLT2 inhibitors. Cardiovasc. Diabetol. 2023, 22, 24. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.L.; Miao, Z.M.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; et al. Influence of NT-proBNP on Efficacy of Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. JACC Heart Fail. 2022, 10, 902–913. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- McGill, J.B.; Subramanian, S. Safety of Sodium-Glucose Co-Transporter 2 Inhibitors. Am. J. Med. 2019, 132, S49–S57.e5. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.D.; Rizkala, A.R.; Gong, J.; Wang, W.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; et al. Angiotensin Receptor Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2017, 5, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Shahzeb Khan, M.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.-J.; Chopra, V.; et al. Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur. J. Heart Fail. 2020, 22, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Seshiah, V.; Swallow, R.; Jones, R.; Rattunde, H.; Woerle, H.J.; Broedl, U.C. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: A 24-week, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2014, 16, 147–158. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A. Risks Associated with SGLT2 Inhibitors: An Overview. Curr. Drug Saf. 2018, 13, 84–91. [Google Scholar] [CrossRef]
- Scheen, A.J. An update on the safety of SGLT2 inhibitors. Expert Opin. Drug Saf. 2019, 18, 295–311. [Google Scholar] [CrossRef]
- Roden, M.; Weng, J.; Eilbracht, J.; Delafont, B.; Kim, G.; Woerle, H.J.; Broedl, U.C. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013, 1, 208–219. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Fadini, G.P.; Bonora, B.M.; Avogaro, A. SGLT2 inhibitors and diabetic ketoacidosis: Data from the FDA Adverse Event Reporting System. Diabetologia 2017, 60, 1385–1389. [Google Scholar] [CrossRef]
- Research C for DE and FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings about Too Much Acid in the Blood and Serious Urinary Tract Infections. FDA. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious (accessed on 27 March 2023).
- Tan, H.; Acharya, S. Perioperative cessation of sodium-glucose cotransporter-2 inhibitors: 72 hours or seven days. Anaesth. Intensive Care 2018, 46, 425. [Google Scholar]
- Vardeny, O.; Vaduganathan, M. Practical Guide to Prescribing Sodium-Glucose Cotransporter 2 Inhibitors for Cardiologists. JACC Heart Fail. 2019, 7, 169–172. [Google Scholar] [CrossRef]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.-E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients with Heart Failure with and Without Diabetes. JAMA 2020, 323, 1353–1368. [Google Scholar] [CrossRef]
- Hao, R.; Myroniuk, T.; McGuckin, T.; Manca, D.; Campbell-Scherer, D.; Lau, D.; Yeung, R.O. Underuse of Cardiorenal Protective Agents in High-Risk Diabetes Patients in Primary Care: A Cross-Sectional Study. BMC Prim. Care 2022, 23, 124. [Google Scholar] [CrossRef]
- Campbell, D.B.; Campbell, D.J.T.; Au, F.; Beall, R.F.; Ronksley, P.E.; Chew, D.S.; Ogundeji, Y.; Manns, B.J.; Hemmelgarn, B.R.; Tonelli, M. Patterns and Patients’ Characteristics Associated with Use of Sodium/Glucose Cotransporter 2 Inhibitors Among Adults with Type 2 Diabetes: A Population-Based Cohort Study. Can. J. Diabetes 2022, 47, 58–65.e2. [Google Scholar] [CrossRef]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Ilyas, I.; Zheng, X.; Luo, S.; Little, P.J.; Kamato, D.; Sahebkar, A.; Wu, W.; Weng, J.; et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: From pharmacology to pre-clinical and clinical therapeutics. Theranostics 2021, 11, 4502–4515. [Google Scholar] [CrossRef]
- Irace, C.; Casciaro, F.; Scavelli, F.B.; Oliverio, R.; Cutruzzolà, A.; Cortese, C.; Gnasso, A. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc. Diabetol. 2018, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K. Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Feinkohl, I.; Keller, M.; Robertson, C.M.; Morling, J.R.; Williamson, R.M.; Nee, L.D.; McLachlan, S.; Sattar, N.; Welsh, P.; Reynolds, R.M.; et al. Clinical and subclinical macrovascular disease as predictors of cognitive decline in older patients with type 2 diabetes: The Edinburgh type 2 diabetes study. Diabetes Care 2013, 36, 2779–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.C.H.; Chan, M.C.Y. SGLT2 Inhibitors: The Next Blockbuster Multifaceted Drug? Medicina 2023, 59, 388. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Fragasso, G.; Petrie, M.; Mullens, W.; Ferrari, R.; Thum, T.; Bauersachs, J.; Anker, S.D.; Ray, R.; Çavuşoğlu, Y.; et al. Sodium–glucose co-transporter 2 inhibitors in heart failure: Beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
| SGLT2 Inhibitors | Therapeutic Indication | Dosing |
|---|---|---|
| Canagliflozin |
| 100 mg once a day |
| Dapagliflozin |
| 10 mg once a day |
| 10 mg once a day | |
| 10 mg once a day | |
| Empagliflozin |
| 10 mg once a day |
| 10 mg once a day | |
| Ertugliflozin |
| 5 mg or 10 mg once a day |
| Sotagliflozin |
| 200 mg or 400 mg once a day |
| Bexagliflozin |
| 20 mg once a day |
| Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | Sotagliflozin | Bexagliflozin | |
|---|---|---|---|---|---|---|
| Hypotension | uncommon | uncommon | Very common | common | common | unknown |
| Diabetic ketoacidosis | rare | rare | uncommon | rare | common | - |
| Bone fracture | uncommon | - | - | - | - | - |
| Genital infections | common | common | common | common | common | common |
| Urinary tract infections | common | common | common | common | common | common |
| Fournier’s gangrene | unknown | very rare | rare | unknown | rare | unknown |
| Amputation of lower limbs | uncommon | unknown | unknown | unknown | unknown | rare |
| Hypoglycaemia | very common in combination | very common | very common | common | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreea, M.M.; Surabhi, S.; Razvan-Ionut, P.; Lucia, C.; Camelia, N.; Emil, T.; Tiberiu, N.I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Harms or Unexpected Benefits? Medicina 2023, 59, 742. https://doi.org/10.3390/medicina59040742
Andreea MM, Surabhi S, Razvan-Ionut P, Lucia C, Camelia N, Emil T, Tiberiu NI. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Harms or Unexpected Benefits? Medicina. 2023; 59(4):742. https://doi.org/10.3390/medicina59040742
Chicago/Turabian StyleAndreea, Munteanu Madalina, Swarnkar Surabhi, Popescu Razvan-Ionut, Ciobotaru Lucia, Nicolae Camelia, Tufanoiu Emil, and Nanea Ioan Tiberiu. 2023. "Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Harms or Unexpected Benefits?" Medicina 59, no. 4: 742. https://doi.org/10.3390/medicina59040742
APA StyleAndreea, M. M., Surabhi, S., Razvan-Ionut, P., Lucia, C., Camelia, N., Emil, T., & Tiberiu, N. I. (2023). Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Harms or Unexpected Benefits? Medicina, 59(4), 742. https://doi.org/10.3390/medicina59040742






