Combination of Farnesol with Common Antifungal Drugs: Inhibitory Effect against Candida Species Isolated from Women with RVVC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Identification of Isolates
2.2.1. Conventional Methods
2.2.2. Molecular Assay
2.3. Antifungal Susceptibility Testing
- -
- FLU: MIC ≥ 8 μg/mL—resistance; MIC ≤ 2 μg/mL—susceptible, and MIC = 4 μg/mL—8 μg/mL dose-dependent susceptibility;
- -
- Other azoles: MIC ≤ 0.12 μg/mL—susceptible and MIC ≥ 1 μg/mL—resistant;
- -
- AMB: MIC ≤ 2 μg/mL—susceptible and MIC > 2 μg/mL—resistant.
2.4. Antifungal Activity of Farnesol
2.5. Drug Combination Study
2.6. Cytotoxicity Assay
2.7. Statistical Analysis
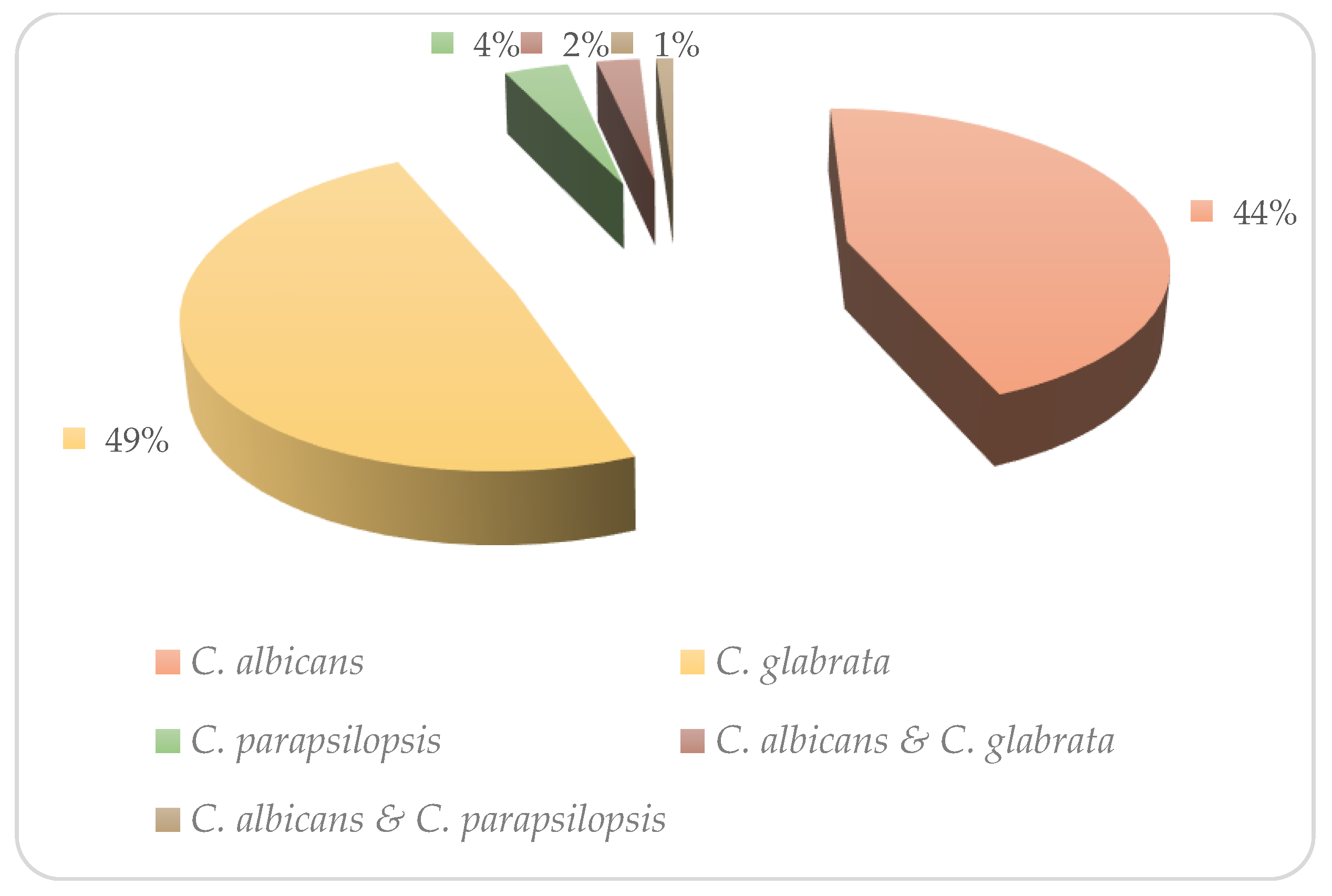
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denning, D.W.; Bromley, M.J. How to Bolster the Antifungal Pipeline: Few Drugs Are Coming to Market, but Opportunities for Drug Development Exist. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, L.; Hawes, S.; Stevens, C.; Koutsky, L.; Eschenbach, D.; Holmes, K. Vulvovaginal Candidiasis: Clinical Manifestations, Risk Factors, Management Algorithm. Obstet. Gynecol. 1998, 92, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, M.V.; Garland, S.M. Genital Candida Species Detected in Samples from Women in Melbourne, Australia, before and after Treatment with Antibiotics. J. Clin. Microbiol. 2006, 44, 3213–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Lema, V.M. Recurrent Vulvo-Vaginal Candidiasis: Diagnostic and Management Challenges in a Developing Country Context. Obstet. Gynecol. Int. J. 2017, 7, 260. [Google Scholar] [CrossRef]
- Lírio, J.; Giraldo, P.C.; Amaral, R.L.; Sarmento, A.C.A.; Costa, A.P.F.; Gonçalves, A.K. Antifungal (Oral and Vaginal) Therapy for Recurrent Vulvovaginal Candidiasis: A Systematic Review Protocol. BMJ Open 2019, 9, e027489. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.Y.; Thian, L.; Than, L. Vulvovaginal candidosis: Contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses 2016, 59, 262–273. [Google Scholar] [CrossRef]
- Li, C.; Xu, Z.; Liu, S.; Huang, Y.; Duan, W.; Wei, X. In Vivo Antifungal Activities of Farnesol Combined with Antifungal Drugs against Murine Oral Mucosal Candidiasis. Biofouling 2021, 37, 818–829. [Google Scholar] [CrossRef]
- Sobel, J.; Sobel, R. Current Treatment Options for Vulvovaginal Candidiasis Caused by Azole-Resistant Candida Species. Expert Opin. Pharmacother. 2018, 19, 971–977. [Google Scholar] [CrossRef]
- Costa, A.F.; Silva, L.C.; Amaral, A.C. Farnesol: An approach on biofilms and nanotechnology. Med. Mycol. 2021, 59, 958–969. [Google Scholar]
- Brilhante, R.S.N.; de Lima, R.A.C.; Caetano, E.P.; Leite, J.J.G.; Castelo-Branco, D.S.C.M.; Riberio, J.F.; Bandeira, T.D.J.P.G.; Cordeiro, R.D.A.; Monteiro, A.J.; Sidrim, J.J.C.; et al. Effect of Farnesol on Growth, Ergosterol Biosynthesis, and Cell Permeability in Coccidioides Posadassi. Antimicrob. Agent Chemother. 2013, 57, 2167–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Y.; Zhou, Y.; Wei, X. Farnesol Induces Apoptosis-like Cell Death in the Pathogenic Funus Aspergillus Flavus. Mycologia 2014, 106, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Delmondes, G.D.A.; Santiago Lemos, I.C.; Dias, D.D.Q.; Da Cunha, G.L.; Araújo, I.M.; Barbosa, R.; Coutinho, H.D.M.; Felipe, C.F.B.; Barbosa-Filho, J.M.; De Lima, N.T.R.; et al. Pharmacological Applications of Farnesol (C15H26O): A Patent Review. Expert Opin. Ther. Pat. 2020, 30, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.; Wickes, B.; Lopez-Ribot, J. Inhibition of Candida Albicans Biofilm Formation by Farnesol, a Quorum-Sensing Molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [Green Version]
- Sebaa, S.; Boucherit-Otmani, Z.; Courtois, P. Effects of Tyrosol and Farnesol on Candida Albicans Biofilm. Mol. Med. Rep. 2019, 19, 3201–3209. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.F.; Černáková, L. Farnesol and Tyrosol: Secondary Metabolites with a Crucial Quorum-Sensing Role in Candida Biofilm Development. Genes 2020, 11, 444. [Google Scholar] [CrossRef] [Green Version]
- Polke, M.; Leonhardt, I.; Kurzai, O.; Jacobsen, I.D. Farnesol Signalling in Candida Albicans—More than Just Communication. Crit. Rev. Microbiol. 2018, 44, 230–243. [Google Scholar] [CrossRef]
- Katragkou, A.; Mccarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In Vitro Interactions between Farnesol and Fluconazole, Amphotericin b or Micafungin against Candida Albicans Biofilms. J. Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef]
- Onder, S.; Oz, Y. In Vitro Effects of Farnesol Alone and in Combination with Antifungal Drugs against Aspergillus Clinical Isolates. Med. Mycol. J. 2021, 62, 5–10. [Google Scholar] [CrossRef]
- Bozó, A.; Domán, M.; Majoros, L.; Kardos, G.; Varga, I.; Kovács, R. The in Vitro and in Vivo Efficacy of Fluconazole in Combination with Farnesol against Candida Albicans Isolates Using a Murine Vulvovaginitis Model. J. Microbiol. 2016, 54, 753–760. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial Strategies for Combating Invasive Fungal Infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination with Fluconazole against Candida Albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Boas, D.V.; Haynes, K.; Henriques, M. The MNN2 Gene Knockout Modulates the Antifungal Resistance of Biofilms of Candida Glabrata. Biomolecules 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Arastehfar, A.; Fang, W.; Pan, W.; Liao, W.; Yan, L.; Boekhout, T. Identification of Nine Cryptic Species of Candida albicans, C. glabrata, and C. parapsilosis Complexes Using One-Step Multiplex PCR. BMC Infect. Dis. 2018, 18, 480. [Google Scholar] [CrossRef] [Green Version]
- Nikoomanesh, F.; Roudbarmohammadi, S.; Khoobi, M.; Haghighi, F.; Roudbary, M. Design and Synthesis of Mucoadhesive Nanogel Containing Farnesol: Investigation of the Effect on HWP1, SAP6 and Rim101 Genes Expression of Candida Albicans in Vitro. Artif. Cells Nanomed. Biotechnol. 2019, 47, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Zare-Bidaki, M.; L Maleki, A.; Ghanbarzadeh, N. Expression pattern of drug-resistance genes ERG11 and TAC1 in Candida albicans Clinical isolates. Mol. Biol. Rep. 2022, 49, 11625–11633. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; ApprovedStandard—Third Edition: M27-A3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Nikoomanesh, F.; Roudbarmohammadi, S.; Bashardoust, B.; Zareei, M. Effect of Farnesol on Responsive Gene Expressions in Hyphal Morphogenesis Transformation of Candida Albicans. Infect. Epidemiol. Microbiol. 2018, 4, 73–77. [Google Scholar]
- Meletiadis, J.; Mouton, J.W.; Meis, J.F.; Verweij, P.E. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 2003, 47, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Alipour, R.; Fatemi, A.; Alsahebfosul, F.; Andalib, A.; Pourazar, A. Autologous Plasma versus Fetal Calf Serum as a Supplement for the Culture of Neutrophils. BMC Res. Notes 2020, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kumar, A.; Roudbary, M.; Mohammadi, R.; Černáková, L. Overview on the Infections Related to Rare Candida Species. Pathogens 2022, 11, 963. [Google Scholar] [CrossRef]
- Roudbary, M.; Roudbarmohammadi, S.H.; Bakhshi, B.; Farhadi, Z.; Nikoomanesh, F. Identification of Candida Species Isolated Form Iranian Women Eith Vaginal Candidasis by PCR-RFLP Method. Eur. J. Exp. Biol. 2013, 3, 365–369. [Google Scholar]
- Alves, A.M.C.V.; Cruz-Martins, N.; Rodrigues, C.F. Marine Compounds with Anti-Candida Sp. Activity: A Promised “Land” for New Antifungals. J. Fungi 2022, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Zhang, T.; Xue, Y.; Xu, H.; An, R. Risk Factors of Vulvovaginal Candidiasis among Women of Reproductive Age in Xi’an: A Cross-Sectional Study. Biomed. Res. Int. 2018, 7, 9703754. [Google Scholar]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, 216–445. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.F.; Gonçalves, B.; Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. The Effectiveness of Voriconazole in Therapy of Candida Glabrata’s Biofilms Oral Infections and Its Influence on the Matrix Composition and Gene Expression. Mycopathologia 2017, 182, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Nyirjesy, P.; Brookhart, C.; Lazenby, G.; Schwebke, G.; Sobel, J.D. Vulvovaginal Candidiasis: A Review of the Evidence for the 2021 Centers for Disease Control and Prevention of Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 2022, 74, S162–S168. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, V.E.; Stafylaki, D.; Nioti, E.; Hamilos, G.; Kasimati, A. Epidemiology and antifungal susceptibility patterns of Candida isolates from Greek women with vulvovaginal candidíases. Mycoses 2019, 62, 692–697. [Google Scholar] [CrossRef]
- Guzel, A.B.; Ilkit, M.; Burgut, R.; Urunsak, I.F.; Ozgunen, F.T. An Evaluation of Risk Factors in Pregnant Women with Candida Vaginitis and the Diagnostic Value of Simultaneous Vaginal and Rectal Sampling. Mycopathologia 2011, 172, 25–36. [Google Scholar] [CrossRef]
- Bitew, A.; Abebaw, Y. Vulvovaginal Candidiasis: Species Distribution of Candida and Their Antifungal Susceptibility Pattern. BMC Womens Health 2018, 18, 94. [Google Scholar] [CrossRef]
- Arastehfar, A.; Kargar, M.L.; Mohammadi, S.R.; Roudbary, M.; Ghods, N.; Haghighi, L.; Daneshnia, F.; Tavakoli, M.; Jafarzadeh, J.; Hedayati, M.T.; et al. A High Rate of Recurrent Vulvovaginal Candidiasis and Therapeutic Failure of Azole Derivatives among Iranian Women. Front. Microbiol. 2021, 12, 819. [Google Scholar] [CrossRef]
- Mohammadi-Ghalehbin, B.; Javanpour Heravi, H.; Arzanlou, M.; Sarvi, M. Prevalence and Antibiotic Resistance Pattern of Candida Spp. Isolated from Pregnant Women Referred to Health Centers in Ardabil, Iran. J. Ardabil. Univ. Med. Sci. 2017, 16, 409–421. [Google Scholar]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Sustr, V.; Foessleitner, P.; Kiss, H.; Farr, A. Vulvovaginal Candidosis: Current Concepts, Challenges and Perspectives. J. Fungi 2020, 6, 267. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Vaezi, A.; Dannaoui, E.; Chowdhary, A.; Nasiry, D.; Faeli, L.; Meis, J.F.; Badali, H. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses 2018, 61, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum Sensing in the Demorphic Fungus Candida Albicans Is Mediated by Farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [Green Version]
- Sachivkina, N.; Podoprigora, I.; Bokov, D. Morphological characteristics of Candida albicans, Candida krusei, Candida guilliermondii, and Candida glabrata biofilms, and response to farnesol. Vet. World 2021, 14, 1608–1614. [Google Scholar] [CrossRef]
- Yu, L.H.; Wei, X.; Ma, M.; Chen, X.J.; Xu, S.B. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob. Agents Chemother. 2012, 56, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Nagy, F.; Vitális, E.; Jakab, Á.; Borman, A.M.; Forgács, L.; Tóth, Z.; Majoros, L.K.R. In Vitro and in Vivo Effect of Exogenous Farnesol Exposure Against Candida Auris. Front. Microbiol. 2020, 20, 957. [Google Scholar] [CrossRef]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar]
- Décanis, N.; Tazi, N.; Correia, A.; Vilanova, M.; Rouabhia, M. Farnesol, a Fungal Quorum-Sensing Molecule Triggers Candida Albicans Morphological Changes by Downregulating the Expression of Different Secreted Aspartyl Proteinase Genes. Open Microbiol. J. 2011, 5, 119–126. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Alves, D.F.; Henriques, M. Combination of Posaconazole and Amphotericin b in the Treatment of Candida Glabrata Biofilms. Microorganisms 2018, 6, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, R.A.; Teixeira, C.E.C.; Brilhante, R.S.N.; Castelo-Branco, D.S.C.M.; Paiva, M.A.N.; Giffoni Leite, J.J.; Lima, D.T.; Monteiro, A.J.; Sidrim, J.J.C.; Rocha, M.F.G. Minimum Inhibitory Concentrations of Amphotericin B, Azoles and Caspofungin against Candida Species Are Reduced by Farnesol. Med. Mycol. 2013, 51, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Qian, F.; Xu, W.; Zhang, Z.; Wei, X. In Vitro Inhibitory Effects of Farnesol and Interaction between Farnesol and Antifungals against Biofilm of C Andida Albicans Resistance Strains. Biofouling 2017, 33, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.F.; de Alencar Júnior, J.G.; de Lima Honorato, R.; dos Santos, A.T.L.; Pereira da Silva, J.C.; Gusmão da Silva, T.; Leal, A.L.A.B.; Rocha, J.E.; de Freitas, T.S.; Tavares Vieira, T.A.; et al. Antifungal Activity of Farnesol Incorporated in Liposomes and Associated with Fluconazole. Chem. Phys. Lipids 2020, 233, 33058818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Prasad, R. The Quorum-Sensing Molecule Farnesol Is a Modulator of Drug Efflux Mediated by ABC Multidrug Transporters and Synergizes with Drugs in Candida Albicans. Antimicrob. Agents Chemother. 2011, 55, 4834–4843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Černáková, L.; Dižová, S.; Gášková, D.; Jančíková, I.; Bujdáková, H. Impact of Farnesol as a Modulator of Efflux Pumps in a Fluconazole-Resistant Strain of Candida Albicans. Microb. Drug Resist. 2019, 25, 805–812. [Google Scholar] [CrossRef]
- Dekkerová, J.; Černáková, L.; Kendra, S.; Borghi, E.; Ottaviano, E.; Willinger, B.; Bujdáková, H. Farnesol Boosts the Antifungal Effect of Fluconazole and Modulates Resistance in Candida Auris through Regulation of the CDR1 and ERG11 Genes. J. Fungi 2022, 8, 783. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Shirtliff, M.; James, C.; Meiller, T. Effect of Farnesol on Candida Dubliniensis Biofilm Formation and Fluconazole Resistance. FEMS Yeast Res. 2006, 6, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, B.Y.; Feyzullazade, N.; Dağ, I.; Şengel, T. The investigation of in vitro effects of farnesol at different cancer cell lines. Microsc. Res. Tech. 2022, 85, 2760–2775. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, Y.; Fan, S.; Vitagliano, A.; Liu, X.; Liao, Y.; Liang, Y.; Vitale, S.G. Clinical Characteristics and Antifungal Susceptibility of Candida Nivariensis from Vulvovaginal Candidiasis. Gynecol. Obstet. Investig. 2019, 85, 1. [Google Scholar] [CrossRef]
| Candida Species | Sequences (5′->3′) | Amplicons |
|---|---|---|
| C. albicans | F5′AGATTATTGCCATGCCCTGAG3′ R5′CCATGTCGAACGTAGCGTATGC3′ | 606 bp |
| C. glabrata | F5′ACCGTGCTTGCCTCTACA3′ R5′GACATCTGAGCCTCGTCTGA3′ | 212 bp |
| C. tropicalis | F5′AGAACAAGAAAACAGTGAAGCAA3′ R5′CCATGTCGAACGTAGCGTATGC3 | 126 bp |
| C. parapsilosis | F5′TACACCAAGCGACTCAGC3′ R5′ACCAGCTGCTTTGACTTG3′ | 490 bp |
| C. krusei | F5′GGCGTTGTCCATCCAATG3′ R5′CAGGAGAATTGCTGTTCCC3′ | 1159 bp |
| C. dubliniensis | F5′GTCGGACATATACCTCCAACTC3′ R5′CCATGTCGAACGTAGCGTAT3′ | 718 bp |
| Candida Species | Antifungal Drug | Sensitive (S) | Dose-Dependent | Resistance (R) | |||
|---|---|---|---|---|---|---|---|
| n | % | N | % | n | % | ||
| C. albicans n = 35 | FLU | 11 | 31.4 | 1 | 2.1 | 23 | 65.7 |
| ITZ | 18 | 51.4 | - | - | 17 | 48.5 | |
| VOR | 17 | 48.5 | - | - | 18 | 51.4 | |
| AMB | 35 | 100 | - | - | - | - | |
| CTZ | 13 | 37.1 | - | - | 22 | 62.8 | |
| C. glabrata n = 39 | FLU | 9 | 23 | 2 | 5.1 | 28 | 71.8 |
| ITZ | 16 | 41 | - | - | 23 | 59 | |
| VOR | 14 | 35.9 | - | - | 25 | 64.1 | |
| AMB | 35 | 89.7 | - | - | 4 | 10.2 | |
| CTZ | 13 | 33.3 | - | - | 26 | 66.6 | |
| C. parapsilosis n = 3 | FLU | 2 | 66.6 | - | - | 1 | 33.3 |
| ITZ | 3 | 100 | - | - | - | - | |
| VOR | 2 | 66.6 | - | - | 1 | 33.3 | |
| AMB | 1 | 33.3 | - | - | 2 | 66.6 | |
| CTZ | 1 | 33.3 | - | - | 2 | 66.6 | |
| Isolates | Median MIC Values | Interaction Analysis | ||||
|---|---|---|---|---|---|---|
| MIC Alone | MIC in Combination | Median FICI | Type of Interaction | |||
| FLU (µg/L) | FAR (µM) | FLU (µg/L) | FAR (µM) | |||
| C. albicans | 64 (8–64) | 300 | 8 (2–8) | 150 | 0.5 | Synergy |
| C. glabrata | 64 (8–64) | 300 | 8 (2–16) | 300 | 0.9 | Indifferent |
| C. parapsilosis | 32 (8–32) | 300 | 4 (2–8) | 150 | 0.35 | Synergy |
| ITRA (µg/L) | FAR (µM) | ITRA (µg/L) | FAR (µM) | |||
| C. albicans | 8 (1–8) | 300 | 4 (1–8) | 150 | 0.5 | Synergy |
| C. glabrata | 8 (2–8) | 300 | 8 (2–8) | 300 | 1.01 | Indifferent |
| C. parapsilosis | 8 (2–8) | 300 | 4 (1–4) | 150 | 0.25 | Synergy |
| VOR (µg/L) | FAR (µM) | VOR (µg/L) | FAR (µM) | |||
| C. albicans | 16 (2–16) | 300 | 8 (1–8) | 150 | 0.75 | Indifferent |
| C. glabrata | 16 (2–16) | 300 | 8 (2–16) | 300 | 0.75 | Indifferent |
| C. parapsilosis | 8 (2–16) | 300 | 4 (1–4) | 150 | 0.5 | Synergy |
| AmB (µg/L | FAR (µM) | AmB (µg/L) | FAR (µM) | |||
| C. albicans | 2 (0.031–2) | 300 | 2 (0.031–2) | 150 | – | – |
| C. glabrata | 2 (0.031–2) | 300 | 1 (0.031–2) | 300 | 1.25 | Indifferent |
| C. parapsilosis | 2 (0.031–2) | 300 | 1 (0.031–2) | 150 | 0.35 | Synergy |
| CTZ (µg/L) | FAR (µM) | CTZ (µg/L) | FAR (µM) | |||
| C. albicans | 16 (2–16) | 300 | 4 (1–4) | 150 | 1.75 | Indifferent |
| C. glabrata | 16 (2–16) | 300 | 8 (2–16) | 300 | 0.9 | Indifferent |
| C. parapsilosis | 8 (2–16) | 300 | 2 (0.5–4) | 150 | 1.25 | Indifferent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoomanesh, F.; Falahatinejad, M.; Černáková, L.; dos Santos, A.L.S.; Mohammadi, S.R.; Rafiee, M.; Rodrigues, C.F.; Roudbary, M. Combination of Farnesol with Common Antifungal Drugs: Inhibitory Effect against Candida Species Isolated from Women with RVVC. Medicina 2023, 59, 743. https://doi.org/10.3390/medicina59040743
Nikoomanesh F, Falahatinejad M, Černáková L, dos Santos ALS, Mohammadi SR, Rafiee M, Rodrigues CF, Roudbary M. Combination of Farnesol with Common Antifungal Drugs: Inhibitory Effect against Candida Species Isolated from Women with RVVC. Medicina. 2023; 59(4):743. https://doi.org/10.3390/medicina59040743
Chicago/Turabian StyleNikoomanesh, Fatemeh, Mahsa Falahatinejad, Lucia Černáková, André Luis Souza dos Santos, Shahla Roudbar Mohammadi, Mitra Rafiee, Célia Fortuna Rodrigues, and Maryam Roudbary. 2023. "Combination of Farnesol with Common Antifungal Drugs: Inhibitory Effect against Candida Species Isolated from Women with RVVC" Medicina 59, no. 4: 743. https://doi.org/10.3390/medicina59040743








