Orthotopic Liver Transplantation of a SARS-CoV-2 Negative Recipient from a Positive Donor: The Border between Uncertainty and Necessity in a Pandemic Era- Case Report and Overview of the Literature
Abstract
1. Introduction
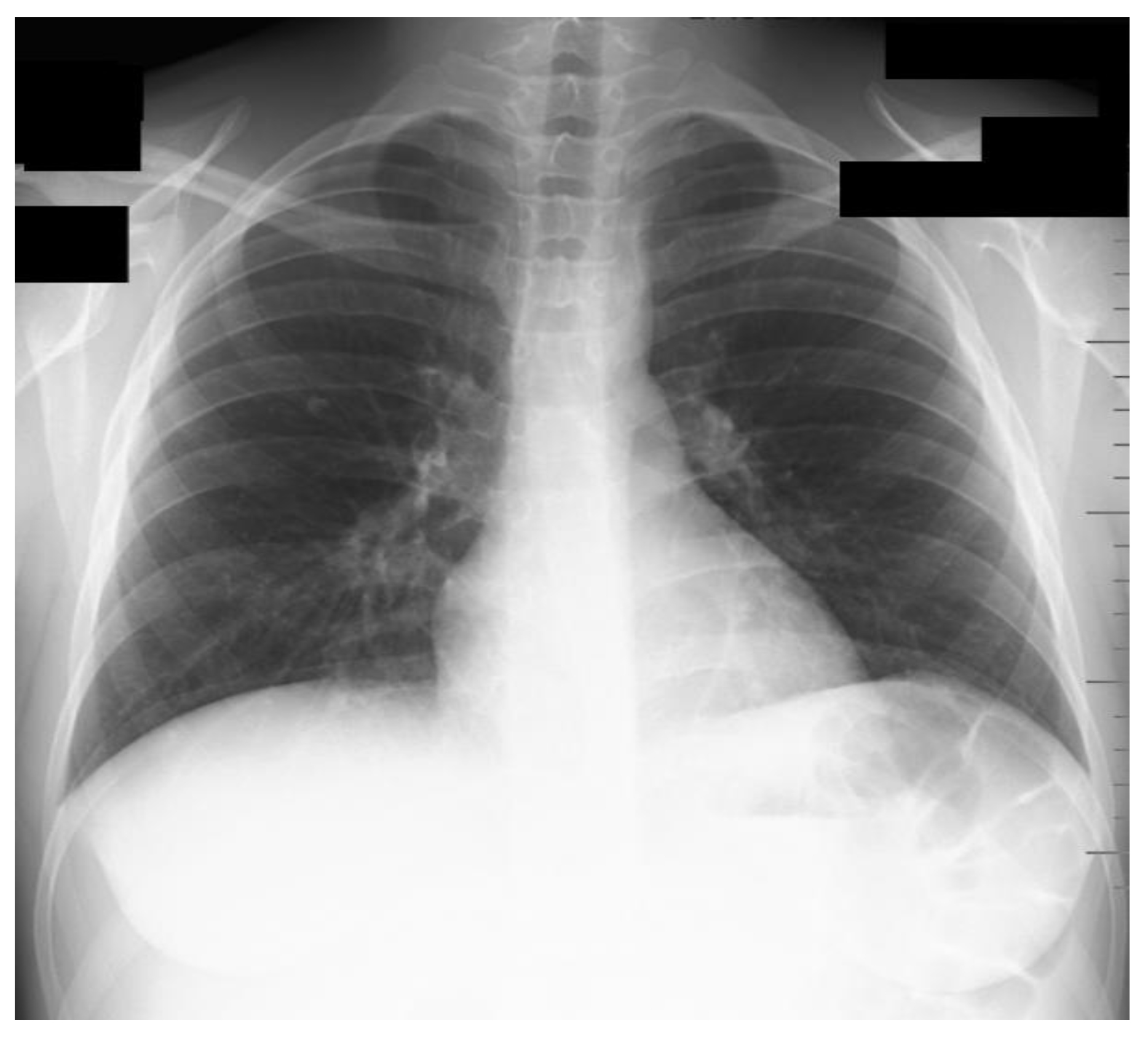
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Lin, S.X.; Tao, J.; Wei, X.Q.; Liu, Y.T.; Chen, Y.M.; Wu, B. Study of liver cirrhosis over ten consecutive years in Southern China. World J. Gastroenterol. WJG 2014, 20, 13546. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Isac, S.; Jelea, D.; Martac, C.; Stefan, M.G.; Cotorogea-Simion, M.; Buzatu, C.G.S.; Ingustu, D.; Abdulkareem, I.; Vasilescu, C.; et al. COVID-19 Pandemic Was Associated with Lower Activity but Not Higher Perioperative Mortality in a Large Eastern European Center. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e935809-1. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, G.; Bekele, F.; Tolossa, T.; Fetensa, G.; Turi, E.; Getachew, M.; Abdisa, E.; Assefa, L.; Afeta, M.; Demisew, W.; et al. Impact of COVID-19 pandemic on chronic diseases care follow-up and current perspectives in low resource settings: A narrative review. Int. J. Physiol. Pathophysiol. Pharmacol. 2021, 13, 86. [Google Scholar] [PubMed]
- Horton, R. Offline: COVID-19 and the NHS—“A national scandal”. Lancet 2020, 395, 1022. [Google Scholar] [CrossRef]
- Ahmed, O.; Brockmeier, D.; Lee, K.; Chapman, W.C.; Doyle, M.B.M. Organ donation during the COVID-19 pandemic. Am. J. Transplant. 2020, 20, 3081. [Google Scholar] [CrossRef]
- Søreide, K.; Hallet, J.; Matthews, J.B.; Schnitzbauer, A.A.; Line, P.D.; Lai, P.B.S.; Otero, J.; Callegaro, D.; Warner, S.G.; Baxter, N.N.; et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br. J. Surg. 2020, 107, 1250. [Google Scholar] [CrossRef]
- Singh, S.; Khan, A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 among Patients with Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020, 159, 768. [Google Scholar] [CrossRef]
- Barnes, E. Infection of liver hepatocytes with SARS-CoV-2. Nat. Metab. 2022, 4, 301–302. [Google Scholar] [CrossRef]
- Pavel, B.; Moroti, R.; Spataru, A.; Popescu, M.R.; Panaitescu, A.M.; Zagrean, A.-M. Neurological Manifestations of SARS-CoV2 Infection: A Narrative Review. Brain Sci. 2022, 12, 1531. [Google Scholar] [CrossRef]
- Cerrada-Romero, C.; Berastegui-Cabrera, J.; Camacho-Martínez, P.; Goikoetxea-Aguirre, J.; Pérez-Palacios, P.; Santibáñez, S.; Blanco-Vidal, M.J.; Valiente, A.; Alba, J.; Rodríguez-Álvarez, R.; et al. Excretion and viability of SARS-CoV-2 in feces and its association with the clinical outcome of COVID-19. Sci. Rep. 2022, 12, 7397. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.Y.; Ding, Y.H.; Nie, K.; Dong, Y.M.; Xu, J.H.; Yang, M.L.; Liu, M.Q.; Wei, L.; Nasser, M.I.; Xu, L.Y.; et al. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed. Pharmacother. 2021, 133, 111064. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Cossiga, V.; Loperto, I.; Esposito, I.; Ortolani, R.; Fiorentino, A.; Pontillo, G.; De Coppi, L.; Cozza, V.; Lanza, A.G.; et al. COVID-19 in liver transplant recipients: Incidence, hospitalization and outcome in an Italian prospective double-centre study. Sci. Rep. 2022, 12, 4831. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Tevethia, H.V.; Premkumar, M.; Arab, J.P.; Candia, R.; Kumar, K.; Kumar, P.; Sharma, M.; Rao, P.N.; Reddy, D.N. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 101025. [Google Scholar] [CrossRef]
- Punga, D.; Isac, S.; Paraipan, C.; Cotorogea, M.; Stefan, A.; Cobilinschi, C.; Vacaroiu, I.A.; Tulin, R.; Ionescu, D.; Droc, G. Impact of COVID-19 Infection on Liver Transplant Recipients: Does It Make Any Difference? Cureus J. Med. Sci. 2022, 14, 22687. [Google Scholar] [CrossRef] [PubMed]
- Millson, C.; Considine, A.; Cramp, M.E.; Holt, A.; Hubscher, S.; Hutchinson, J.; Jones, K.; Leithead, J.; Masson, S.; Menon, K.; et al. Adult liver transplantation: UK clinical guideline—Part 2: Surgery and post-operation. Frontline Gastroenterol. 2020, 11, 385–396. [Google Scholar] [CrossRef]
- Belli, L.S.; Fondevila, C.; Cortesi, P.A.; Conti, S.; Karam, V.; Adam, R.; Coilly, A.; Ericzon, B.G.; Loinaz, C.; Cuervas-Mons, V.; et al. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients with COVID-19: Results from the ELITA/ELTR Multi-center European Study. Gastroenterology 2021, 160, 1151. [Google Scholar] [CrossRef]
- Cheng, G.S.; Evans, S.E. The paradox of immunosuppressants and COVID-19. Eur. Respir. J. 2021, 59, 2102828. [Google Scholar] [CrossRef]
- Peghin, M.; Grossi, P.A. COVID-19 positive donor for solid organ transplantation. J. Hepatol. 2022, 77, 1198–1204. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Weng, J. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2022, 44, 695–709. [Google Scholar] [CrossRef]
- Schold, J.D.; Koval, C.E.; Wee, A.; Eltemamy, M.; Poggio, E.D. Utilization and outcomes of deceased donor SARS-CoV-2-positive organs for solid organ transplantation in the United States. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22, 2217–2227. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, E.M.; Coniglio, A.C.; Milano, C.; Schroder, J.; Bryner, B.S.; Spencer, P.J.; Haney, J.C.; Klapper, J.; Glass, C.; Pavlisko, E.; et al. Transplanting thoracic COVID-19 positive donors: An institutional protocol and report of the first 14 cases. J. Heart Lung Transplant. 2022, 41, 1376. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.K.; Amit, I.; Alan, H.; Weingarten, N.; Helmers, R.M.; Pavan, A. Abstract 12504: Outcomes of COVID-19 Positive Donor Heart Transplantation in the United States. Circulation 2022, 146, A12504. [Google Scholar]
- Perlin, D.V.; Dymkov, I.N.; Terentiev, A.V.; Perlina, A.V. Is Kidney Transplantation from a COVID-19-Positive Deceased Donor Safe for the Recipient? Transplant. Proc. 2021, 53, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Liton, E.; Morgan, M. The PiCCO monitor: A review. Anaesth. Intensive Care 2012, 40, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Kates, O.S.; Stock, P.G.; Ison, M.G.; Allen, R.D.M.; Burra, P.; Jeong, J.C.; Kute, V.; Muller, E.; Nino-Murcia, A.; Wang, H.; et al. Ethical review of COVID-19 vaccination requirements for transplant center staff and patients. Am. J. Transplant. 2022, 22, 371–380. [Google Scholar] [CrossRef]
- Vallamkondu, J.; John, A.; Wani, W.Y.; Ramadevi, S.P.; Jella, K.K.; Reddy, P.H.; Kandimalla, R. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165889. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.; Tsang, J.O.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, 14–23. [Google Scholar] [CrossRef]
- Zhao, B.; Ni, C.; Gao, R.; Wang, Y.; Yang, L.; Wei, J.; Lv, T.; Liang, J.; Zhang, Q.; Xu, W.; et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 2020, 11, 771–775. [Google Scholar] [CrossRef]
- Chang, L.; Yan, Y.; Wang, L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus. Med. Rev. 2020, 34, 75. [Google Scholar] [CrossRef]
- Chiem, C.; Alghamdi, K.; Nguyen, T.; Han, J.H.; Huo, H.; Jackson, D. The Impact of COVID-19 on Blood Transfusion Services: A Systematic Review and Meta-Analysis. Transfus. Med. Hemotherapy 2022, 49, 107. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.C.; Andersson, M.I.; Arancibia-Carcamo, C.V.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beneke, T.; Bibi, S.; Brooks, T.; Carroll, M. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020, 5, 181. [Google Scholar]
- Schoot, T.S.; Kerckhoffs, A.P.M.; Hilbrands, L.B.; Van Marum, R.J. Immunosuppressive Drugs and COVID-19: A Review. Front. Pharmacol. 2020, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Lui, S.L.; Ramassar, V.; Urmson, J.; Halloran, P.F. Mycophenolate mofetil reduces production of interferon-dependent major histocompatibility complex induction during allograft rejection, probably by limiting clonal expansion. Transpl. Immunol. 1998, 6, 23–32. [Google Scholar] [CrossRef]
- Sajgure, A.; Kulkarni, A.; Joshi, A.; Sajgure, V.; Pathak, V.; Melinkeri, R.; Pathak, S.; Agrawal, S.; Naik, M.; Rajurkar, M.; et al. Safety and efficacy of mycophenolate in COVID-19: A nonrandomised prospective study in western India. Lancet Reg. Health Southeast Asia 2023, 11, 100154. [Google Scholar] [CrossRef]
- Yin, S.; Wang, X.; Song, T. Tacrolimus Use and COVID-19 Infection in Patients After Solid Organ Transplantation. Gastroenterology 2021, 161, 728. [Google Scholar] [CrossRef]
- Hage, R.; Schuurmans, M.M. Calcineurin Inhibitors and COVID-19. Reumatol. Clin. 2022, 18, 314. [Google Scholar] [CrossRef]
- Bremer, S.; Vethe, N.T.; Bergan, S. Monitoring Calcineurin Inhibitors Response Based on NFAT-Regulated Gene Expression. Personalized Immunosuppression in Transplantation: Role of Biomarker Monitoring and Therapeutic Drug Monitoring. Br. J. Clin. Pharmacol. 2016, 11, 259–290. [Google Scholar]
- Yanny, B.; Alkhero, M.; Alani, M.; Stenberg, D.; Saharan, A.; Saab, S. Post-COVID-19 Cholangiopathy: A Systematic Review. J. Clin. Exp. Hepatol. 2022. [Google Scholar] [CrossRef]
- Bernal, R.B.; Medina-Morales, E.; Goyes, D.; Patwardhan, V.; Bonder, A.; Lai, Q. Management of Autoimmune Liver Diseases after Liver Transplantation. Transplantology 2021, 2, 162–182. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Droc, G.; Martac, C.; Buzatu, C.G.; Jipa, M.; Punga, M.D.; Isac, S. Orthotopic Liver Transplantation of a SARS-CoV-2 Negative Recipient from a Positive Donor: The Border between Uncertainty and Necessity in a Pandemic Era- Case Report and Overview of the Literature. Medicina 2023, 59, 836. https://doi.org/10.3390/medicina59050836
Droc G, Martac C, Buzatu CG, Jipa M, Punga MD, Isac S. Orthotopic Liver Transplantation of a SARS-CoV-2 Negative Recipient from a Positive Donor: The Border between Uncertainty and Necessity in a Pandemic Era- Case Report and Overview of the Literature. Medicina. 2023; 59(5):836. https://doi.org/10.3390/medicina59050836
Chicago/Turabian StyleDroc, Gabriela, Cristina Martac, Cristina Georgiana Buzatu, Miruna Jipa, Maria Daniela Punga, and Sebastian Isac. 2023. "Orthotopic Liver Transplantation of a SARS-CoV-2 Negative Recipient from a Positive Donor: The Border between Uncertainty and Necessity in a Pandemic Era- Case Report and Overview of the Literature" Medicina 59, no. 5: 836. https://doi.org/10.3390/medicina59050836
APA StyleDroc, G., Martac, C., Buzatu, C. G., Jipa, M., Punga, M. D., & Isac, S. (2023). Orthotopic Liver Transplantation of a SARS-CoV-2 Negative Recipient from a Positive Donor: The Border between Uncertainty and Necessity in a Pandemic Era- Case Report and Overview of the Literature. Medicina, 59(5), 836. https://doi.org/10.3390/medicina59050836






