Biliary and Vascular Complications after Liver Transplantation–From Diagnosis to Treatment
Abstract
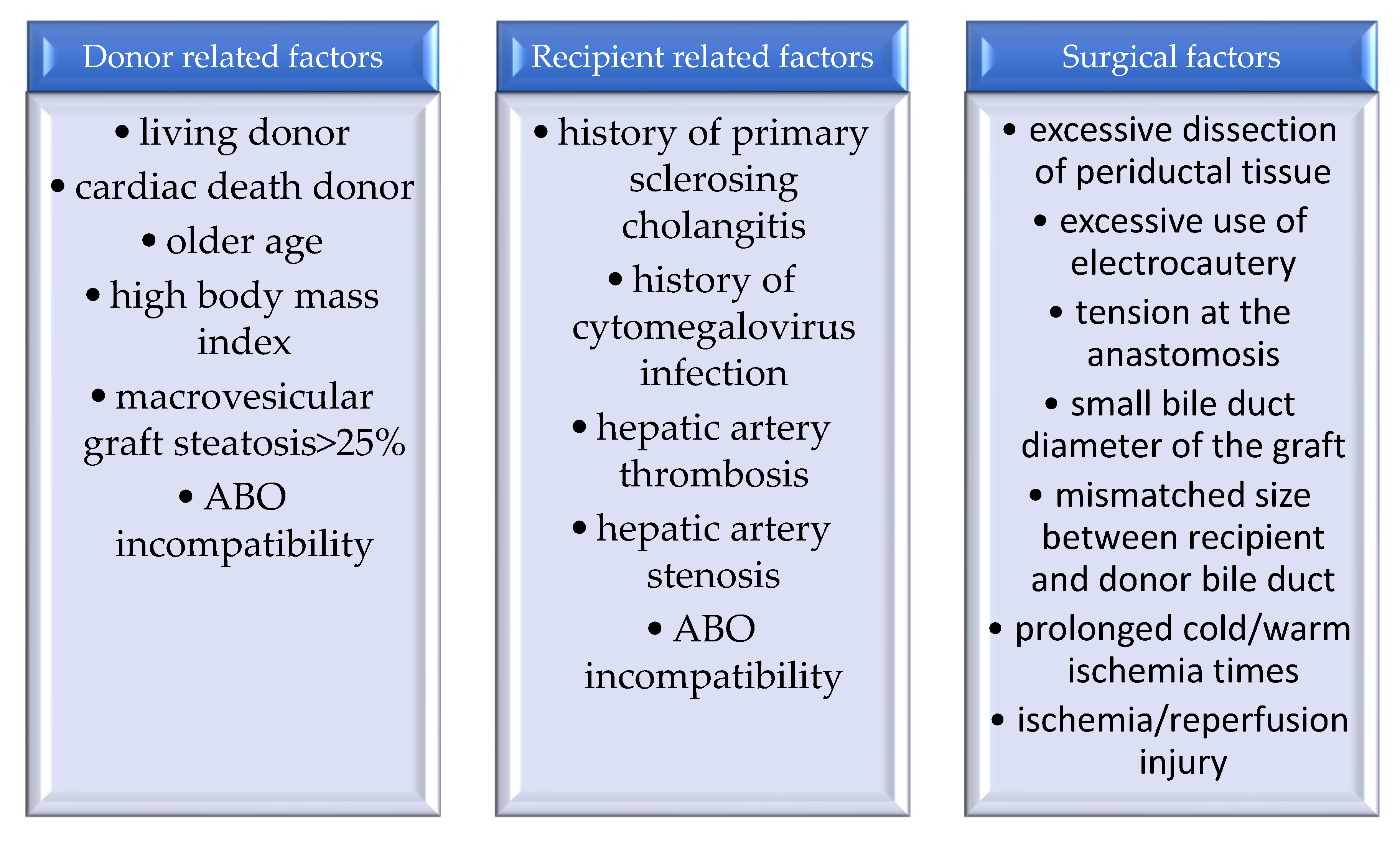
:1. Introduction
2. Biliary Complications
2.1. Biliary Strictures
2.2. Biliary Fistulas
2.3. Bilioma and Abscess
- Type I, true sphincter stenosis.
- Type II, the association of a structural dysfunction with motility disorder.
- Type III, functional biliary type pain [15,81]. Roma III criteria sustain the elimination of SOD type III and replacement with the terminology “functional biliary or pancreatic SOD” [68]. Biliary manometry can be used for diagnosis. The treatment of this condition usually involves only endoscopic sphincterotomy and in case of fibrosis of the sphincter apparatus, placement of stents [80,81,82,83].
3. Vascular Complications
3.1. Hepatic Artery Thrombosis
3.2. Portal Vein Thrombosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Boeva, I.; Karagyozov, P.I.; Tishkov, I. Post-liver transplant biliary complications: Current knowledge and therapeutic advances. World J. Hepatol. 2021, 13, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K.; Camilleri, M.; Fitz, G.; Kalloo, A.N.; Shanahan, F.; Wang, T.C. Yamada’s Textbook of Gastroenterology, 6th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 2129–2145. [Google Scholar]
- Ionescu, V.A.; Diaconu, C.C.; Bungau, S.; Jinga, V.; Gheorghe, G. Current approaches in the allocation of liver transplantation. J. Pers. Med. 2022, 12, 1661. [Google Scholar] [CrossRef]
- Clavien, P.A.; Breitenstein, S.; Belghiti, J.; Chari, R.S.; Llovet, J.M.; Lo, C.M.; Morse, M.A.; Takayama, T.; Vauthey, J.N. Malignant Liver Tumors: Current and Emerging Therapies, 3rd ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 307–346. [Google Scholar]
- United Network for Organ Sharing Organ Procurement and Transplantation Network 2019. Available online: https://optn.transplant.hrsa.gov. (accessed on 10 February 2023).
- Meier, R.P.H.; Kelly, Y.; Braun, H.; Maluf, D.; Freise, C.; Ascher, N.; Roberts, J.; Roll, G. Comparison of biliary complications rates after brain death, donation after circulatory death, and living-donor liver transplantation: A single-center cohort study. Transpl. Int. 2022, 35, 10855. [Google Scholar] [CrossRef]
- Kvietkauskas, M.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Machine Perfusion of Extended Criteria Donor Organs: Imunological Aspects. Front. Immunol. 2020, 11, 192. [Google Scholar] [CrossRef]
- Olschewski, P.; Gass, P.; Ariyakhagorn, V.; Jasse, K.; Hunold, G.; Menzel, M.; Schoning, W.; Schmitz, V.; Neuhaus, P.; Puhl, G. The influence of storage temperature during machine perfusion on perservation quality of marginal donor livers. Cryobiology 2010, 60, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Yeh, H.; Ozer, S.; Martins, P.N.; Farmer, A.; Wu, W.; Saeidi, N.; op den Dries, S.; Berendsen, T.A.; Smith, R.N.; et al. Subnormothermic machine perfusion for Ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transpl. 2014, 14, 1400–1409. [Google Scholar] [CrossRef]
- Piardi, T.; Lhuaire, M.; Bruno, O.; Memeo, R.; Pessaux, P.; Kianmanesh, R.; Sommacale, D. Vascular complications following liver transplantation: A literature review of advances in 2015. World J. Hepatol. 2016, 8, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, R.; Maruzelli, L.; Caruso, S.; Marrone, G.; Carollo, V.; Spada, M.; Luca, A.; Gridelli, B. Interventional radiology procedures in pediatric patients with complications after liver transplantation. Radiographics 2009, 29, 567–584. [Google Scholar] [CrossRef]
- Duffy, J.P.; Hong, J.C.; Farmer, D.G.; Ghobrial, R.M.; Yersiz, H.; Hiatt, J.R.; Busuttil, R.W. Vascular complications of orthotopic liver transplantation: Experience in more than 4,200 patients. J. Am. Coll. Surg. 2009, 208, 896–903. [Google Scholar] [CrossRef]
- Zhong, J.; Smith, C.; Walker, P.; Sheridan, M.; Guthrie, A.; Albazaz, R. Imaging post liver transplantation part I: Vascular complications. Clin. Radiol. 2020, 75, 845–853. [Google Scholar] [CrossRef]
- Khalaf, H. Vascular complications after deceased and living donor liver transplantation: A single-center experience. Transpl. Proc. 2010, 42, 865–870. [Google Scholar] [CrossRef]
- Pawlak, J.; Grodzicki, M.; Leowska, E.; Malkowski, P.; Michalowicz, B.; Nyckowski, P.; Rowinski, O.; Pacho, R.; Zieniewicz, K.; Andrezejewska, M.; et al. Vascular complications after liver transplantation. Transpl. Proc. 2003, 35, 2313–2315. [Google Scholar] [CrossRef] [PubMed]
- Moy, B.T.; Birk, J.W. A Review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J. Clin. Transl. Hepatol. 2019, 7, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Girotra, M.; Soota, K.; Klair, J.S.; Dang, S.M.; Aduli, F. Endoscopic management of post-liver transplant biliary complications. World J. Gastrointest. Endosc. 2015, 7, 446–459. [Google Scholar] [CrossRef]
- D’Antiga, L. Pediatric Hepatology and Liver Transplantation, 1st ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; p. 3. [Google Scholar]
- Fasullo, M.; Patel, M.; Khanna, L.; Shah, T. Post-transplant biliary complications: Advances in pathophysiology, diagnosis, and treatment. BMJ Open Gastroenterol. 2022, 9, e000778. [Google Scholar] [CrossRef]
- Clavien, P.A.; Trotter, J.F. Medical Care of the Liver Transplant Patient, 4th ed.; Wiley: Hoboken, NJ, USA, 2012; p. 1. [Google Scholar]
- Daniel, K.; Said, A. Early biliary complications after liver transplantation. Clin. Liver Dis. 2017, 10, 63–67. [Google Scholar] [CrossRef]
- Pinna, A.D.; Ercolani, G. Abdominal Solid Organ Transplantation: Immunology, Indications, Techniques and Early Complications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; p. 5. [Google Scholar]
- Nemes, B.; Gaman, G.; Doros, A. Biliary complications after liver transplantation. Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.F.; Hua, X.W.; Cui, X.L.; Xia, Q. Liver transplantation for hepatocellular carcinoma: Recent advances in China. J. Dig. Dis. 2014, 15, 51–53. [Google Scholar] [CrossRef]
- Wan, P.; Yu, X.; Xia, Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: A systematic review and meta-analysis. Liver Transpl. 2014, 20, 425. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Baker, T.; Goodrich, N.P.; Freise, C.; Hong, J.C.; Kumer, S.; Abt, P.; Cotterell, A.H.; Samstein, B.; Everhart, J.E.; et al. Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: A report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl. 2013, 19, 259. [Google Scholar] [CrossRef]
- Villa, N.A.; Harrison, M.E. Management of Biliary strictures after liver transplantation. Gastroenterol. Hepatol. 2015, 11, 316–318. [Google Scholar]
- Pascher, A.; Neuhaus, P. Biliary complications after deceased-donor orthotopic liver transplantation. J. Hepatobiliary Pancreat. Surg. 2006, 13, 487–496. [Google Scholar] [CrossRef]
- Sharma, S.; Gurakar, A.; Jabbour, N. Biliary strictures following liver transplantation: Past, present and preventive strategies. Liver Transpl. 2008, 14, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Jarlot-Gas, C.; Muscari, F.; Mokrane, F.Z.; Del Bello, A.; Culetto, A.; Buscail, E.; Pere, G.; Fares, N.; Peron, J.M.; Cuellar, E.; et al. Management of anastomotic biliary stricture after liver transplantation and impact on survival. HPB 2021, 23, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Qiao, L.L.; Du, Z.Q.; Zhang, J.; Wang, M.Z.; Wang, T.; Liu, W.M.; Zhang, L.; Dong, J.; Wu, Z.; et al. T-tube vs no T-tube for biliary tract reconstruction in adult orthotopic liver transplantation: An updated systematic review and meta-analysis. World J. Gastroenterol. 2021, 27, 1507–1523. [Google Scholar] [CrossRef]
- Glowka, T.R.; Karlstetter, C.; Weismuller, T.J.; Vilz, T.O.; Strassburg, C.P.; Kalff, J.C.; Manekeller, S. Intensified Endoscopic Evaluation for Biliary Complications After Orthotopic Liver Transplantation. Ann. Transpl. 2021, 26, e928907. [Google Scholar] [CrossRef]
- Kochhar, G.; Parungao, J.M.; Hanouneh, I.A.; Parsi, M.A. Biliary complications following liver transplantation. World J. Gastroenterol. 2013, 19, 2841–2846. [Google Scholar] [CrossRef]
- Wojcicki, M.; Milkiewicz, P.; Silva, M. Biliary Tract Complications after Liver Transplantation: A Review. Dig. Surg. 2008, 25, 245–257. [Google Scholar] [CrossRef]
- Scatton, O.; Meunier, B.; Cherqui, D.; Boillot, O.; Sauvanet, A.; Boudjema, K.; Launois, B.; Fagniez, P.L.; Belghiti, J.; Wolff, P. Randomized trial of choledochocholedo-chostomy with or without a T tube in orthotopic liver transplantation. Ann. Surg. 2001, 233, 432–437. [Google Scholar] [CrossRef]
- Mutignani, M.; Albert, J.G.; Fabbri, C. Endotherapy in Biliopancreatic Diseases: ERCP Meets EUS; Springer: Basel, Switzerland, 2020; pp. 483–489. [Google Scholar]
- Poley, J.W.; Lekkerkerker, M.N.; Metselaar, H.J.; Kuipers, E.J.; Bruno, M.J. Clinical outcome of progressive stenting in patients with anastomotic strictures after orthotopic liver transplantation. Endoscopy 2013, 45, 567–570. [Google Scholar] [CrossRef]
- Park, J.B.; Kwon, C.H.D.; Choi, G.S.; Chun, J.M.; Jung, G.O.; Kim, S.J.; Joh, J.W.; Lee, S.K. Prolonged cold ischemic time is a risk factor for biliary strictures in duct-to-duct biliary reconstruction in living donor liver trans-plantation. Transplantation 2008, 86, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.H.; Lee, S.K. Biliary Strictures after Liver Tranplantation. Gut Liver 2011, 5, 133–142. [Google Scholar] [CrossRef]
- Bennet, W.; Zimmerman, M.; Campsen, J.; Mandell, M.S.; Bak, T.; Wachs, M.; Kam, I. Choledochoduodenostomy is a safe alternative to Roux-en-Y choledochojejunostomy for biliary reconstruction in liver transplantation. World J. Surg. 2009, 33, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Botros, M.; Aziz, A.; Guorgui, J.G.; Agopian, V.G.; Farmer, D.G.; Busuttil, R.W.; Kaldas, F.M. Nonanastomotic biliary strictures after liver transplantation. Am. Surg. 2020, 86, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- De Vries, Y.; von Meijenfeldt, F.A.; Porte, R.J. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Roos, F.J.M.; Poley, J.W.; Polak, W.G.; Metselaar, H.J. Biliary complications after liver transplantation: Recent developments in etiology, diagnosis and endoscopic treatment. Best. Pract. Res. Clin. Gastroenterol. 2017, 31, 227–235. [Google Scholar] [CrossRef]
- Meurisse, N.; Pirenne, J.; Monbaliu, D. Non-anastomotic strictures after transplanting a liver graft with an accidentally ligated and unflushed common bile duct: A case report. Int. J. Surg. Case Rep. 2017, 41, 200–204. [Google Scholar] [CrossRef]
- Lee, H.W.; Shah, N.H.; Lee, S.K. An Update on Endoscopic Management of Post-Liver Transplant Biliary Complications. Clin. Endosc. 2017, 50, 451–463. [Google Scholar] [CrossRef]
- Aepli, P.; St John, A.; Gupta, S.; Hourigan, L.F.; Vaughan, R.; Efthymiou, M.; Kaffes, A. Success and complications of an intra-ductal fully covered self-expanding metal stent (ID-FCSEMS) to treat anastomotic biliary strictures (AS) after orthotopic liver transplantation (OLT). Surg. Endosc. 2017, 31, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Coté, G.A.; Slivka, A.; Tarnasky, P.; Mullady, D.K.; Elmunzer, B.J.; Elta, G.; Fogel, E.; Lehman, G.; McHenry, L.; Romagnuolo, J.; et al. Effect of covered metallic stents compared with plastic stents on benign biliary stricture resolution: A randomized clinical trial. JAMA 2016, 315, 1250–1257. [Google Scholar] [CrossRef]
- Kumar, K.Y.S.; Mathew, J.S.; Balakrishnan, D.; Bharathan, V.K.; Thankamony Amma, B.S.P.; Gopalakrishnan, U.; Narayana Menon, R.; Dhar, P.; Vayoth, S.O.; Sudhindran, S. Intraductal transanastomotic stenting in duct-to-duct biliary reconstruction after living-donor liver transplantation: A randomized trial. J. Am. Coll. Surg. 2017, 225, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.G.; Filmann, N.; Elsner, J.; Moench, C.; Trojan, J.; Bojunga, J.; Sarrazin, C.; Friedrich-Rust, M.; Herrmann, E.; Bechstein, W.O.; et al. Long-term follow-up of endoscopic therapy in stenosis of the bilio-biliary anastomosis associated with orthotopic liver transplantation. Liver Transpl. 2013, 19, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, I.W.; Schwaighofer, H.; Koch, R.; Nachbaur, K.; Koenigsrainer, A.; Margreiter, R.; Vogel, W. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl. 2006, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, H.; Weiss, K.H.; Gotthardt, D.; Adler, G.; Stremmel, W.; Schaible, A.; Dogan, A.; Stiehl, A.; Sauer, P. Is stenting necessary after balloon dilation of post-transplantation biliary strictures? Results of a prospective comparative study. Endoscopy 2008, 40, 746. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Simon, A.; Díaz-Gonzalez, A.; Thuluvath, P.J.; Cárdenas, A. Endoscopic retrograde cholangiography for biliary anastomotic strictures after liver transplantation. Clin. Liver Dis. 2014, 18, 913–926. [Google Scholar] [CrossRef]
- Aabakken, L.; Bretthauer, M.; Line, P.D. Double balloon enteroscopy for endoscopic retrograde cholangiography in patients with a Roux en Y anastomosis. Endoscopy 2007, 39, 1068. [Google Scholar] [CrossRef]
- Jue, T.L.; Imperial, J.C. Management of post liver transplant biliary strictures: A work in progress. Gastrointest. Endosc. 2008, 67, 886. [Google Scholar] [CrossRef]
- Verdonk, R.C.; Buis, C.I.; van der Jagt, E.J.; Gouw, A.S.; Limburg, A.J.; Slooff, M.J.; Kleibeuker, J.H.; Porte, R.J.; Haagsma, E.B. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl. 2007, 13, 725. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Asham, E.H.; Goldstein, L.; Han, S.H.; Saab, S.; Tong, M.J.; Busuttil, R.W.; Durazo, F.A. Endoscopic treatment with multiple stents for post-liver-transplantation nonanastomotic biliary strictures. Gastrointest. Endosc. 2009, 69, 1236. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Biggins, S.W.; Bagatelos, K.C.; Ostroff, J.W. Predictors of endoscopic treatment outcomes in the management of biliary problems after liver transplantation at a high-volume academic center. Gastrointest. Endosc. 2011, 73, 37. [Google Scholar] [CrossRef]
- Guichelaar, M.M.; Benson, J.T.; Malinchoc, M.; Krom, R.A.; Wiesner, R.H.; Charlton, M.R. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am. J. Transpl. 2003, 3, 885. [Google Scholar] [CrossRef]
- Martins, F.P.; De Paulo, G.A.; Contini, M.L.C.; Ferrari, A.P. Metal versus plastic stents for anastomotic biliary strictures after liver transplantation: A randomized controlled trial. Gastrointest. Endosc. 2018, 87, 131. [Google Scholar] [CrossRef] [PubMed]
- Tal, A.O.; Finkelmeier, F.; Filmann, N.; Kylänpää, L.; Udd, M.; Parzanese, I.; Cantù, P.; Dechêne, A.; Penndorf, V.; Schnitzbauer, A.; et al. Multiple plastic stents versus covered metal stent for treatment of anastomotic biliary strictures after liver transplantation: A prospective, randomized, multicenter trial. Gastrointest. Endosc. 2017, 86, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Tringali, A.; Tarantino, I.; Barresi, L.; Traina, M.; Bonato, G.; Cintolo, M.; Hassan, C.; Mutignani, M.; Adler, D.G. Multiple plastic versus fully covered metal stents for managing post-liver transplantation anastomotic biliary strictures: A meta-analysis of randomized controlled trials. Ann. Gastroenterol. 2019, 32, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Zeair, S.; Butkiewicz, F.; Butkiewicz, J.; Stasiuk, R. Application of fully covered self-expandable metallic stents with and without antimigration waist versus repeated plastic biliary stent placement in management of anastomotic biliary strictures after orthotopic liver transplantation. Ann. Transpl. 2017, 22, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.S.; Aly, R.A.; Sherif, A.E. Impact of MRCP findings on the management of biliary strictures in post-living donor liver transplant. Egypt. J. Radiol. Nucl. Med. 2019, 50, 19. [Google Scholar] [CrossRef]
- Akamatsu, N.; Sugawara, Y.; Hashimoto, D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: A systematic review of the incidence, risk factors and outcome. Transpl. Int. 2011, 24, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.D.; Draganov, P.V. Endoscopic management of biliary strictures after liver transplantation. World J. Gastroenterol. 2009, 15, 3725–3733. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Weinstein, J.; Black, S.; Spain, J.; Brady, P.S.; Dowell, J.D. Surgical and endovascular treatment of hepatic arterial complications following liver transplant. Clin. Transpl. 2014, 28, 1305–1312. [Google Scholar] [CrossRef]
- Porrett, P.M.; Hsu, J.; Shaked, A. Late surgical complications following liver transplantation. Liver Transpl. 2009, 15 (Suppl. S2), S12–S18. [Google Scholar] [CrossRef]
- Saad, W.E.; Davies, M.G.; Sahler, L.; Lee, D.E.; Patel, N.C.; Kitanosono, T.; Sasson, T.; Waldman, D.L. Hepatic artery stenosis in liver transplant recipients: Primary treatment with percutaneous transluminal angioplasty. J. Vasc. Interv. Radiol. 2005, 16, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Pfau, P.R.; Kimmey, M.B.; Ginsberg, G.G. Biliary complications after liver transplantation: The role of endoscopy. Endoscopy 2005, 37, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Yan, S.; Zheng, S. Bile Leakage after Liver Transplantation. Open. Med. 2017, 12, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lu, Q.; Luo, Y. Vascular complications after adult living donor liver transplantation: Evaluation with ultrasonography. World J. Gastroenterol. 2016, 22, 1617–1626. [Google Scholar] [CrossRef]
- Spier, B.J.; Pfau, P.R.; Lorenze, K.R.; Knechtle, S.J.; Said, A. Risk factors and outcomes in post-liver transplantation bile duct stones and casts: A case-control study. Liver Transpl. 2008, 14, 1461–1465. [Google Scholar] [CrossRef]
- Sheng, R.; Ramirez, C.B.; Zajko, A.B.; Campbell, W.L. Biliary stones and sludge in liver transplant patients: A 13-year experience. Radiology 1996, 198, 243–247. [Google Scholar] [CrossRef]
- Farouk, M.; Branum, G.D.; Watters, C.R.; Cucchiaro, G.; Helms, M.; McCann, R.; Bollinger, R.; Meyers, W.C. Bile compositional changes and cholesterol stone formation following orthotopic liver transplantation. Transplantation 1991, 52, 727–730. [Google Scholar]
- Maheshwari, A.; Maley, W.; Li, Z.; Thuluvath, P.J. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007, 13, 1645–1653. [Google Scholar] [CrossRef]
- Rerknimitr, R.; Sherman, S.; Fogel, E.L.; Kalayci, C.; Lumeng, L.; Chalasani, N.; Kwo, P.; Lehman, G.A. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: Endoscopic findings and results of therapy. Gastrointest. Endosc. 2002, 55, 224–231. [Google Scholar] [CrossRef]
- Gürakar, A.; Wright, H.; Camci, C.; Jaboour, N. The application of SpyScope® technology in evaluation of pre and post liver transplant biliary problems. Turk. J. Gastroenterol. 2010, 21, 428–432. [Google Scholar] [CrossRef]
- Nam, K.; Lee, S.K.; Song, T.J.; Park, D.H.; Lee, S.S.; Seo, D.W.; Kim, M.H. Percutaneous transhepatic cholangioscopy for biliary complications after liver transplantation: A single center experience. J. Hepatobiliary Pancreat. Sci. 2016, 23, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; Finkelmeier, F.; Friedrich-Rust, M.; Kronenberger, B.; Trojan, J.; Zeuzem, S.; Sarrazin, C. Identifying indications for percutaneous (PTC) vs. endoscopic ultrasound (EUS)-guided “Rendezvous” procedure in biliary obstruction and incomplete endo-scopic retrograde cholangiography (ERC). Z. Für Gastroenterol. 2014, 52, 1157–1163. [Google Scholar]
- Sarhan, M.D.; Osman, A.M.A.; Mohamed, M.A.; Abdelaziz, O.; Serour, D.K.; Mansour, D.A.; Mogawer, S.; Helmy, A.S.; El-Shazli, M.A.; Hosny, A.A. Biliary Complications in Recipients of Living-Donor Liver Transplant: A Single-Center Review of 120 Patients. Exp. Clin. Transpl. 2017, 15, 648. [Google Scholar]
- Doskhanov, M.; Kausova, G.; Chotmanov, A.; Baimakhanov, B.; Askeev, B. Biliary complications after liver transplantation. Georgian Med. News 2019, 296, 7–11. [Google Scholar] [CrossRef]
- Bistritz, L.; Bain, V.G. Sphincter of Oddi dysfunction: Managing the patient with chronic biliary pain. World J. Gastroenterol. 2006, 12, 3793–3802. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, J.; Li, X.; Zhang, C.; Zhou, X.; Zhang, C. Biliary tract reconstruction with or without T-tube in orthotopic liver transplantation: A systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 529–538. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Ellis, S.M.; Karani, J.B.; Ryan, S.M. Hepatic artery stenosis following liver transplantation: Significance of the tardus parvus waveform and the role of microbubble contrast media in the detection of a focal stenosis. Clin. Radiol. 2002, 57, 789–799. [Google Scholar] [CrossRef]
- Hejazi Kenari, S.K.; Zimmerman, A.; Eslami, M.; Saidi, R.F. Current state of art management for vascular complications after liver transplantation. Middle East. J. Dig. Dis. 2014, 6, 121–130. [Google Scholar] [CrossRef]
- Park, G.C.; Moon, D.B.; Kang, S.H.; Ahn, C.S.; Hwang, S.; Kim, K.H.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Yoon, Y.I.; et al. Overcoming Hepatic Artery Thrombosis After Living Donor Liver Transplantations: An Experience from Asan Medical Center. Ann. Transpl. 2019, 24, 588–593. [Google Scholar] [CrossRef]
- Abdelaziz, O.; Hosny, K.; Amin, A.; Emadeldin, S.; Uemoto, S.; Mostafa, M. Endovascular management of early hepatic artery thrombosis after living donor liver transplantation. Transpl. Int. 2012, 25, 847–856. [Google Scholar] [CrossRef]
- Perez-Saborino, B.; Pacheco-Sanchez, D.; Barrera-Rebollo, A.; Asensio-Diaz, E.; Pinto-Fuentes, P.; Sarmentero-Prieto, J.C.; Rodriguez-Vielva, P.; Martinez-Diaz, R.; Gonzalo-Martin, M.; Rodríguez, M.; et al. Incidence, Management, and Rezults of Vascular Complications After Liver Transplantation. Tranpl. Proc. 2011, 43, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Stange, B.J.; Glanemann, M.; Nuessler, N.C.; Settmacher, U.; Steinmüller, T.; Neuhaus, P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003, 9, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.; Davies, M.G.; Saad, N.E.; Westesson, K.E.; Patel, N.C.; Sahler, L.G.; Lee, D.E.; Kitanosono, T.; Sasson, T.; Waldman, D.L. Catheter thrombolysis of thrombosed hepatic arteries in liver transplant recipients: Predictors of success and role of thrombolysis. Vasc. Endovasc. Surg. 2007, 41, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Stokes, K.; Sebastian, A.; Wright, H.I.; Kohli, V. Endovascular treatment of hepatic artery thrombosis following liver transplantation. Transpl. Int. 2010, 23, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Panaro, F.; Gallix, B.; Bouyabrine, H.; Ramos, J.; Addeo, P.; Testa, G.; Carabalona, J.P.; Pageaux, G.; Domergue, J.; Navarro, F. Liver transplantation and spontaneous neovascularization after arterial thrombosis: “The neovascularized liver”. Transpl. Int. 2011, 24, 949–957. [Google Scholar] [CrossRef]
- Diaconu, C.; Balaceanu, A.; Costache, C. Prevalence of hypoxic hepatitis in heart failure patients. J. Hepatol. 2014, 60 (Suppl. S1), S515. [Google Scholar] [CrossRef]
- Pareja, E.; Cortes, M.; Navarro, R.; Sanjuan, F.; López, R.; Mir, J. Vascular complications after orthotopic liver transplantation: Hepatic artery thrombosis. Transpl. Proc. 2010, 42, 2970–2972. [Google Scholar] [CrossRef]
- Fouzas, I.; Sklavos, A.; Bismpa, K.; Paxiadakis, I.; Antoniadis, N.; Giakoustidis, D.; Katsiki, E.; Tatsou, N.; Mouloudi, E.; Karapanagiotou, A.; et al. Hepatic artery thrombosis after orthotopic liver transplantation: 3 patients with collateral formation and conservative treatment. Transpl. Proc. 2012, 44, 2741–2744. [Google Scholar] [CrossRef]
- Woo, D.H.; Laberge, J.M.; Gordon, R.L.; Wilson, M.W.; Kerlan, R.K. Management of portal venous complications after liver transplantation. Tech. Vasc. Interv. Radiol. 2007, 10, 233–239. [Google Scholar]
- Huang, T.L.; Cheng, Y.F.; Chen, T.Y.; Tsang, L.L.; Ou, H.Y.; Yu, C.Y.; Wang, C.C.; Wang, S.H.; Lin, C.L.; Cheung, H.K.; et al. Doppler ultrasound evaluation of postoperative portal vein stenosis in adult living donor liver transplantation. Transpl. Proc. 2010, 42, 879–881. [Google Scholar]
- Lee, S.J.; Kim, K.W.; Kim, S.Y.; Park, Y.S.; Lee, J.; Kim, H.J.; Lee, J.S.; Song, G.W.; Hwang, S.; Lee, S.G. Contrast-enhanced sonography for screening of vascular complication in recipients following living donor liver transplantation. J. Clin. Ultrasound 2013, 41, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Rennert, J.; Dornia, C.; Georgieva, M.; Roehrl, S.; Fellner, C.; Schleder, S.; Stroszczynski, C.; Jung, E.M. Identification of early complications following liver transplantation using contrast enhanced ultrasound (CEUS). First results. J. Gastrointest. Liver Dis. 2012, 21, 407–412. [Google Scholar]
| Biliary Complications | Type |
|---|---|
| Biliary strictures | Anastomotic strictures Non-anastomotic strictures |
| Bile leaks | Anastomotic leaks Non-anastomotic leaks |
| Bile duct filling defects | Bile duct stones Bile duct casts |
| Other complications | Sphincter of Oddi dysfunction Biloma |
| Vascular Complications | Types |
|---|---|
| Arterial complications | Hepatic artery thrombosis (HAT) Hepatic artery stenosis (HAS) Hepatic artery pseudoaneurysm (HAP) Hepatic artery rupture (HAR) |
| Portal vein complications | Portal vein thrombosis (PVT) Portal vein stenosis (PVS) |
| Caval anastomosis complications | Caval resection and end-to-end cavo-caval anastomosis Piggy-back |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, G.; Diaconu, C.C.; Bungau, S.; Bacalbasa, N.; Motas, N.; Ionescu, V.-A. Biliary and Vascular Complications after Liver Transplantation–From Diagnosis to Treatment. Medicina 2023, 59, 850. https://doi.org/10.3390/medicina59050850
Gheorghe G, Diaconu CC, Bungau S, Bacalbasa N, Motas N, Ionescu V-A. Biliary and Vascular Complications after Liver Transplantation–From Diagnosis to Treatment. Medicina. 2023; 59(5):850. https://doi.org/10.3390/medicina59050850
Chicago/Turabian StyleGheorghe, Gina, Camelia Cristina Diaconu, Simona Bungau, Nicolae Bacalbasa, Natalia Motas, and Vlad-Alexandru Ionescu. 2023. "Biliary and Vascular Complications after Liver Transplantation–From Diagnosis to Treatment" Medicina 59, no. 5: 850. https://doi.org/10.3390/medicina59050850







