Evaluation of Ocular Diameter Parameters Using Swept-Source Optical Coherence Tomography
Abstract
:1. Introduction
2. Methods
Subjects
3. Data Acquisition
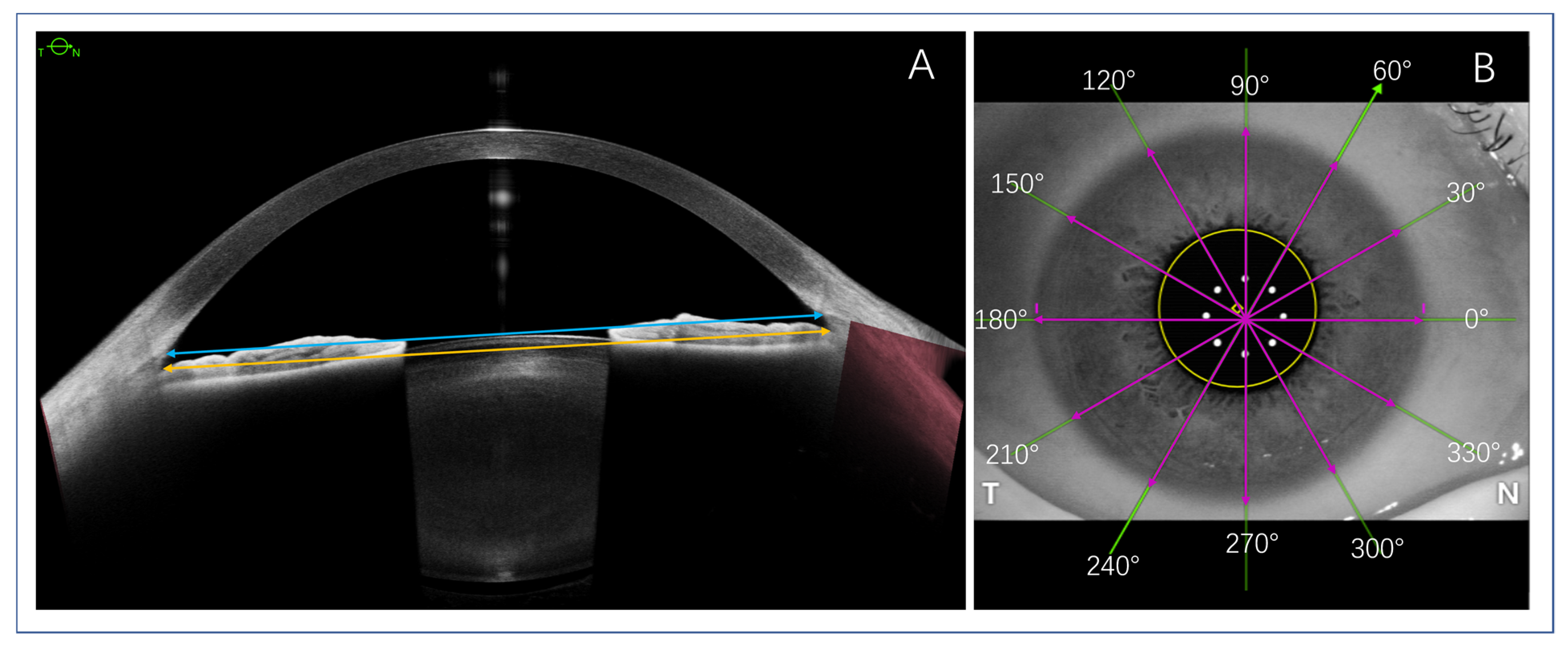
4. ATA, STS, and WTW Parameter Measurements
5. ACIOL and ICL Size Calculations
6. Statistical Analysis
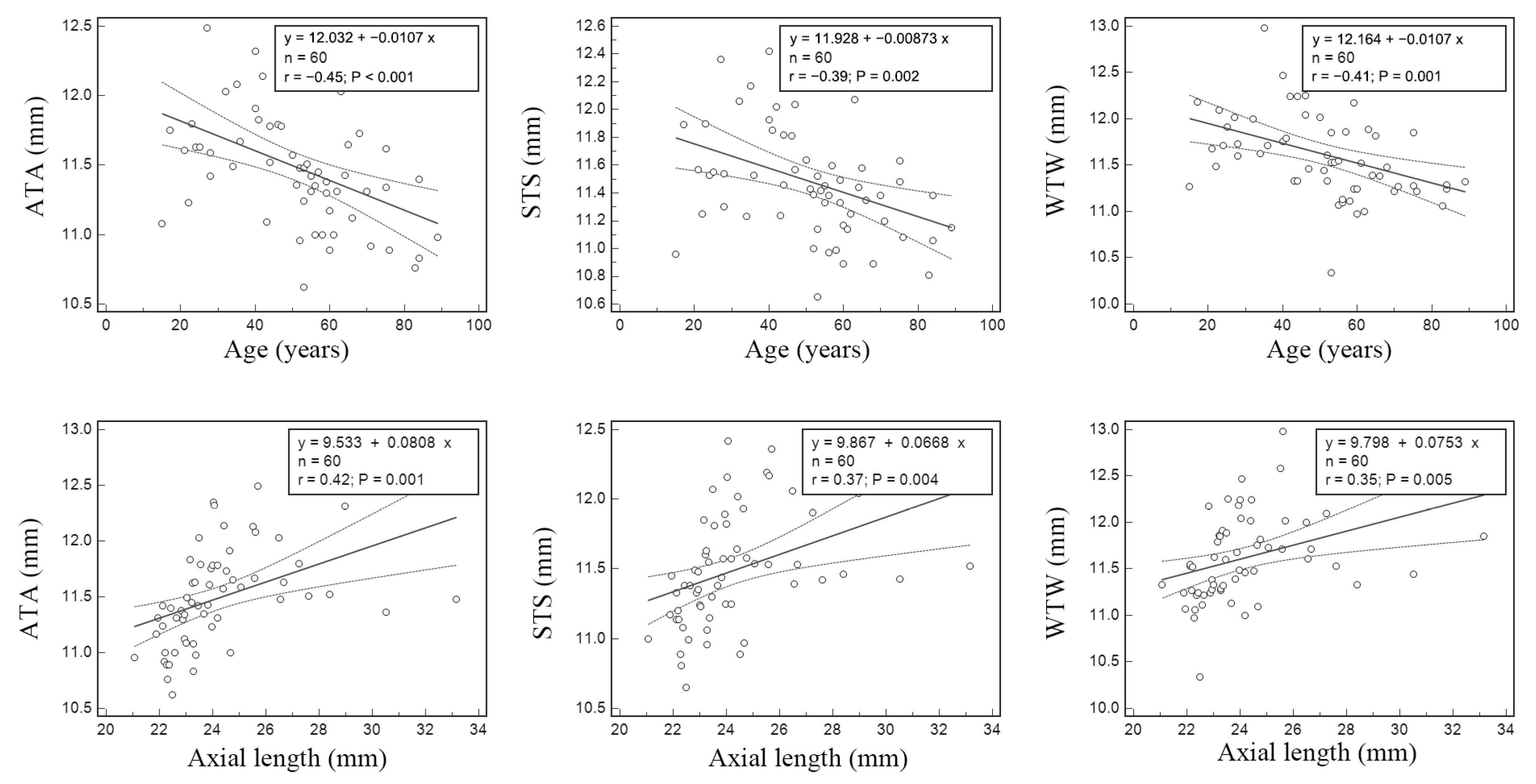
7. Results
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahapatra, S.; Mannem, N. Anterior chamber intraocular lens—An effective alternative in traumatic and surgical aphakia in the era of scleral-fixated intraocular lens. Indian J. Ophthalmol. 2021, 69, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Bruner, C.; Skanchy, D.F.; Wooten, J.P.; Chuang, A.Z.; Kim, G. Anterior chamber lens sizing: Comparison of white-to-white and scleral spur-to-scleral spur methods. J. Cataract. Refract. Surg. 2020, 46, 95–101. [Google Scholar] [PubMed]
- Sanders, D.R.; Vukich, J. Comparison of Implantable Contact Lens and Laser Assisted In Situ Keratomileusis for Moderate to High Myopia. Cornea 2003, 22, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Meta-analysis and review: Effectiveness, safety, and central port design of the intraocular collamer lens. Clin. Ophthalmol. 2016, 10, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. The Implantable Collamer Lens with a central port: Review of the literature. Clin. Ophthalmol. 2018, 12, 2427–2438. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Lee, J.E. Traumatic dislocation of posterior chamber phakic intraocular lens into the Berger’s space. Korean J. Ophthalmol. 2016, 30, 396–397. [Google Scholar] [CrossRef]
- He, J.; Zhang, L.; Zheng, F.; Fang, X. Case report: Dislocation into vitreous cavity and removal of a posterior chamber phakic in-traocular lens. Front. Med. 2022, 8, 792253. [Google Scholar] [CrossRef]
- Kojima, T.; Kitazawa, Y.; Nakamura, T.; Kamiya, K.; Ichikawa, K.; Igarashi, A.; Shimizu, K. Multicenter survey on implantable collamer lens dislocation. PLoS ONE 2022, 17, e0264015. [Google Scholar] [CrossRef]
- Yildirim, T.M.; Khoramnia, R.; Son, H.S.; Mayer, C.S.; Łabuz, G.; Munro, D.J.; Auffarth, G.U. Reasons for explantation of phakic intra-ocular lenses and associated perioperative complications: Cross-sectional explant registry analysis. BMC Ophthalmol. 2021, 21, 80. [Google Scholar] [CrossRef]
- Chen, X.; Han, T.; Zhao, W.; Wang, X.; Xu, Y.; Cheng, M.; Zhou, X. Effect of the difference between the white-to-white and sulcus-to-sulcus on vault and the relat-ed factors after ICL implantation. Ophthalmol. Ther. 2021, 10, 947–955. [Google Scholar] [CrossRef]
- Huang, G.; Gonzalez, E.; Peng, P.H.; Lee, R.; Leeungurasatien, T.; He, M.; Lin, S.C. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: Narrow vs open iridocorneal angles. Arch. Ophthalmol. 2011, 129, 1283–1290. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, M.K.; Wee, W.R.; Lee, J.H. Effects of White-to-White Diameter and Anterior Chamber Depth on Implantable Collamer Lens Vault and Visual Outcome. J. Refract. Surg. 2009, 25, 730–738. [Google Scholar] [CrossRef]
- Montés-Micó, R.; Tañá-Rivero, P.; Aguilar-Córcoles, S.; Ruiz-Santos, M.; Rodríguez-Carrillo, M.D.; Ruiz-Mesa, R. Angle-to-angle and spur-to-spur distance analysis with high-resolution optical coherence tomography. Eye Vis. 2020, 7, 42. [Google Scholar] [CrossRef]
- Kojima, T.; Yokoyama, S.; Ito, M.; Horai, R.; Hara, S.; Nakamura, T.; Ichikawa, K. Optimization of an implantable collamer lens sizing method using high-frequency ultra-sound biomicroscopy. Am. J. Ophthalmol. 2012, 153, 632–637.e1. [Google Scholar] [CrossRef]
- Buckenham Boyle, A.; Namkung, S.; Shew, W.; Gokul, A.; McGhee, C.N.J.; Ziaei, M. Repeatability and agreement of white-to-white measurements between slit-scanning tomography, infrared biometry, dual rotating Scheimpflug camera/Placido disc tomog-raphy, and swept source anterior segment optical coherence tomography. PLoS ONE 2021, 16, e0254832. [Google Scholar] [CrossRef]
- Baumeister, M.; Terzi, E.; Ekici, Y.; Kohnen, T. Comparison of manual and automated methods to determine horizontal corneal diameter. J. Cataract. Refract. Surg. 2004, 30, 374–380. [Google Scholar] [CrossRef]
- Nakamura, T.; Isogai, N.; Kojima, T.; Yoshida, Y.; Sugiyama, Y. Optimization of implantable collamer lens sizing based on swept-source anterior segment optical coherence tomography. J. Cataract. Refract. Surg. 2020, 46, 742–748. [Google Scholar] [CrossRef]
- Chen, S.; Potsaid, B.; Li, Y.; Lin, J.; Hwang, Y.; Moult, E.M.; Zhang, J.; Huang, D.; Fujimoto, J.G. High speed, long range, deep penetration swept source OCT for structural and angiographic imaging of the anterior eye. Sci. Rep. 2022, 12, 992. [Google Scholar] [CrossRef]
- Matarazzo, F.; Day, A.C.; Fernandez-Vega Cueto, L.; Maurino, V. Vertical implantable collamer lens (ICL) rotation for the man-agement of high vault due to lens oversizing. Int. Ophthalmol. 2018, 38, 2689–2692. [Google Scholar] [CrossRef]
- Wei, R.; Li, M.; Aruma, A.; Knorz, M.C.; Yang, D.; Yu, Y.; Wang, X.; Choi, J.; Yao, P.; Zhou, X. Factors leading to realignment or ex-change after implantable collamer lens implantation in 10 258 eyes. J. Cataract. Refract. Surg. 2022, 48, 1190–1196. [Google Scholar] [CrossRef]
- Sakata, L.M.; Lavanya, R.; Friedman, D.S.; Aung, H.T.; Seah, S.K.; Foster, P.J.; Aung, T. Assessment of the scleral spur in anterior segment optical coherence tomography images. Arch. Ophthalmol. 2008, 126, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; He, W.; Meng, J.; Qian, D.; Lu, Y.; Zhu, X. Evaluation of the White-to-White Distance in 39,986 Chinese Cataractous Eyes. Investig. Opthalmol. Vis. Sci. 2021, 62, 7. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R. Ethnicity and ocular imaging. Eye 2010, 25, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, G.; Hassan, Z.; Szalai, E.; Berta, A.; Modis, L. Comparative analysis of white-to-white and angle-to-angle distance measurements with partial coherence interferometry and optical coherence tomography. J. Cataract. Refract. Surg. 2010, 36, 1862–1866. [Google Scholar] [CrossRef]
- Petermeier, K.; Suesskind, D.; Altpeter, E.; Schatz, A.; Messias, A.; Gekeler, F.; Szurman, P. Sulcus anatomy and diameter in pseudophakic eyes and correlation with bio-metric data: Evaluation with a 50 MHz ultrasound biomicroscope. J. Cataract. Refract. Surg. 2012, 38, 986–991. [Google Scholar] [CrossRef]
- Werner, L.; Izak, A.M.; Pandey, S.K.; Apple, D.J.; Trivedi, R.H.; Schmidbauer, J.M. Correlation between different measurements within the eye relative to phakic intraocular lens implantation. J. Cataract. Refract. Surg. 2004, 30, 1982–1988. [Google Scholar] [CrossRef]
- Baikoff, G.; Jodai, H.J.; Bourgeon, G. Measurement of the internal diameter and depth of the anterior chamber: IOLMaster versus anterior chamber optical coherence tomographer. J. Cataract. Refract. Surg. 2005, 31, 1722–1728. [Google Scholar] [CrossRef]
- Oh, J.; Shin, H.-H.; Kim, J.-H.; Kim, H.-M.; Song, J.-S. Direct Measurement of the Ciliary Sulcus Diameter by 35-Megahertz Ultrasound Biomicroscopy. Ophthalmology 2007, 114, 1685–1688. [Google Scholar] [CrossRef]
- Salouti, R.; Nowroozzadeh, M.H.; Zamani, M.; Ghoreyshi, M.; Salouti, R. Comparison of horizontal corneal diameter measure-ments using Galilei, EyeSys and Orbscan II systems. Clin. Exp. Optom. 2009, 92, 429–433. [Google Scholar] [CrossRef]
- Abass, A.; Lopes, B.T.; Eliasy, A.; Wu, R.; Jones, S.; Clamp, J.R.A., Jr.; Elsheikh, A. Three-dimensional non-parametric method for limbus detection. PLoS ONE 2018, 13, e0207710. [Google Scholar] [CrossRef]
- Namkung, S.; Boyle, A.B.; Li, Y.; Gokul, A.; McGhee, C.; Ziaei, M. Repeatability and agreement of horizontal corneal diameter measurements between scanning-slit topography, dual rotating scheimpflug camera with placido disc tomography, placido disc topography, and optical coherence tomography. Cornea 2021, 41, 1392–1397. [Google Scholar] [CrossRef]
- Dong, J.; Yao, J.; Chang, S.; Kanclerz, P.; Khoramnia, R.; Wang, X. Comparison Study of the Two Biometers Based on Swept-Source Optical Coherence Tomography Technology. Diagnostics 2022, 12, 598. [Google Scholar] [CrossRef]
- Esaki, K.; Ishikawa, H.; Liebmann, J.M.; Greenfield, D.S.; Uji, Y.; Ritch, R. Angle recess area decreases with age in normal Japanese. Jpn. J. Ophthalmol. 2000, 44, 46–51. [Google Scholar] [CrossRef]
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 51 ± 18 | 15–89 |
| AL (mm) | 24.19 ± 2.20 | 21.05–33.16 |
| CCT (μm) | 522 ± 34 | 447–589 |
| ACD (mm) | 3.39 ± 0.43 | 1.99–4.20 |
| LT (mm) | 4.14 ± 0.49 | 2.90–5.29 |
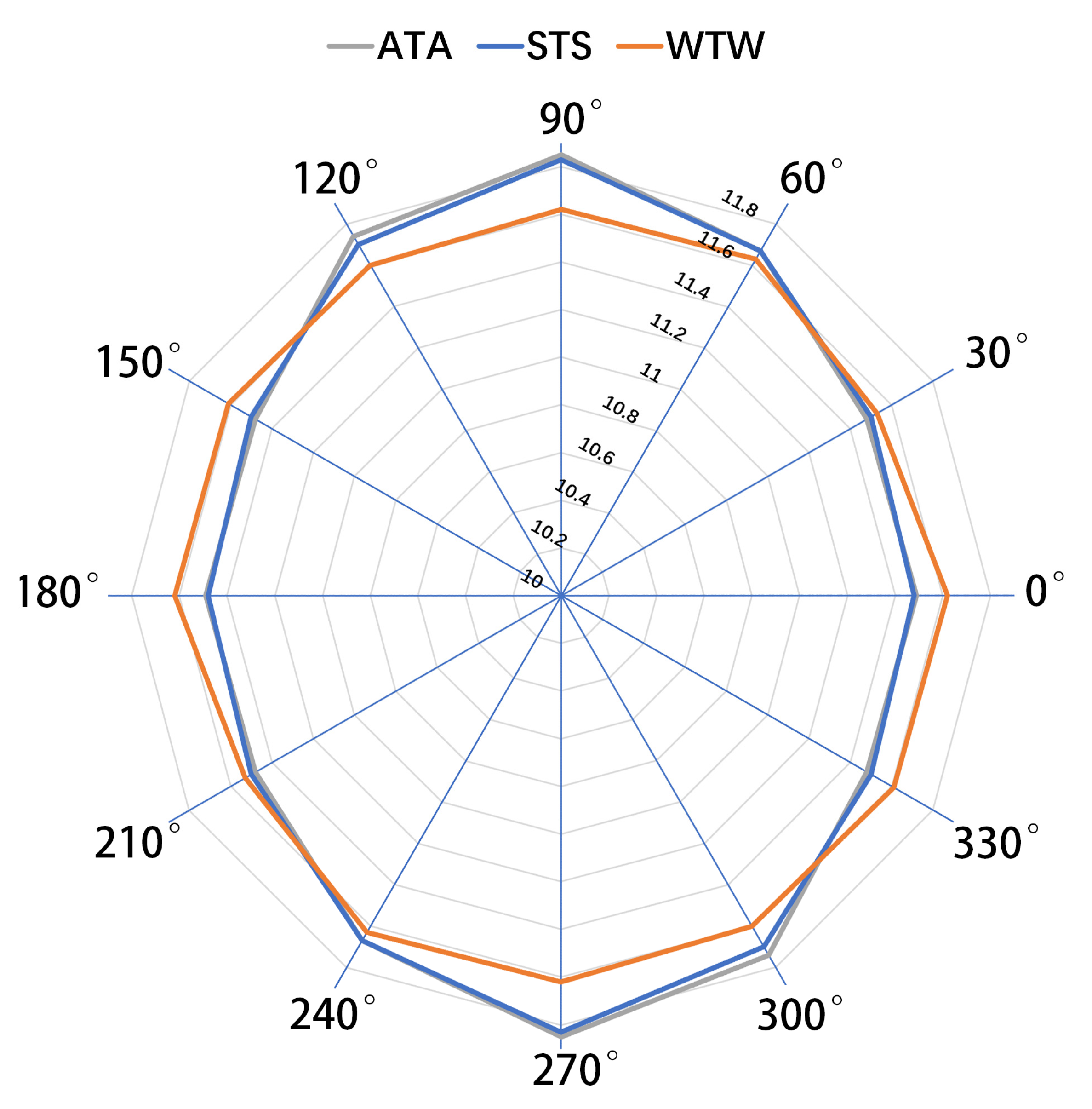
| ATA 90°–270° (mm) | 11.85 ± 0.48 | 10.61–12.93 |
| ATA 60°–240° (mm) | 11.67 ± 0.47 | 10.70–12.69 |
| ATA 30°–210° (mm) | 11.48 ± 0.45 | 10.70–12.52 |
| ATA 0°–180° (mm) | 11.49 ± 0.43 | 10.62–12.49 |
| ATA 150°–330° (mm) | 11.48 ± 0.43 | 10.62–12.61 |
| ATA 120°–300° (mm) | 11.74 ± 0.43 | 10.91–12.74 |
| STS 90°–270° (mm) | 11.83 ± 0.47 | 10.62–12.80 |
| STS 60°–240° (mm) | 11.67 ± 0.45 | 10.95–12.63 |
| STS 30°–210° (mm) | 11.50 ± 0.43 | 10.80–12.45 |
| STS 0°–180° (mm) | 11.48 ± 0.40 | 10.65–12.42 |
| STS 150°–330° (mm) | 11.50 ± 0.40 | 10.82–12.44 |
| STS 120°–300° (mm) | 11.70 ± 0.42 | 10.72–12.56 |
| WTW 90°–270° (mm) | 11.62 ± 0.37 | 10.95–12.58 |
| WTW 60°–240° (mm) | 11.63 ± 0.43 | 10.92–12.79 |
| WTW 30°–210° (mm) | 11.53 ± 0.44 | 10.40–12.81 |
| WTW 0°–180° (mm) | 11.62 ± 0.47 | 10.34–12.98 |
| WTW 150°–330° (mm) | 11.61 ± 0.40 | 10.48–12.58 |
| WTW 120°–300° (mm) | 11.60 ± 0.35 | 10.83–12.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Yao, J.; Chang, S.; Kanclerz, P.; Khoramnia, R.; Wang, X. Evaluation of Ocular Diameter Parameters Using Swept-Source Optical Coherence Tomography. Medicina 2023, 59, 899. https://doi.org/10.3390/medicina59050899
Dong J, Yao J, Chang S, Kanclerz P, Khoramnia R, Wang X. Evaluation of Ocular Diameter Parameters Using Swept-Source Optical Coherence Tomography. Medicina. 2023; 59(5):899. https://doi.org/10.3390/medicina59050899
Chicago/Turabian StyleDong, Jing, Jinhan Yao, Shuimiao Chang, Piotr Kanclerz, Ramin Khoramnia, and Xiaogang Wang. 2023. "Evaluation of Ocular Diameter Parameters Using Swept-Source Optical Coherence Tomography" Medicina 59, no. 5: 899. https://doi.org/10.3390/medicina59050899





