Fetal Biometric Assessment and Infant Developmental Prognosis of the Tadalafil Treatment for Fetal Growth Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Study Population
2.2. Ultrasound Procedures
2.3. Maternal Hemodynamics
2.4. The Kyoto Scale of Psychological Development (KSPD) Test
2.5. Statistics
3. Results
3.1. Characteristics of Participants
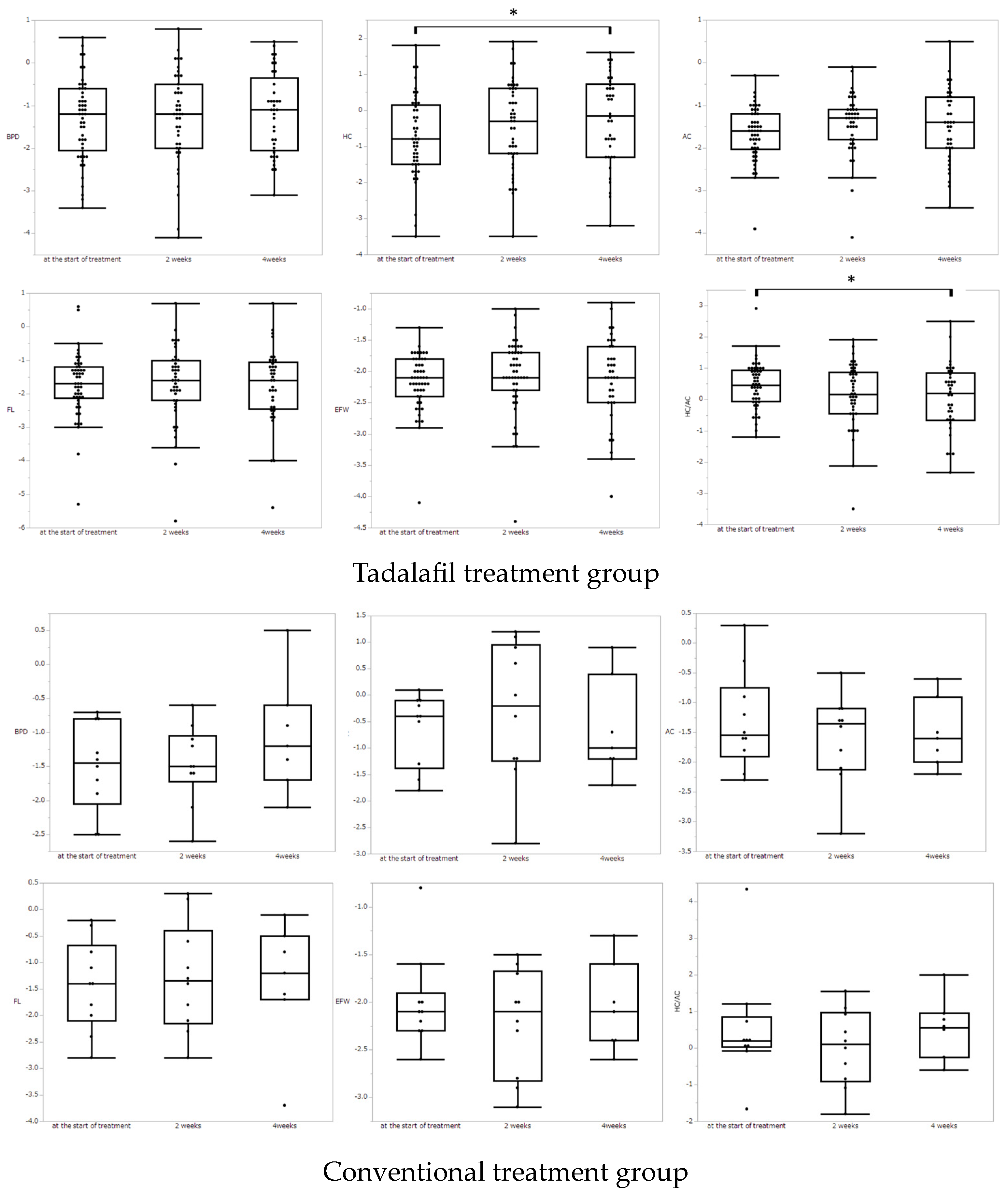
3.2. Fetal Biometric Measurements
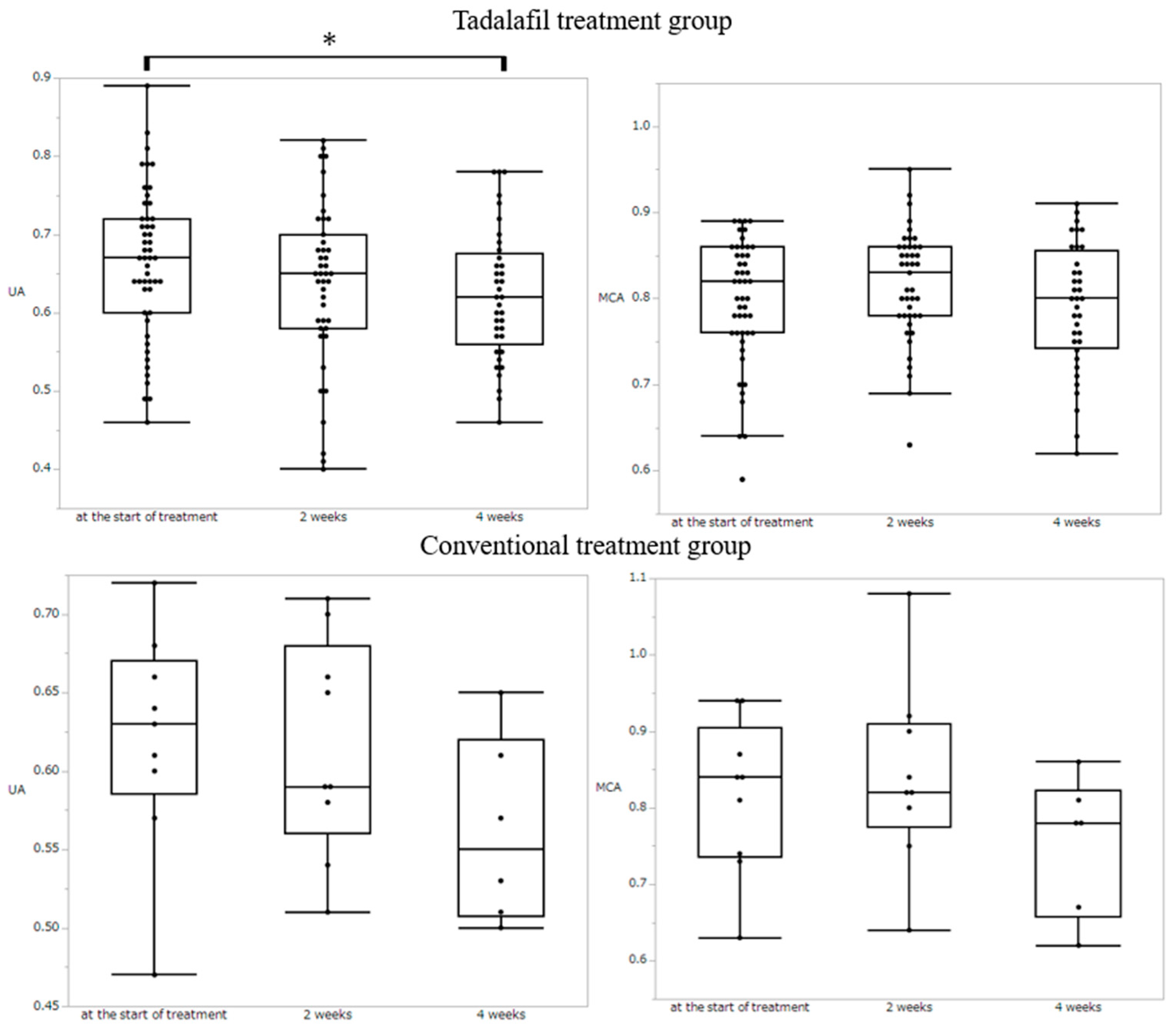
3.3. Doppler Study
3.4. Maternal Hemodinamics
3.5. KSPD Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lees, C.; Marlow, N.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Duvekot, J.; Frusca, T.; Diemert, A.; Ferrazzi, E.; et al. Perinatal Morbidity and Mortality in Early-Onset Fetal Growth Restriction: Cohort Outcomes of the Trial of Randomized Umbilical and Fetal Flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol. 2013, 42, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.A.; Grunau, R.E.; McAuliffe, F.M.; Pinnamaneni, R.; Foran, A.; Alderdice, F.A. Early Childhood Neurodevelop-ment After Intrauterine Growth Restriction: A Systematic Review. Pediatrics 2015, 135, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Fernandes, M.; Fazel, M.; Kennedy, S.; Villar, J.; Stein, A. Differential Effect of Intrauterine Growth Restriction on Childhood Neurodevelopment: A Systematic Review. BJOG 2015, 122, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Challis, D. Diagnosis and Management of Fetal Growth Restriction: The Role of Fetal Therapy. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Flenady, V.; Koopmans, L.; Middleton, P.; Frøen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major Risk Factors for Stillbirth in High-Income Countries: A Systematic Review and Meta-analysis. Lancet 2011, 377, 1331–1340. [Google Scholar] [CrossRef]
- Iliodromiti, S.; Mackay, D.F.; Smith, G.C.S.; Pell, J.P.; Sattar, N.; Lawlor, D.A.; Nelson, S.M. Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland. PLoS Med. 2017, 14, e1002228. [Google Scholar] [CrossRef]
- Abecassis, A.; Wainstock, T.; Sheiner, E.; Pariente, G. Perinatal Outcome and Long-Term Gastrointestinal Morbidity of Offspring of Women With Celiac Disease. J. Clin. Med. 2019, 8, 1924. [Google Scholar] [CrossRef]
- Korkalainen, N.; Räsänen, J.; Kaukola, T.; Kallankari, H.; Hallman, M.; Mäkikallio, K. Fetal Hemodynamics and Adverse Outcome in Primary School-aged Children with Fetal Growth Restriction: A Prospective Longitudinal Study. Acta Obstet. Gynecol. Scand. 2017, 96, 69–77. [Google Scholar] [CrossRef]
- Hanson, M. The Birth and Future Health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” Are Associated with Disorders of Deep Placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Meher, S.; Duley, L.; Hunter, K.; Askie, L. Antiplatelet Therapy Before or After 16 Weeks’ Gestation for Preventing Preeclampsia: An Individual Participant Data Meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 121–128.e2. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; McLeod, A.; Windrim, R.C.; Kingdom, J. Antithrombotic Therapy for Improving Maternal or Infant Health Outcomes in Women Considered At Risk of Placental Dysfunction. Cochrane Database Syst. Rev. 2010, 16, CD006780. [Google Scholar]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The Role of Aspirin Dose on the Prevention of Preeclampsia and Fetal Growth Restriction: Systematic Review and Meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120.e6. [Google Scholar] [CrossRef]
- Costantine, M.M.; Cleary, K. Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric Fetal Pharmacology Research Units Network. Pravastatin for the Prevention of Preeclampsia in High-Risk Pregnant Women. Obstet. Gynecol. 2013, 121, 349–353. [Google Scholar] [CrossRef]
- Carreras, E.; Alijotas-Reig, J.; Mendoza, M.; Ferrer-Oliveras, R.; Bonacina, E.; Garcia-Manau, P.; Rodo, C. Evaluating the Effect of Pravastatin in Early-Onset Fetal Growth Restriction: A Nonrandomized and Historically Controlled Pilot Study. Am. J. Perinatol. 2021, 38, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Williams, D.J.; Cheed, V.; Middleton, L.J.; Ahmad, S.; Wang, K.; Vince, A.T.; Hewett, P.; Spencer, K.; Khan, K.S.; et al. Pravastatin for Early-Onset Pre-eclampsia: A Randomised, Blinded, Placebo-Controlled Trial. BJOG 2020, 127, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Ganzevoort, W.; Alfirevic, Z.; Von Dadelszen, P.; Kenny, L.; Papageorghiou, A.; Van Wassenaer-Leemhuis, A.; Gluud, C.; Mol, B.W.; Baker, P.N. STRIDER: Sildenafil Therapy In Dismal Prognosis Early-Onset Intrauterine Growth Restriction—A Protocol for a Systematic Review with Individual Participant Data and Aggregate Data Meta-analysis and Trial Sequential Analysis. Syst. Rev. 2014, 3, 23. [Google Scholar] [CrossRef]
- Sharp, A.; Cornforth, C.; Jackson, R.; Harrold, J.; Turner, M.A.; Kenny, L.C.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P.; et al. Maternal Sildenafil for Severe Fetal Growth Restriction (STRIDER): A Multicentre, Randomised, Placebo-Controlled, Double-Blind Trial. Lancet Child Adolesc. Health 2018, 2, 93–102. [Google Scholar] [CrossRef]
- Sharp, A.; Jackson, R.; Cornforth, C.; Harrold, J.; Turner, M.A.; Kenny, L.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P.; et al. A Prediction Model for Short-Term Neonatal Outcomes in Severe Early-Onset Fetal Growth Restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 241, 109–118. [Google Scholar] [CrossRef]
- Groom, K.M.; McCowan, L.M.; Mackay, L.K.; Lee, A.C.; Gardener, G.; Unterscheider, J.; Sekar, R.; Dickinson, J.E.; Muller, P.; Reid, R.A.; et al. Strider NZAus: A Multicentre Randomised Controlled Trial of Sildenafil Therapy in Early-Onset Fetal Growth Restriction. BJOG 2019, 126, 997–1006. [Google Scholar] [CrossRef]
- Groom, K.M.; Ganzevoort, W.; Alfirevic, Z.; Lim, K.; Papageorghiou, A.T.; Baker, P.; von Dadelszen, P.; Gluud, C.; Jakobsen, J.; Kenny, L.; et al. STRIDER Consortium. Clinicians should stop prescribing sildenafil for fetal growth restriction (FGR): Comment from the STRIDER Consortium. Ultrasound Obs. Gynecol. 2018, 52, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Sharp, A.; Cornforth, C.; Jackson, R.; Mousa, H.; Stock, S.; Harrold, J.; Turner, M.A.; Kenny, L.C.; Baker, P.N.; et al. Effect of Sildenafil on Maternal Hemodynamics in Pregnancies Complicated by Severe Early-Onset Fetal Growth Restriction: Planned Subgroup Analysis From a Multicenter Randomized Placebo-Controlled Double-Blind Trial. Ultrasound Obstet. Gynecol. 2020, 55, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, F.; Richter, A.E.; Lely, A.T.; Hoebeek, F.E.; Elvan-Taspinar, A.; Bos, A.F.; Ganzevoort, W.; Pels, A.; Lemmers, P.M.; Kooi, E.M.W. Prenatal Use of Sildenafil in Fetal Growth Restriction and Its Effect on Neonatal Tissue Oxygenation-A Retrospective Analysis of Hemodynamic Data from Participants of the Dutch Strider Trial. Front. Pediatr. 2020, 8, 595693. [Google Scholar] [CrossRef] [PubMed]
- Pels, A.; Derks, J.; Elvan-Taspinar, A.; van Drongelen, J.; de Boer, M.; Duvekot, H.; van Laar, J.; van Eyck, J.; Al-Nasiry, S.; Sueters, M.; et al. Maternal Sildenafil vs. Placebo in Pregnant Women with Severe Early-Onset Fetal Growth Restriction: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e205323. [Google Scholar] [CrossRef]
- Maki, S.; Tanaka, H.; Tsuji, M.; Furuhashi, F.; Magawa, S.; Kaneda, M.K.; Nii, M.; Tanaka, K.; Kondo, E.; Tamaru, S.; et al. Safety Evaluation of Tadalafil Treatment for Fetuses with Early-Onset Growth Restriction (TADAFER): Results from the Phase II Trial. J. Clin. Med. 2019, 8, 856. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Umekawa, T.; Maekawa, Y.; Tanaka, H.; Nii, M.; Murabayashi, N.; Osato, K.; Kamimoto, Y.; Ikeda, T. Retrospective Study of Tadalafil for Fetal Growth Restriction: Impact on Maternal and Perinatal Outcomes. J. Obstet. Gynaecol. Res. 2017, 43, 291–297. [Google Scholar] [CrossRef]
- Kubo, M.; Tanaka, H.; Maki, S.; Nii, M.; Murabayashi, N.; Osato, K.; Kamimoto, Y.; Umekawa, T.; Kondo, E.; Ikeda, T. Safety and Dose-Finding Trial of Tadalafil Administered for Fetal Growth Restriction: A Phase-1 Clinical Study. J. Obstet. Gynaecol. Res. 2017, 43, 1159–1168. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Umekawa, T.; Maki, S.; Kubo, M.; Nii, M.; Tanaka, K.; Tanaka, H.; Osato, K.; Kamimoto, Y.; Kondo, E.; et al. Tadalafil Improves L-NG-Nitroarginine Methyl Ester-Induced Preeclampsia with Fetal Growth Restriction-Like Symptoms in Pregnant Mice. Am. J. Hypertens. 2017, 31, 89–96. [Google Scholar] [CrossRef]
- Tachibana, R.; Umekawa, T.; Yoshikawa, K.; Owa, T.; Magawa, S.; Furuhashi, F.; Tsuji, M.; Maki, S.; Shimada, K.; Kaneda, M.K.; et al. Tadalafil Treatment in Mice for Preeclampsia with Fetal Growth Restriction has Neuro-benefic Effects in Offspring through Modulating Prenatal Hypoxic Conditions. Sci. Rep. 2019, 9, 234. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Tanaka, K.; Tanaka, H.; Maki, S.; Enomoto, N.; Takakura, S.; Nii, M.; Toriyabe, K.; Katsuragi, S.; Ikeda, T. Tadalafil Treatment Ameliorates Hypoxia and Alters Placental Expression of Proteins Downstream of MTOR Signaling in Fetal Growth Restriction. Medicina 2020, 56, 722. [Google Scholar] [CrossRef]
- Rotella, D.P. Phosphodiesterase 5 inhibitors: Current status and potential applications. Nat. Rev. Drug Discov. 2002, 1, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Wharton, J.; Grimminger, F.; Ghofrani, H.A. Phosphodiesterase inhibitors for the treatment of pulmonary hypertension. Eur. Respir. J. 2008, 32, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Forgue, S.T.; Patterson, B.E.; Bedding, A.W.; Payne, C.D.; Phillips, D.L.; Wrishko, R.E.; Mitchell, M.I. Tadalafil pharmacokinetics in healthy subjects. Br. J. Clin. Pharmacol. 2006, 61, 280–288. [Google Scholar] [CrossRef]
- Walton, R.B.; Reed, L.C.; Estrada, S.M.; Schmiedecke, S.S.; Villazana-Kretzer, D.L.; Napolitano, P.G.; Ieronimakis, N. Evaluation of sildenafil and tadalafil for reversing constriction of fetal arteries in a human placenta perfusion model. Hypertension 2018, 72, 167–176. [Google Scholar] [CrossRef]
- Khong, T.Y.; De Wolf, F.; Robertson, W.B.; Brosens, I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-forgestational age infants. Br. J. Obstet. Gynaecol. 1986, 93, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Helou, S.M.; Hudak, M.L.; Jones, M.D. Cerebral Blood Flow Response to Hypercapnia in Immature Fetal Sheep. Am. J. Physiol. 1991, 261, H1366–H1370. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Arduini, D. Fetal Cardiac Function in Intrauterine Growth Retardation. Am. J. Obstet. Gynecol. 1991, 165, 876–882. [Google Scholar] [CrossRef]
- Hecher, K.; Bilardo, C.M.; Stigter, R.H.; Ville, Y.; Hackelöer, B.J.; Kok, H.J.; Senat, M.V.; Visser, G.H.A. Monitoring of Fetuses with Intrauterine Growth Restriction: A Longitudinal Study. Ultrasound Obstet. Gynecol. 2001, 18, 564–570. [Google Scholar] [CrossRef]
- Arduini, D.; Rizzo, G.; Romanini, C. Changes of Pulsatility Index From Fetal Vessels Preceding the Onset of Late Decelerations in Growth-Retarded Fetuses. Obstet. Gynecol. 1992, 79, 605–610. [Google Scholar]
- Turan, O.M.; Turan, S.; Gungor, S.; Berg, C.; Moyano, D.; Gembruch, U.; Nicolaides, K.; Harman, C.R.; Baschat, A.A. Progression of Doppler Abnormalities in Intrauterine Growth Restriction. Ultrasound Obstet. Gynecol. 2008, 32, 160–167. [Google Scholar] [CrossRef]
- Baschat, A.A. Neurodevelopment Following Fetal Growth Restriction and Its Relationship with Antepartum Parameters of Placental Dysfunction. Ultrasound Obstet. Gynecol. 2011, 37, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Daimon, A.; Kamiya, C.A.; Iwanaga, N.; Ikeda, T.; Nakanishi, N.; Yoshimatsu, J. Management of pulmonary vasodilator therapy in three pregnancies with pulmonary arterial hypertension. J. Obstet. Gynaecol. Res. 2017, 43, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Minakami, H.; Maeda, T.; Fujii, T.; Hamada, H.; Iitsuka, Y.; Itakura, A.; Itoh, H.; Iwashita, M.; Kanagawa, T.; Kanai, M.; et al. Guidelines for Obstetrical Practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J. Obstet. Gynaecol. Res. 2014, 40, 1469–1499. [Google Scholar] [CrossRef]
- Shinozuka, N. Fetal Biometry and Fetal Weight Estimation: JSUM Standardization. Ultrasound Rev. Obstet. Gynecol. 2002, 2, 156–161. [Google Scholar] [CrossRef]
- Shinozuka, N.; Masuda, H.; Kagawa, H. Standard Values of Ultrasonographic Fetal Biometry. J. Med. Ultrason. 1996, 23, 879–888. [Google Scholar]
- Shinozuka, N.; Akamatsu, N.; Sato, S. Ellipse Tracing Fetal Growth Assessment Using Abdominal Circumference: JSUM Standardization Committee for Fetal Measurements. J. Med. Ultrasound 2000, 8, 87–94. [Google Scholar]
- Kuno, A.; Akiyama, M.; Yanagihara, T.; Hata, T. Comparison of Fetal Growth in Singleton, Twin, and Triplet Pregnancies. Hum. Reprod. 1999, 14, 1352–1360. [Google Scholar] [CrossRef]
- Chitty, L.S.; Altman, D.G.; Henderson, A.; Campbell, S. Charts of Fetal Size: 2. Head Measurements. Br. J. Obstet. Gynaecol. 1994, 101, 35–43. [Google Scholar] [CrossRef]
- Chitty, L.S.; Altman, D.G.; Henderson, A.; Campbell, S. Charts of Fetal Size: 4. Femur Length. Br. J. Obstet. Gynaecol. 1994, 101, 132–135. [Google Scholar] [CrossRef]
- Mari, G.; Abuhamad, A.Z.; Cosmi, E.; Segata, M.; Altaye, M.; Akiyama, M. Middle Cerebral Artery Peak Systolic Velocity: Technique and Variability. J. Ultrasound Med. 2005, 24, 425–430. [Google Scholar] [CrossRef]
- Divon, M.Y.; Chamberlain, P.F.; Sipos, L.; Manning, F.A.; Platt, L.D. Identification of the Small for Gestational Age Fetus with the Use of Gestational Age-Independent Indices of Fetal Growth. Am. J. Obstet. Gynecol. 1986, 155, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, M.; Ek, S.; Tambyrajia, R. Screening for Fetal Growth Restriction: A Mathematical Model of the Effect of Time Interval and Ultrasound Error. Obstet. Gynecol. 1998, 92, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Society for the Kyoto Scale of Psychological Development Test. Shinpan K Shiki Hattatsu Kensahou 2001 Nenban [The Kyoto Scale of Psychological Development Test 2001]; Nakanishiya Shuppan: Kyoto, Japan, 2008. (In Japanese) [Google Scholar]
- Scherjon, S.A.; Smolders-DeHaas, H.; Kok, J.H.; Zondervan, H.A. The “Brain-Sparing” Effect: Antenatal Cerebral Doppler Findings in Relation to Neurologic Outcome in Very Preterm Infants. Am. J. Obstet. Gynecol. 1993, 169, 169–175. [Google Scholar] [CrossRef]
- Kiserud, T.; Kessler, J.; Ebbing, C.; Rasmussen, S. Ductus Venosus Shunting in Growth-Restricted Fetuses and the Effect of Umbilical Circulatory Compromise. Ultrasound Obstet. Gynecol. 2006, 28, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Tchirikov, M.; Kertschanska, S.; Schröder, H.J. Obstruction of Ductus Venosus Stimulates Cell Proliferation in Organs of Fetal Sheep. Placenta 2001, 22 (Suppl. A), 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tchirikov, M.; Kertschanska, S.; Stürenberg, H.; Schröder, H. Liver Blood Perfusion as a Possible Instrument for Fetal Growth Regulation. Placenta 2002, 23, S153–S158. [Google Scholar] [CrossRef]
- Trudinger, B.J.; Giles, W.B.; Cook, C.M.; Bombardieri, J.; Collins, L. Fetal Umbilical Artery Flow Velocity Waveforms and Placental Resistance: Clinical Significance. Br. J. Obstet. Gynaecol. 1985, 92, 23–30. [Google Scholar] [CrossRef]
- Trudinger, B.J.; Giles, W.B. Elaboration of Stem Villous Vessels in Growth Restricted Pregnancies with Abnormal Umbilical Artery Doppler Waveforms. Br. J. Obstet. Gynaecol. 1996, 103, 487–489. [Google Scholar] [CrossRef]
- Kunihiko, N.; Toru, H.; Tomoko, S.; Nakai, K.; Hosokawa, T.; Satoh, H. Comparison of Kyoto Scale of Psychological Development and Bayley Scales of Infant Development second edition among Japanese infants. J. Spec. Educ. Res. 2013, 2, 17–24. [Google Scholar]
- Miller, S.L.; Huppi, P.S.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef]
- Maki, S.; Kato, I.; Enomoto, N.; Takakura, S.; Nii, M.; Tanaka, K.; Tanaka, H.; Hori, S.; Matsuda, K.; Ueda, Y.; et al. Developmental Evaluation of Infants Who Have Received Tadalafil In Utero for Fetal Growth Restriction. J. Clin. Med. 2020, 9, 1448. [Google Scholar] [CrossRef] [PubMed]
- Motoki, T.; Chigusa, Y.; Tomotaki, S.; Kawamura, Y.; Taki, M.; Yamaguchi, K.; Mandai, M.; Mogami, H. Clinical Features of Neuro-developmental Outcomes in Children with Preterm Severe Fetal Growth Restriction: A Retrospective Observational Study. JMA J. 2022, 5, 341–348. [Google Scholar] [PubMed]
- Barker, D.; Osmond, C.; Winter, P.; Margetts, B.; Simmonds, S. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 9, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Karmer, M.S. The epidemiology of low birthweight. Nestle Nutr. Inst. Workshop Ser. 2013, 74, 1–10. [Google Scholar]
| Tadalafil Group (n = 50) | Control Group (n = 10) | |
|---|---|---|
| Maternal characteristics | ||
| Age (y) | 33 (28–35) | 32 (30–36) |
| Height (cm) | 156 (151–160) | 156 (154–162) |
| BMI | 21.1 (19.8–23.1) | 19.8 (21.0–18.5) |
| Body weight before pregnancy (kg) | 50.0 (46.0–55.6) | 48.5 (46.3–51.7) |
| Primipara | 24 | 2 |
| Smoking | 2 | 1 |
| Gestational age at the start of treatment (weeks) | 30 (27–32) | 31 (30–32) |
| Incidence of HDP | 8 | 1 |
| Gestational age at delivery (weeks) | 37 (35–37) | 37 (34–38) |
| Fetal characteristics | ||
| EFBW at the start of treatment (g) | 1089 (748–1389) | 1348 (1148–1482) |
| Z-score | −2.2 (−2.4 to −1.9) | −2.1 (−2.3 to −2.0) |
| BPD at the start of treatment (cm) | 7.03 (6.45–7.75) | 7.12 (6.97–7.55) |
| Z-score | −1.1 (−1.9 to −0.63) | −1.5 (−1.9 to −0.9) |
| HC at the start of treatment (cm) | 25.8 (22.8–27.1) | 26.5 (26.2–27.6) |
| Z-score | −0.8 (−1.5–0.1) | −0.4 (−1.1 to −0.1) |
| AC at the start of treatment (cm) | 21.7 (19.1–24.0) | 23.8 (22.3–25.3) |
| Z-score | −1.6 (−2 to −1.2) | −1.6 (−1.8 to −1.0) |
| FL at the start of treatment (cm) | 4.93 (4.27–5.32) | 5.34 (5.00–5.53) |
| Z-score | −1.7 (−2.1 to −1.3) | −1.4 (−2.0 to −0.9) |
| Maternal Vital Sign | Tadalafil Group (n = 50) | Conventional Treatment Group (n = 10) | p-Value | |
|---|---|---|---|---|
| At the start of treatment | sBP (mmHg) | 108 (102–120) | 108.5 (103.75–121.75) | 0.76 |
| dBP (mmHg) | 62 (56–69) | 71 (59.25–78) | 0.86 | |
| MAP (mmHg) | 77.33 (71.33–85) | 85 (72.75–93) | 0.18 | |
| HR (bpm) | 78 (70–90) | 83.5 (76.25–88.5) | 0.32 | |
| 2 weeks of treatment | sBP (mmHg) | 106.5 (100–112.25) | 110 (98.75–119.5) | 0.64 |
| dBP (mmHg) | 58.5 (50.75–65) | 69 (59.75–74.25) | 0.018 | |
| MAP (mmHg) | 75.33 (67.83–79.75) | 84 (72.5–88.5) | 0.076 | |
| HR (bpm) | 76 (70–84.25) | 74 (68.5–78.5) | 0.56 | |
| 4 weeks of treatment | sBP (mmHg) | 107 (102.5–114.5) | 103 (99–113) | 0.30 |
| dBP (mmHg) | 59.5 (55.25–66.75) | 60 (56–66) | 0.87 | |
| MAP (mmHg) | 75 (70.83–83.67) | 73 (72–82) | 0.62 | |
| HR (bpm) | 76 (67.25–81.75) | 70 (66–82) | 0.47 | |
| 1.5 Years of CA | 3 Years Old | |
|---|---|---|
| (n = 37) | (n = 19) | |
| P–M | 89 (76–94) | 96 (77–102) |
| C–A | 91 (80–102) | 86 (79–96) |
| L–S | 94 (84–99) | 92 (77–96) |
| Total | 88 (82–100) | 87 (76–98) |
| 1.5 Years CA (n = 37) | ||||
| DQ | P–M | C–A | L–S | Total Area |
| <70 | 7 (19%) | 3 (8%) | 7 (19%) | 4 (11%) |
| 70–85 | 10 (27%) | 10 (27%) | 4 (11%) | 10 (27%) |
| >85 | 20 (54%) | 24 (65%) | 26 (70%) | 23 (62%) |
| Data are presented as n (%) | ||||
| 3 Years Old (n = 19) | ||||
| DQ | P–M | C–A | L–S | Total Area |
| <70 | 3 (16%) | 4 (21%) | 3 (16%) | 3 (16%) |
| 70–85 | 4 (21%) | 2 (11%) | 5 (26%) | 5 (26%) |
| >85 | 12 (63%) | 13 (68%) | 11 (58%) | 11 (58%) |
| Data are presented as n (%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, M.; Maki, S.; Enomoto, N.; Okamoto, K.; Kitamura, A.; Magawa, S.; Takakura, S.; Nii, M.; Tanaka, K.; Yodoya, N.; et al. Fetal Biometric Assessment and Infant Developmental Prognosis of the Tadalafil Treatment for Fetal Growth Restriction. Medicina 2023, 59, 900. https://doi.org/10.3390/medicina59050900
Tsuji M, Maki S, Enomoto N, Okamoto K, Kitamura A, Magawa S, Takakura S, Nii M, Tanaka K, Yodoya N, et al. Fetal Biometric Assessment and Infant Developmental Prognosis of the Tadalafil Treatment for Fetal Growth Restriction. Medicina. 2023; 59(5):900. https://doi.org/10.3390/medicina59050900
Chicago/Turabian StyleTsuji, Makoto, Shintaro Maki, Naosuke Enomoto, Kota Okamoto, Asa Kitamura, Shoichi Magawa, Sho Takakura, Masafumi Nii, Kayo Tanaka, Noriko Yodoya, and et al. 2023. "Fetal Biometric Assessment and Infant Developmental Prognosis of the Tadalafil Treatment for Fetal Growth Restriction" Medicina 59, no. 5: 900. https://doi.org/10.3390/medicina59050900
APA StyleTsuji, M., Maki, S., Enomoto, N., Okamoto, K., Kitamura, A., Magawa, S., Takakura, S., Nii, M., Tanaka, K., Yodoya, N., Tanaka, H., Sawada, H., Kondo, E., Hirayama, M., & Ikeda, T. (2023). Fetal Biometric Assessment and Infant Developmental Prognosis of the Tadalafil Treatment for Fetal Growth Restriction. Medicina, 59(5), 900. https://doi.org/10.3390/medicina59050900







