Oxygen Saturation in Hospitalized COVID-19 Patients and Its Relation to Colchicine Treatment: A Retrospective Cohort Study with an Updated Systematic Review
Abstract
1. Introduction
2. Research Design and Methodology
2.1. Study Population
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Study Drug (Exposure)
2.3. Data Collection
2.3.1. The Primary Outcome
2.3.2. The Secondary Outcomes
2.4. Statistical Analysis
Sample Size Calculation
2.5. Systematic Review
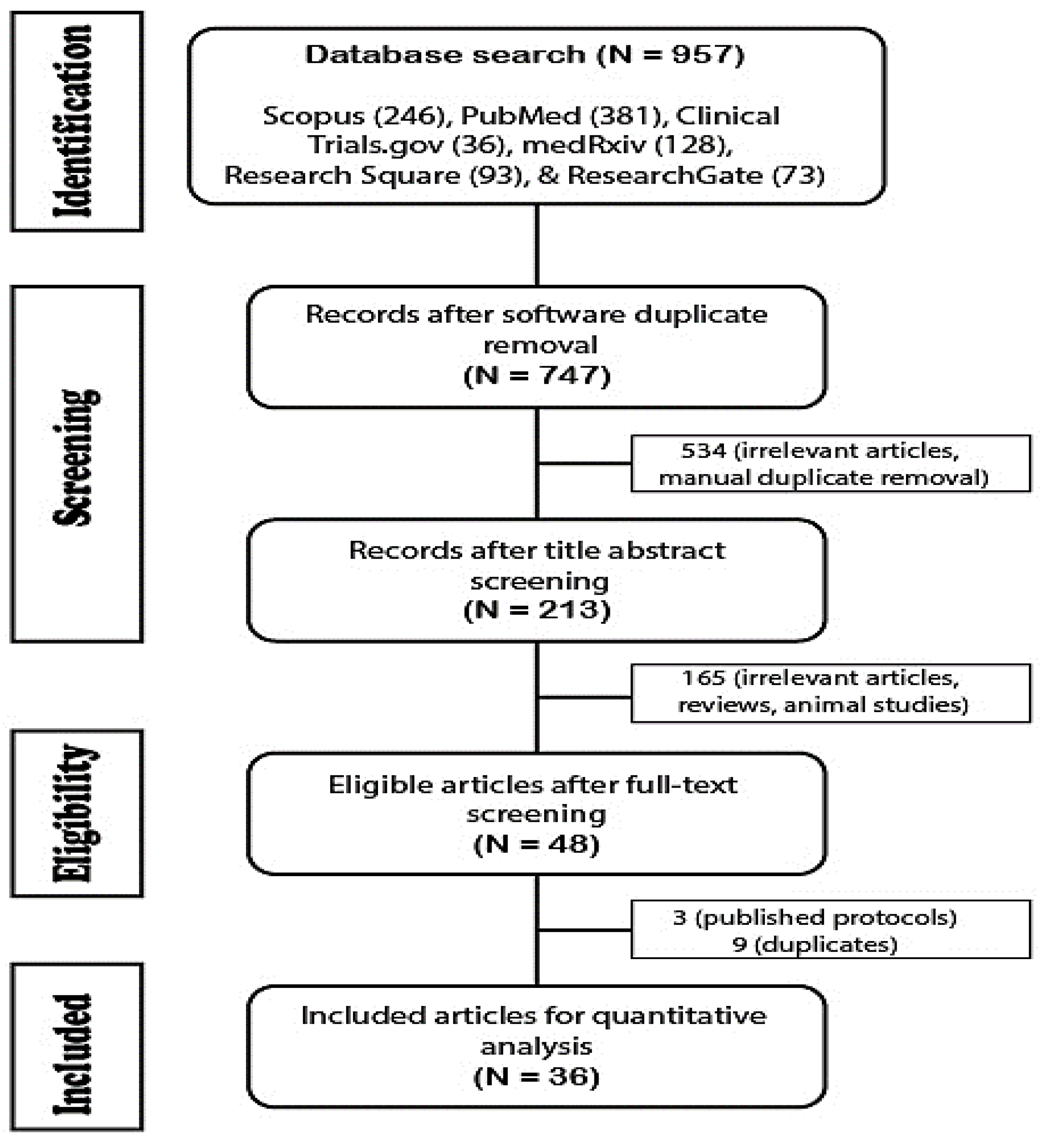
2.5.1. Database Search and Study Selection
2.5.2. Screening and Data Extraction
3. Results
3.1. Outcomes
3.2. Adjusting Variables
3.3. Systematic Review
4. Discussion
4.1. Based on Our Study Analysis
4.2. Based on Our Systematic Review Analysis
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| CVD | Cardiovascular disease |
| COPD | Chronic obstructive pulmonary disorder |
| COVID-19 | Coronavirus associated disease 2019 |
| DAMA | Discharge against medical advice |
| DVT | Deep vein thrombosis |
| FDA | Food and drug administration |
| HCV | Hepatitis C virus |
| HR | Hazard ratio |
| ILD | Interstitial lung disease |
| LOS | Length of stay |
| MoHP | Egyptian ministry of health and population |
| MV | Mechanical ventilation |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
| SARS-CoV-2 | The severe acute respiratory syndrome coronavirus |
| SGPT | Serum glutamic pyruvic transaminase |
| SGOT | Serum glutamic oxaloacetic transaminase |
| SpO2 | Peripheral oxygen saturation |
| UV | Ultraviolet |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 9 April 2023).
- Montealegre-Gómez, G.; Garavito, E.; Gómez-López, A.; Rojas-Villarraga, A.; Parra-Medina, R. Colchicine: A Potential Therapeutic Tool against COVID-19. Experience of 5 Patients. Reumatol. Clín. (Engl. Ed.) 2021, 17, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for Potential Pathogenesis of SARS-CoV-2 Infection—A Review of Immune Changes in Patients with Viral Pneumonia. Emerg. Microbes Infect. 2020, 9, 727. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Slobodnick, A.; Shah, B.; Krasnokutsky, S.; Pillinger, M.H. Update on Colchicine, 2017. Rheumatology 2018, 57, i4–i11. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on Mechanisms of Action and Therapeutic Uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Benhamou, O.-M.; Geva, S.; Jacobs, M.; Drew, J.; Waldman, M.; Kalchiem-Dekel, O. The Use of Colchicine in Respiratory Diseases. Curr. Respir. Med. Rev. 2014, 9, 300–304. [Google Scholar] [CrossRef]
- Elshafei, M.N.; Khalil, A.; El-Bardissy, A.; Danjuma, M.; Ahmed, M.B.; Mohamed, M.F.H. The Efficacy of Colchicine in the Management of Coronavirus Disease 2019: A Protocol for Systematic Review and Meta-Analysis. Medicine 2020, 99, e21911. [Google Scholar] [CrossRef]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Golpour, M.; Mousavi, T.; Alimohammadi, M.; Mosayebian, A.; Shiran, M.; Alizadeh Navaei, R.; Rafiei, A. The Effectiveness of Colchicine as an Anti-Inflammatory Drug in the Treatment of Coronavirus Disease 2019: Meta-Analysis. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211031763. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.I.; Bonjorno, L.P.; Giannini, M.C.; Amaral, N.B.; Menezes, P.I.; Dib, S.M.; Gigante, S.L.; Benatti, M.N.; Rezek, U.C.; Emrich-Filho, L.L.; et al. Beneficial Effects of Colchicine for Moderate to Severe COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Clinical Trial. RMD Open 2021, 7, e001455. [Google Scholar] [CrossRef] [PubMed]
- Toro-Huamanchumo, C.J.; Benites-Meza, J.K.; Mamani-García, C.S.; Bustamante-Paytan, D.; Gracia-Ramos, A.E.; Diaz-Vélez, C.; Barboza, J.J. Efficacy of Colchicine in the Treatment of COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2615. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.G.; Patel, T.; Chavda, P.D.; Patel, P. Efficacy and Safety of Colchicine in COVID-19: A Meta-Analysis of Randomised Controlled Trials. RMD Open 2021, 7, e001746. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Colchicine in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet Respir. Med. 2021, 9, 1419–1426. [Google Scholar] [CrossRef]
- Masoud, H.; Elassal, G.; Hakim, M.; Shawky, A.; Zaky, S.; Baki, A.; Abdelbary, A.; Hassany, M.; Mohsen, A.; Taema, K.; et al. Management Protocol for COVID-19 Patients COVID-19 Ministry of Health and Population, Egypt. Version 1.5/September 2021. 2021. Available online: https://www.researchgate.net/publication/354694237_Management_Protocol_for_COVID-19_Patients_COVID-19_Ministry_of_Health_and_Population_Egypt_version_15_September_2021/ (accessed on 25 February 2023).
- Cochrane Risk of Bias Tool for Randomized Controlled Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials/ (accessed on 2 May 2023).
- Shamsrizi, P.; Gladstone, B.P.; Carrara, E.; Luise, D.; Cona, A.; Bovo, C.; Tacconelli, E. Variation of Effect Estimates in the Analysis of Mortality and Length of Hospital Stay in Patients with Infections Caused by Bacteria-Producing Extended-Spectrum Beta-Lactamases: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e030266. [Google Scholar] [CrossRef]
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2013136. [Google Scholar] [CrossRef]
- Absalón-Aguilar, A.; Rull-Gabayet, M.; Pérez-Fragoso, A.; Mejía-Domínguez, N.R.; Núñez-Álvarez, C.; Kershenobich-Stalnikowitz, D.; Sifuentes-Osornio, J.; Ponce-de-León, A.; González-Lara, F.; Martín-Nares, E.; et al. Colchicine Is Safe Though Ineffective in the Treatment of Severe COVID-19: A Randomized Clinical Trial (COLCHIVID). J. Gen. Intern. Med. 2022, 37, 4–14. [Google Scholar] [CrossRef]
- Mareev, V.Y.; Orlova, Y.A.; Plisyk, A.G.; Pavlikova, E.P.; Akopyan, Z.A.; Matskeplishvili, S.; Malahov, P.S.; Krasnova, T.N.; Seredenina, E.M.; Potapenko, A.V.; et al. Proactive Anti-Inflammatory Therapy with Colchicine in the Treatment of Advanced Stages of New Coronavirus Infection. The First Results of the COLORIT Study. Kardiologiya 2021, 61, 15–27. [Google Scholar] [CrossRef]
- Salehzadeh, F.; Pourfarzi, F.; Ataei, S. The Impact of Colchicine on the COVID-19 Patients; A Clinical Trial Study. Res. Sq. 2020, 33, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Orlandini, A.; Castellana, N.; Caccavo, A.; Corral, P.; Corral, G.; Chacón, C.; Lamelas, P.; Botto, F.; Díaz, M.L.; et al. Effect of Colchicine vs Usual Care Alone on Intubation and 28-Day Mortality in Patients Hospitalized with COVID-19: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2141328. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Roura-Piloto, A.E.; Moral-Escudero, E.; Bernal, E.; Albendín-Iglesias, H.; Pérez-Martínez, M.T.; Noguera-Velasco, J.A.; Cebreiros-López, I.; Hernández-Vicente, Á.; Vázquez-Andrés, D.; et al. Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID). Int. J. Gen. Med. 2021, 14, 5517–5526. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.; Abu-Fanne, R.; Bdeir, K.; Maraga, E.; Higazi, M.; Cines, D.B.; Heyman, S.N.; Higazi, A.A.R. Divergent Impacts of Tocilizumab and Colchicine in COVID-19-Associated Coagulopathy: The Role of Alpha-Defensins. Br. J. Haematol. 2022, 196, 923–927. [Google Scholar] [CrossRef]
- Alsultan, M.; Obeid, A.; Alsamarrai, O.; Anan, M.T.; Bakr, A.; Soliman, N.; Kurdy, M.; Mosa, M.H.; Saleh, Z.; Hujij, F.; et al. Efficacy of Colchicine and Budesonide in Improvement Outcomes of Patients with Coronavirus Infection 2019 in Damascus, Syria: A Randomized Control Trial. Interdiscip. Perspect. Infect. Dis. 2021, 2021, 2129006. [Google Scholar] [CrossRef]
- Pourdowlat, G.; Saghafi, F.; Mozafari, A.; Sahebnasagh, A.; Abedini, A.; Nabi Meybodi, M.; Salehi Nezamabadi, A.; Mousavinasab, S.R.; Kiani, A.; Raji, H.; et al. Efficacy and Safety of Colchicine Treatment in Patients with COVID-19: A Prospective, Multicenter, Randomized Clinical Trial. Phytother. Res. 2022, 36, 891–898. [Google Scholar] [CrossRef]
- Rabbani, A.; Rafique, A.; Wang, X.; Campbell, D.; Wang, D.; Brownell, N.; Capdevilla, K.; Garabedian, V.; Chaparro, S.; Herrera, R.; et al. Colchicine for the Treatment of Cardiac Injury in Hospitalized Patients with Coronavirus Disease-19. Front. Cardiovasc. Med. 2022, 9, 876718. [Google Scholar] [CrossRef]
- Gorial, F.I.; Maulood, M.F.; Abdulamir, A.S.; Alnuaimi, A.S.; Abdulrrazaq, M.K.; Bonyan, F.A. Randomized Controlled Trial of Colchicine Add on to the Standard Therapy in Moderate and Severe Corona Virus Disease-19 Infection. Ann. Med. Surg. 2022, 77, 103593. [Google Scholar] [CrossRef]
- Cecconi, A.; Martinez-Vives, P.; Vera, A.; Lavilla Olleros, C.; Barrios, A.; Fonseca Aizpuru, E.; Roquero, P.; Hernandez Muñiz, S.; Olivera, M.J.; Ciudad, M.; et al. Efficacy of Short-Course Colchicine Treatment in Hospitalized Patients with Moderate to Severe COVID-19 Pneumonia and Hyperinflammation: A Randomized Clinical Trial. Sci. Rep. 2022, 12, 9208. [Google Scholar] [CrossRef]
- El Sayed, R.G.; Hafez, A.F.; Mohammed Haggag, A.M.A.; Alhadidy, M.A. Effect of Combined Use of Ivermectin and Colchicine in COVID-19 Patients. Egypt. J. Anaesth. 2022, 38, 365–372. [Google Scholar] [CrossRef]
- Jalal, A.M.; Aref, S.F.; Albustany, D.A. Effectiveness of Colchicine among Patients with COVID-19 Infection: A Randomized, Open-Labeled, Clinical Trial. Indian J. Rheumatol. 2022, 17, 136–141. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Jolly, S.S.; Belley-Cote, E.P.; Whitlock, R.P.; Rangarajan, S.; Xu, L.; Heenan, L.; Bangdiwala, S.I.; Luz Diaz, M.; Diaz, R.; et al. Colchicine and the Combination of Rivaroxaban and Aspirin in Patients Hospitalised with COVID-19 (ACT): An Open-Label, Factorial, Randomised, Controlled Trial. Lancet Respir. Med. 2022, 10, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Datta, P.K.; Islam, K.; Haque, M.; Mahmud, R.; Mallik, U.; Hasan, P.; Haque, M.; Faruq, I.; Sharif, M.; et al. Efficacy of Colchicine in Patients with Moderate COVID-19: A Double-Blinded, Randomized, Placebo-Controlled Trial. PLoS ONE 2022, 17, e0277790. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.Z.; Farooq, U.; Ashraf, S.; Zeb, S.; Gillani, S.Y.; Malik, S.; Ali, R.; Irshad, R.; Mehmood, Z.; Abbas, Y.; et al. Colchicine Anti-Inflammatory Therapy for Non-Intensive Care Unit Hospitalized COVID-19 Patients: Results from a Pilot Open-Label, Randomized Controlled Clinical Trial. J. Physiol. Pharmacol. 2022, 73, 413–420. [Google Scholar] [CrossRef]
- Perricone, C.; Scarsi, M.; Brucato, A.; Pisano, P.; Pigatto, E.; Becattini, C.; Cingolani, A.; Tiso, F.; Prota, R.; Tomasoni, L.R.; et al. Treatment with COLchicine in Hospitalized Patients Affected by COVID-19: The COLVID-19 Trial. Eur. J. Intern. Med. 2023, 107, 30–36. [Google Scholar] [CrossRef]
- Kasiri, H.; Ghazaiean, M.; Rouhani, N.; Naderi-behdani, F.; Ghazaeian, M.; Ghodssi-Ghassemabadi, R. The Effects of Colchicine on Hospitalized COVID-19 Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Investig. Med. 2023, 71, 124–131. [Google Scholar] [CrossRef]
- Sunil Naik, K.; Andhalkar, N.; Pendse, S. Effect of Colchicine and Aspirin given Together in Patients with Moderate COVID-19. Contemp. Clin. Trials Commun. 2023, 32, 101070. [Google Scholar] [CrossRef]
- Amaral, N.B.; Rodrigues, T.S.; Giannini, M.C.; Lopes, M.I.; Bonjorno, L.P.; Menezes, P.I.S.O.; Dib, S.M.; Gigante, S.L.G.; Benatti, M.N.; Rezek, U.C.; et al. Colchicine Reduces the Activation of NLRP3 Inflammasome in COVID-19 Patients. Inflamm. Res. 2023, 1–5. [Google Scholar] [CrossRef]
- Scarsi, M.; Piantoni, S.; Colombo, E.; Airó, P.; Richini, D.; Miclini, M.; Bertasi, V.; Bianchi, M.; Bottone, D.; Civelli, P.; et al. Association between Treatment with Colchicine and Improved Survival in a Single-Centre Cohort of Adult Hospitalised Patients with COVID-19 Pneumonia and Acute Respiratory Distress Syndrome. Ann. Rheum. Dis. 2020, 79, 1286–1289. [Google Scholar] [CrossRef]
- Sandhu, T.; Tieng, A.; Chilimuri, S.; Franchin, G. A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 8865954. [Google Scholar] [CrossRef]
- Brunetti, L.; Diawara, O.; Tsai, A.; Firestein, B.L.; Nahass, R.G.; Poiani, G.; Schlesinger, N. Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19. J. Clin. Med. 2020, 9, 2961. [Google Scholar] [CrossRef]
- García-Posada, M.; Aruachan-Vesga, S.; Mestra, D.; Humánez, K.; Serrano-Coll, H.; Cabrales, H.; Faccini, Á.; Mattar, S. Clinical Outcomes of Patients Hospitalized for COVID-19 and Evidence-Based on the Pharmacological Management Reduce Mortality in a Region of the Colombian Caribbean. J. Infect. Public Health 2021, 14, 696–701. [Google Scholar] [CrossRef]
- Manenti, L.; Maggiore, U.; Fiaccadori, E.; Meschi, T.; Antoni, A.D.; Nouvenne, A.; Ticinesi, A.; Cerundolo, N.; Prati, B.; Delsante, M.; et al. Reduced Mortality in COVID-19 Patients Treated with Colchicine: Results from a Retrospective, Observational Study. PLoS ONE 2021, 16, e0248276. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, M.A.; Cardona Arango, D.; Betancur, J.F.; Ortiz, S.; Holguín, H.; Arias Arias, C.; Muñoz Palacio, B.J.; Amarillo, M.; Llano, J.F.; Montoya, P. Clinical Outcome of Patients with COVID-19 Pneumonia Treated with Corticosteroids and Colchicine in Colombia. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, Ö.; Erden, A.; Güven, S.C.; Armaǧan, B.; Sahiner, E.S.; Kurtipek, A.C.; Inan, O.; Gemcioglu, E.; Ateş, I.; Omma, A.; et al. Reducing Length of Hospital Stay with Colchicines. J. Infect. Dev. Ctries. 2022, 16, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Doğan, A.; Karakök, T.; Gezer, Y. Efficacy of Colchicine Treatment in COVID-19 Patients: A Case-Control Study. Arch. Clin. Exp. Med. 2022, 7, 11–14. [Google Scholar] [CrossRef]
- Korra, E.E.A.; AbdelFattah, E.; Ahmed, M. Use of Colchicine in COVID-19 Hospitalized Patients. Egypt. J. Chest Dis. Tuberc. 2022, 71, 290. [Google Scholar] [CrossRef]
- Nazir, U.; Dismang, N.; Shi, X. 223: Colchicine in the Prevention of ARDS in Hospitalized COVID-19 Patients: A Retrospective Analysis. Crit. Care Med. 2022, 50, 96. [Google Scholar] [CrossRef]
- Qenawy, L.M.; Kamel, Y.Y.; El-Dash, H.A.; Hassen, H.A.; Abed, H.A. Effect of Colchicine in Treating Severe COVID-19 Patients on Hospital Discharge: Retrospective Cohort Study. Egypt. J. Hosp. Med. 2022, 89, 4251–4259. [Google Scholar] [CrossRef]
- Villamañán, E.; Sobrino, C.; Carpio, C.; Mateos, C.; Larrubia, Y.; Zamarrón, E.; Armada, E.; Llorente, J.; Martínez, A.; Gómez-cerezo, J.; et al. Colchicine and COVID-19: Could There Still Be Hope for This Old Low-Cost Drug? Multicenter Observational Study in Hospitalized Patients. J. Clin. Infect. Dis. Pract. 2022, 7, 5–9. [Google Scholar]
- Sáenz-Aldea, M.; Salgado-Barreira, Á.; Taracido Trunk, M.; Piñeiro-Lamas, M.; Herdeiro, M.T.; Portela-Romero, M.; Saez, M.; Figueiras, A. Colchicine and Risk of Hospitalization Due to COVID-19: A Population-based Study. J. Med. Virol. 2023, 95, e28496. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.A.; Ismail, N.S.M.; Yahia, I.S.; Aboshanab, K.M. Potential Role of Colchicine in Combating COVID-19 Cytokine Storm and Its Ability to Inhibit Protease Enzyme of Sars-Cov-2 as Conferred by Molecular Docking Analysis. Medicina 2022, 58, 20. [Google Scholar] [CrossRef]
- Shah, T.; McCarthy, M.; Nasir, I.; Archer, H.; Ragheb, E.; Kluger, J.; Kashyap, N.; Paredes, C.; Patel, P.; Lu, J.; et al. Colchicine and High-Intensity Rosuvastatin in the Treatment of Non-Critically Ill Patients Hospitalised with COVID-19: A Randomised Clinical Trial. BMJ Open 2023, 13, e067910. [Google Scholar] [CrossRef] [PubMed]
- Beran, A.; Mhanna, M.; Wahood, W.; Ghazaleh, S.; Sajdeya, O.; Kalifa, M.; Ayesh, H.; Srour, O.; Mhanna, A.S.; Altorok, N.; et al. Colchicine Treatment in SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Am. J. Ther. 2021, 29, E95–E98. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. Meta-Analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19. Am. J. Cardiol. 2021, 145, 170. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. Colchicine for Community-Treated Patients with COVID-19 (COLCORONA): A Phase 3, Randomised, Double-Blinded, Adaptive, Placebo-Controlled, Multicentre Trial. Lancet Respir. Med. 2021, 9, 924–932. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Yatoo, M.I.; Natesan, S.; Megawati, D.; Singh, K.P.; Michalak, I.; Dhama, K. A Comprehensive Review on Pharmacologic Agents, Immunotherapies and Supportive Therapeutics for COVID-19. Narra J. 2022, 2, e92. [Google Scholar] [CrossRef]
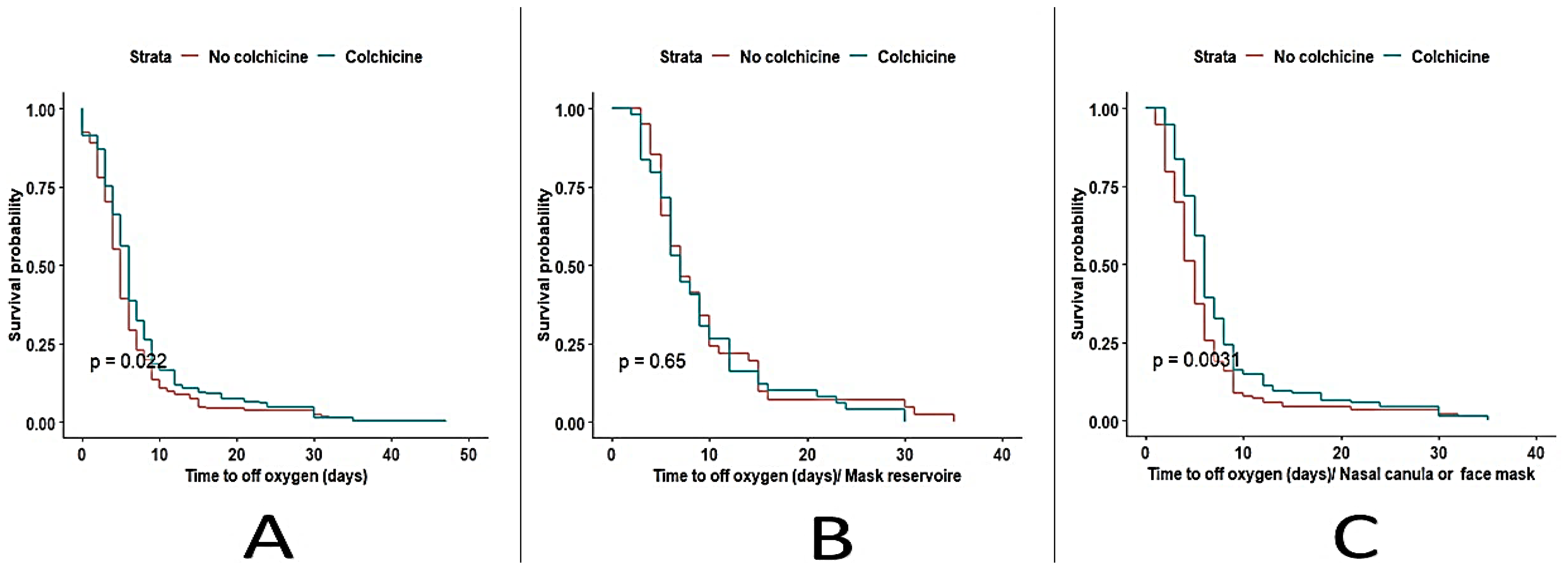
| Characteristics | Colchicine Group (N = 259) | Non-Colchicine Group (N = 256) | Total (N = 515) | p-Value |
|---|---|---|---|---|
| Gender (Female), N (%) | 167 (64.5%) | 164 (64.1%) | 331 (64.3%) | 0.921 |
| Age (years), Mean ± SD | 62.0 ± 12.4 | 59.8 ± 13.9 | 60.9 ± 13.2 | 0.061 |
| Age category (≥50), N (%) | 216 (83.4%) | 195 (76.2%) | 411 (79.8%) | 0.041 |
| Level of consciousness (Alert), N (%) | 235 (90.7%) | 222 (86.7%) | 457 (88.7%) | 0.15 |
| Heart rate (Beat/minute), Median (IQR) | 87 (82, 90) | 88 (81, 90) | 87 (82, 90) | 0.311 |
| Respiratory rate (cycle /minute), Median (IQR)) | 22 (21, 23) | 23 (22, 26) | 22 (22, 24.5) | <0.001 |
| O2 saturation%, Median (IQR) | 88 (80, 91) | 88.0 (80, 92) | 88 (80, 91) | 0.327 |
| Blood pressure, N (%) | 0.601 | |||
| Hypertension | 36 (13.9%) | 30 (11.7%) | 66 (12.8%) | |
| Hypotension | 1 (0.4%) | 2 (0.8%) | 3 (0.6%) | |
| Normal | 222 (85.7%) | 223 (87.1%) | 445 (86.4%) | |
| Feverish, N (%) | 0.832 | |||
| Grade1 | 30 (11.6%) | 29 (11.3%) | 59 (11.5%) | |
| Grade2 | 5 (1.9%) | 7 (2.7%) | 12 (2.3%) | |
| No | 224 (86.5%) | 220 (85.9%) | 444 (86.2%) | |
| Oxygen equipment on day 1, N (%) | 0.281 | |||
| MV (invasive non/invasive) | 5 (1.9%) | 11 (4.3%) | 16 (3.1%) | |
| mask reservoir | 77 (29.7%) | 63 (24.6%) | 140 (27.2%) | |
| oxygen mask/nasal cannula | 153 (59.1%) | 155 (60.5%) | 308 (59.8%) | |
| none | 24 (9.3%) | 27 (10.5%) | 51 (9.9%) | |
| Comorbidities, N (%) | 0.925 | |||
| 0 | 87 (33.6%) | 81 (31.6%) | 168 (32.6%) | |
| 1 | 77 (29.7%) | 74 (28.9%) | 151 (29.3%) | |
| 2 | 59 (22.8%) | 64 (25.0%) | 123 (23.9%) | |
| >2 | 36 (13.9%) | 37 (14.5%) | 73 (14.2%) | |
| Macrolides, N (%) | <0.001 | |||
| Azithromycin | 18 (6.9%) | 53 (20.7%) | 71 (13.8%) | |
| Clarithromycin | 19 (7.3%) | 29 (11.3%) | 48 (9.3%) | |
| None | 222 (85.7%) | 174 (68.0%) | 396 (76.9%) | |
| Anticoagulants, N (%) | 0.163 | |||
| Heparin | 16 (6.2%) | 9 (3.5%) | 25 (4.9%) | |
| LMWH | 229 (88.4%) | 237 (92.6%) | 466 (90.5%) | |
| Oral | 3 (1.2%) | 0 (0.0%) | 3 (0.6%) | |
| None | 11 (4.2%) | 10 (3.9%) | 21 (4.1%) | |
| Corticosteroids, N (%) | 0.06 | |||
| Methylprednisolone | 90 (34.7%) | 69 (27.0%) | 159 (30.9%) | |
| Prednisone | 3 (1.2%) | 1 (0.4%) | 4 (0.8%) | |
| Dexamethasone | 156 (60.2%) | 166 (64.8%) | 322 (62.5%) | |
| None | 10 (3.9%) | 20 (7.8%) | 30 (5.8%) |
| Outcome | Yes, the Colchicine Group (N = 259) | No, the Non-Colchicine Group (N = 256) | Total (N = 515) | p-Value |
|---|---|---|---|---|
| Hospital length of stay (LOS), Days Median (IQR) | 7.0 (5.0, 9.0) | 6.0 (4.0, 8.0) | 6.0 (5.0, 9.0) | 0.021 |
| Days of treatment of supplemental oxygen (alive) Median (IQR) | 6.0 (4.0, 9.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 8.0) | 0.008 |
| In-hospital mortality N (%) | 49 (18.9%) | 55 (21.5%) | 104 (20.2%) | 0.468 |
| Quality Assessment | Outcomes | Dose of Colchicine | Competitor | Study Type, Study Period, Setting | Population, Sample Size, Age. | Author, Year |
|---|---|---|---|---|---|---|
| Randomized trials | ||||||
| Poor | Colchicine has less clinical deterioration rate (p = 0.02). less event-free survival time (p = 0.03). | 1.5 mg loading dose followed by 0.5 mg after one hour, then 0.5 mg b.i.d. for 3 weeks. | Colchicine and SOC vs. SOC. | RCT, open-label 3 April to 27 April 2020 Multicenter, Greece. | Hospitalized COVID-19 105 patients Median age 64 (54–76) years | Deftereos, 2020 [21] |
| Good | Colchicine reduces the length of both, supplemental oxygen therapy (p< 0.001) and hospitalization (p = 0.003). | 0.5 mg t.i.d. for 5 days, followed by 0.5 mg b.i.d. for 5 days. | Colchicine and SOC vs. placebo and SOC | RCT, double-blinded, placebo-controlled. April 2020 to August 2020 Single center, Brazil. | Hospitalized COVID-19, 72 patients Median age 54.5 (42.5–64.5) years. | Lopes, 2021 [14] |
| Fair | No significant difference in 28-day mortality reduction, hospital length of stay (p = 0·44), and risk of mechanical ventilation (p = 0·47). | 1 mg loading dose, followed by 0.5 b.i.d. for 10 days or until discharge. | Colchicine and SOC vs. SOC. | RCT, open-label. November 2020, and March 2021. Multicenter in UK, Indonesia, and Nepal. | Hospitalized COVID-19, 19,423 patients, Mean age 63·4 ± 13·8 years. | Recovery, 2021 [17] |
| Good | No significant difference in death, progression or length of stay (p = 0.67). | 1.5 mg loading dose, followed by 0.5 mg b.i.d. for 10 days | Colchicine vs. placebo. | RCT, triple-blind, placebo-controlled. May 2020 to April 2021. Multicenter, Mexico. | Hospitalized severe COVID-19, 116 patients. Median age 53 (44–62) years. | Absalón-Aguilar, 2021 [22] |
| Poor | Colchicine reduces the SHOCS-COVID score *, the median SHOCS score decreased from 8 to 2 (p = 0.017). | 1 mg for 3 days, followed by 0.5 mg/day. | Colchicine vs. ruxolitinib and secukinumab. | RCT, open label. on day 12 or at discharge before day 12 one center, Russian. | Hospitalized later stage COVID-19, 43 patients Mean age 61.9 ± 10.6 years. | Mareev, 2021 [23] |
| Poor | Colchicine reduces the duration of hospitalization (p = 0.001) and the symptoms (fever) (p = 0.02). | 1 mg o.d. for six days. | Colchicine and SOC vs. SOC. | RCT, open-label, and double-blind. May to June 2020. one hospital, Iran. | Hospitalized COVID-19, 100 patients. Median age 56 years. | Salehzadeh, 2021 [24] |
| Fair | No significant difference in the reduction of mechanical ventilation, and 28-day mortality (p = 0.08). | 1.5 mg loading dose followed by 0.5 mg within 2 h of the initial dose and 0.5 mg b.i.d. for 14 days or discharge. | Colchicine and SOC vs. SOC. | RCT, open-label. April 2020 to March 2021. Multi-center, Argentina. | Hospitalized COVID-19, 1279 patients Mean age 61.8 ± 14.6 years. | Diaz, 2021 [25] |
| Poor | No significant difference in treatment (neither improved the clinical status, nor the inflammatory response) (p = 0.303). | 1.5 mg loading dose for 2 days, followed by 0.5 mg b.i.d. for one week and 0.5 mg o.d. for 28 days. | Colchicine and SOC vs. SOC. SOC (dexamethasone, remdesivir and tocilizumab or baricitinib) | RCT, open-label Four weeks, Madrid, Spain. | Hospitalized COVID-19 patients (non-ICU) 103 patients Mean age 51 ± 12 years. | Pascual-Figal, 2021 [26] |
| Poor | Colchicine inhibits the release of α-Def and D-dimer. | 1 mg b.i.d. for one-day initial dose followed by 0.5 mg b.i.d. for the other 7 days. | Colchicine and SOC vs. SOC. | Randomized, open-label, controlled, clinical trial Hadassah Hospital, Israel. | Hospitalized COVID-19, 16 patients Mean age 51.4 years | Abdeen, 2021 [27] |
| Poor | No significant difference in hospital length days or days on oxygen supplementation. Colchicine decreases mortality (21.4%) vs. supportive care (33.3%) vs. budesonide group (35.7%), (p = 0.67). | 1.5 mg loading dose, followed by 0.5 mg after hour in day 1, then 0.5 mg b.i.d. for 4 days. | Colchicine and supportive care vs. budesonide and supportive care vs. supportive care only. | RCT August 1 to 30. One center, Damascus, Syria. | Hospitalized COVID-19 (non-ventilated). 49 patients. Mean age 50 years. | Alsultan, 2021 [28] |
| Poor | Colchicine improves clinical status distribution on chest CT evaluation (p = 0.048) and reduces pulmonary infiltration (p = 0.026). | 0.5 mg loading dose for 3 days followed by 1 mg for 12 days. | Colchicine and SOC vs. SOC. | RCT March to September 2020. Five hospitals, Iran. | Hospitalized and outpatients, moderate to severe, COVID-19. 202 patients Median age 56 years | Pourdowlat, 2021 [29] |
| Fair | No significant difference in hospitalizations, cause of mortality, and need for ventilation with (p = 0.96, 0.91, and 0.95), respectively. | 0.6 mg b.i.d. for 30 days. | Colchicine and SOC vs. SOC. | RCT May 2020 and March 2021. Multicenter, USA. | Hospitalized COVID-19 with the cardiac disease 93 patients Mean age 71.2 years. | Rabbani, 2022 [30] |
| Poor | Colchicine decreases the time of recovery by an average of 5 days in severe disease and 2 days in moderate (p ≤ 0.001). Did not lower the death rate. | 0.5 mg b.i.d. for 7 days, followed by 0.5 mg o.d. for another 7 days. | Colchicine and SOC vs. SOC. | RCT, open-label. April to August 2021. One hospital, Iraq. | Hospitalized moderate and severe COVID-19 160 patients, Median age 49 [37–60.5] years. | Gorial, 2022 [31] |
| Fair | No significant difference in the combined outcome of (CPAP/BiPAP use, ICU admission, invasive mechanical ventilation, or death) (p = 0.533). | 1 mg loading dose for 5 days followed by 0.5 mg/day. | Colchicine and SOC vs. placebo and SOC. | RCT, observer-blinded endpoint (PROBE). August 2020–March 2021. Four tertiary university hospitals, Spain. | Hospitalized with COVID-19 without oxygen support. 239 patients, Mean age of 65.1 ± 16.0 years. | Cecconi, 2022 [32] |
| Poor | Colchicine combination reduces mortality (p = 0.009), days of oxygen requirement (p = 0.038), and the need for mechanical ventilation (p = 0.020) | 0.5 mg t.i.d. for 5 days, then 0.5 mg b.i.d. for 14 days, or until discharge | 1-Ivermectin + colchicine + SOC. 2-Colchicine + SOC. 3- SOC | RCT. November 2021 to February 2022. University Isolation hospitals, Egypt. | Hospitalized COVID-19, 135 patients. Mean age 57 years. | El Sayed, 2022 [33] |
| Poor | Colchicine has less musculoskeletal (p= 0.001) and respiratory symptoms (p = 0.006), high average SpO2 with oxygen (94.05%), (SpO2: 90.46%) with (p = 0.029), shorter duration at hospitals days (p = 0.009). Less admission to the respiratory care, (p = 0.041). | 0.5 mg b.i.d. for 14 days. | Colchicine and SOC vs. SOC. | RCT, open label. May to June 2021. One Hospital, Iraq. | Hospitalized or at home COVID-19. 80 patients. Age 18–70 years. | Jalal, 2022 [34] |
| Poor | No significant difference in oxygen flow, ventilation, or mortality (p = 0.58). Additionally, in oxygen flow, ventilation, or respiratory mortality (p = 0.58). | 1.2 mg loading dose followed by 0.6 mg two hours later and then 0.6 mg t.i.d. for 28 days. | Rivaroxaban, aspirin and SOC vs. colchicine rivaroxaban, aspirin, and SOC. | RCT open-label, 2 × 2 factorial. October 2020, and February 2022 11 countries. | Hospitalized COVID-19, 2749 patients. Mean age 56.1 years. | Eikelboom, 2022 [35] |
| Fair | No significant difference in the need for mechanical ventilation and death after 14 days (p = 0.171). However, after 28 days’ colchicine reduces mechanical ventilation and death (p = 0.035). | 1.2 mg once initial dose followed by 0.6 mg daily for 13 days. | Colchicine and SOC vs. placebo and SOC. | RCT, blinded placebo controlled. June to November 2020. Dhaka, Bangladesh. | Hospitalized COVID-19, 300 patients Median age 47 (35–55) years. | Rahman, 2022 [36] |
| Poor | No significant difference in the improvement of severe symptoms including cough, shortness of breath, and oxygen requirement with (p = 0.94, 0.69, and 0.28), respectively. | 1.5 mg o.d. for two days initial dose followed by 0.5 mg b.i.d. for 6 days, then 0.5 mg o.d. for other 14 days. | Colchicine and SOC vs. SOC. | RCT, open-labeled. December 2020 to July 2021. Abbottabad, Pakistan. | Hospitalized COVID-19, 96 patients Median age 55.0 (47.5, 68.0) years. | Haroon, 2022 [37] |
| Poor | No significant difference in the day of hospitalization, comorbidities, and oxygen requirements. | Initial dose 0.5 mg t.i.d. for a maximum of 30 days or until hospital discharge. | Colchicine and SOC vs. SOC | RCT open-label April 2020 to May 2021. Multicenter, Italy. | Hospitalized COVID-19 (non-vaccinated). 152 patients Median age of 69.1 ± 13.1 years. | Perricone, 2023 [38] |
| Poor | No significant difference in improving clinical symptoms and decreasing complications in hospitalized COVID-19 patients (p = 0.746). | 2 mg once initial dose followed by 0.5 mg b.i.d. for 7 days. | Colchicine and SOC vs. placebo and SOC | RCT, double-blind, placebo-controlled. February to May 2021 One hospital, Iran. | Hospitalized COVID-19, 106 patients Mean age 54.62 years. | Kasiri, 2023 [39] |
| Poor | Colchicine reduces inflammation and improves symptoms (p = 0.018), and reduces the severity score of CT. | 0.5 mg o.d. | Colchicine, aspirin, and SOC vs. aspirin and SOC. | RCT, open-label. Two hospitals in Mumbai, India. | Hospitalized moderate COVID-19, 122 patients, Age range 40–80 years. | Sunil Naik, 2023 [40] |
| Poor | Colchicine inhibits the NLRP3 inflammasome, lowers levels of Casp1p20 and IL-18 in serum (p < 0.05). Reduces the supplemented oxygen saturation needed and hospitalization days. | 0.5 mg t.i.d. initial dose for 5 days, followed by 0.5 mg b.i.d. for another 5 days. | Colchicine and SOC vs. placebo and SOC | RCT, double-blinded, placebo-controlled. April to August 2020. São Paulo, Brazil. | Hospitalized moderate COVID-19, 72 patients, Median age 55 years. | Amaral, 2023 [41] |
| Observational studies | ||||||
| Good | Colchicine has a better survival rate (p < 0.0001). | 1 mg/day | Colchicine and SOC vs. SOC. | One center observational study from March to April 2020. Italy. | Hospitalized COVID-19 262 patients. Mean age 78.4 (7.5) years non-survivors, 66.6 (13.4) years survivors. | Scarsi, 2020 [42] |
| Good | Colchicine lowers the rate of intubation (p < 0.0001), and mortality (p = 0.0003), and increases the discharge rate (p = 0.0003). Non-significant in mortality and duration of hospitalization in all intubated patients. | 0.6 mg b.i.d. for three days, followed by 0.6 mg o.d. for 12 days. | Colchicine and SOC vs. SOC. | Prospective comparative cohort. March to May 202. One hospital, New York City, USA. | Hospitalized COVID-19 182 patients. Mean age 67.7 ± 12.3 years. | Sandhu, 2020 [43] |
| Poor | Colchicine increases the rate of discharge (p = 0.023), and decreases mortality by day 28 (p = 0.023). | 1.2 mg loading dose for 3 days, followed by 0.6 mg b.i.d. | Colchicine and SOC vs. SOC. | Single-center propensity score matched 1:1 cohort study, March to May 2020. Community Teaching Hospital, USA. | Hospitalized severe COVID-19 66 patients Mean age 61.2 ± 13.0 years. | Brunetti, 2020 [44] |
| Poor | Colchicine combination reduces mortality rate (p < 0.05). No significant differences in clinical severity between the groups (p > 0.05). | For 20 days. | 1-Broad-spectrum antibiotics + low molecular weight heparin (LMWH) + corticosteroids + colchicine. 2-Antibiotic + LMWH + corticosteroids. 3-LMWH + corticosteroids. 4-LMWH + corticosteroids + colchicine. 5-Other treatments (Tocilizumab). | Descriptive observational study. May to August 2020, Private third-level clinic, Colombia. | Hospitalized COVID-19 209 patients. Median age 60 years. | García-Posada, 2021 [45] |
| Good | Colchicine reduces 21-day mortality (p = 0.006), and accelerates recovery. | 1 mg o.d. from 1–21 days or until clinical improvement. | Colchicine and SOC vs. SOC. | Retrospective cohort study, February to April 2020. A tertiary health-care Centre in Parma, Italy. | Hospitalized with COVID-19 with pneumonia, 141 patients Mean age 60.5 (13.4). | Manenti, 2021 [46] |
| Good | Colchicine lowers mortality (p = 0.179). | 0.5 mg b.i.d. for 7 to 14 days. | SOC + colchicine + corticosteroids vs. SOC + corticosteroids vs. SOC alone. | Cross-sectional study March to August 2020. Three clinics in Antioquia, Colombia. | Hospitalized COVID-19, 301 patients. Mean age 56.8 (±17.3) years. | Pinzón, 2021 [47] |
| Good | Colchicine reduces the length of hospital stays (p < 0.001). No significant difference in ICU admission, anti-inflammatory administration, or mortality. Only colchicine (1 mg/day dose) reduces mortality (p = 0.031) and ICU admission rate (p = 0.011) compared with 0.5 mg/day dose. | 0.5 mg/day and 1 mg/day In two separate group | Colchicine and SOC vs. SOC. | Retrospective cohort. August to December 2020. One hospital, Turkey. | Hospitalized COVID-19 336 patients Mean age 62.72 ± 14.37 years. | Karakaş, 2022 [48] |
| Good | No significant difference in decreasing progression concerning admission to the intensive care unit, mortality rate, and treatment failure with (p = 0.174, 1.000, and 0.505), respectively. | 0.5 mg b.i.d. | Colchicine and SOC vs. SOC. | Retrospective case-control study. October 2020 to October 2021. Two centers, Turkey. | Hospitalized COVID-19. 330 patients. Mean age 59.37 ± 14.78 years. | Doğan, 2022 [49] |
| Good | Colchicine resolves symptoms and decreases in duration of hospital stay (p < 0.001), ICU admission (p = 0.013) need for invasive mechanical ventilation (p = 0.025), need for noninvasive mechanical and duration of ICU stay (p > 0.05), and lower mortality rate (p = 0.006). | 1.5 mg initial dose for one day, followed by 0.5 mg b.i.d. on days 2–7 and continuing with 0.5 mg o.d. until completing 14 days. | Colchicine and SOC vs. SOC. | Retrospective study. January to May 2021. One hospital, Egypt. | Hospitalized COVID-19 100 patients. Mean age 58.03 ± 10.59 years. | Korra, 2022 [50] |
| Abstract | Colchicine reduces the duration of hospitalization (p = 0.18). No significant difference in the prevention of ARDS or 28 days mortality. | Treated with colchicine before, or during hospitalization. | Colchicine and SOC vs. SOC. | Retrospective analysis, identified using the Society of Critical Care Medicine COVID-19 registry VIRUS, US. | Hospitalized COVID-19, 108 patients. | Nazir, 2022 [51] |
| Good | Colchicine decreases ICU admission (p = 0.004) and oxygen demand (p = 0.01). The adjusted hazard ratio for hospital death is 0.35, (p < 0.0001). | 0.5 mg b.i.d. within 48 h of declined oxygen saturation. | Colchicine and SOC vs. SOC. | Retrospective, single-center cohort study. November 2020 to January 2021. One hospital, Egypt. | Hospitalized severe COVID-19 153 patients. Mean age 62.65 ± 11.14 years. | Qenawy, 2022 [52] |
| Good | Colchicine lowers the risk of death (p = 0.031). | The median dose was 7.5 mg (3.5–12). | Colchicine and SOC vs. SOC. | Retrospective, multi-center, cohort study. March to June 2020. Two hospitals in Madrid. | Hospitalized COVID-19 (non-ICU). 222 patients. Median age 79 (66–88) years. | Villamañán, 2022 [53] |
| Good | Colchicine does not affect modifying the risk of hospitalization (p = 0.678), preventing (p = 0.291), or decreasing the severity (p = 0.889). | Not mentioned | Colchicine alone or in combination. | Retrospective, case-control, cohort study. June to December 2020, Spain. | Hospitalized COVID-19 86,602 patients (3060 hospitalized, 26 757 not hospitalized for COVID-19, and 56 785 healthy controls) Median age 74 (59−84). | Sáenz-Aldea, 2023 [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharaf, S.; Ashmawy, R.; Saleh, E.; Salama, M.; El-Maradny, Y.A.; Zari, A.; Aly, S.; Tolba, A.; Mahrous, D.; Elsayed, H.; et al. Oxygen Saturation in Hospitalized COVID-19 Patients and Its Relation to Colchicine Treatment: A Retrospective Cohort Study with an Updated Systematic Review. Medicina 2023, 59, 934. https://doi.org/10.3390/medicina59050934
Sharaf S, Ashmawy R, Saleh E, Salama M, El-Maradny YA, Zari A, Aly S, Tolba A, Mahrous D, Elsayed H, et al. Oxygen Saturation in Hospitalized COVID-19 Patients and Its Relation to Colchicine Treatment: A Retrospective Cohort Study with an Updated Systematic Review. Medicina. 2023; 59(5):934. https://doi.org/10.3390/medicina59050934
Chicago/Turabian StyleSharaf, Sandy, Rasha Ashmawy, Eman Saleh, Mayada Salama, Yousra A. El-Maradny, Ali Zari, Shahinda Aly, Ahmed Tolba, Doaa Mahrous, Hanan Elsayed, and et al. 2023. "Oxygen Saturation in Hospitalized COVID-19 Patients and Its Relation to Colchicine Treatment: A Retrospective Cohort Study with an Updated Systematic Review" Medicina 59, no. 5: 934. https://doi.org/10.3390/medicina59050934
APA StyleSharaf, S., Ashmawy, R., Saleh, E., Salama, M., El-Maradny, Y. A., Zari, A., Aly, S., Tolba, A., Mahrous, D., Elsayed, H., Latif, D., Redwan, E. M., & Kamal, E. (2023). Oxygen Saturation in Hospitalized COVID-19 Patients and Its Relation to Colchicine Treatment: A Retrospective Cohort Study with an Updated Systematic Review. Medicina, 59(5), 934. https://doi.org/10.3390/medicina59050934










