Renal Sarcoidosis-like Reaction Induced by PD-1 Inhibitor Treatment in Non-Small Cell Lung Cancer: A Case Report and Literature Review
Abstract
1. Introduction
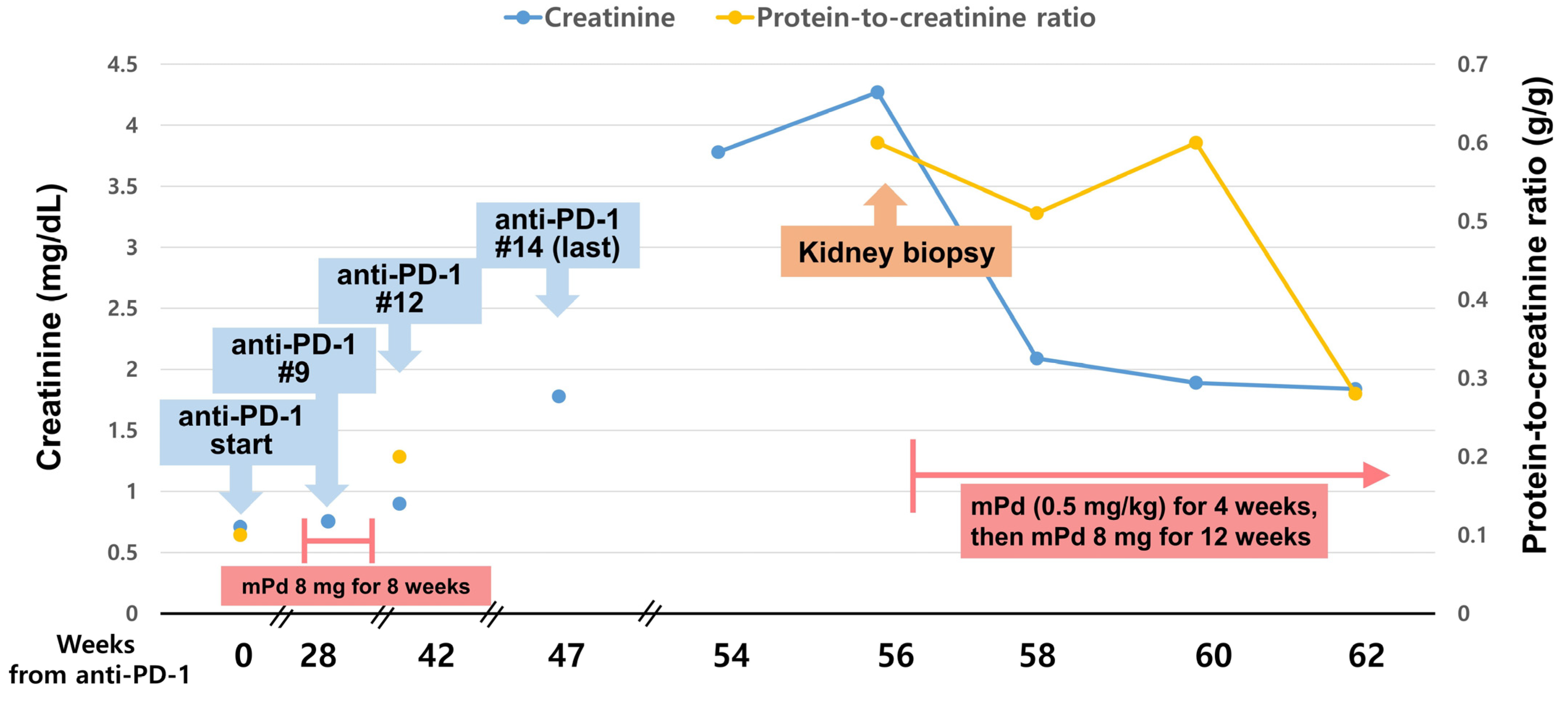
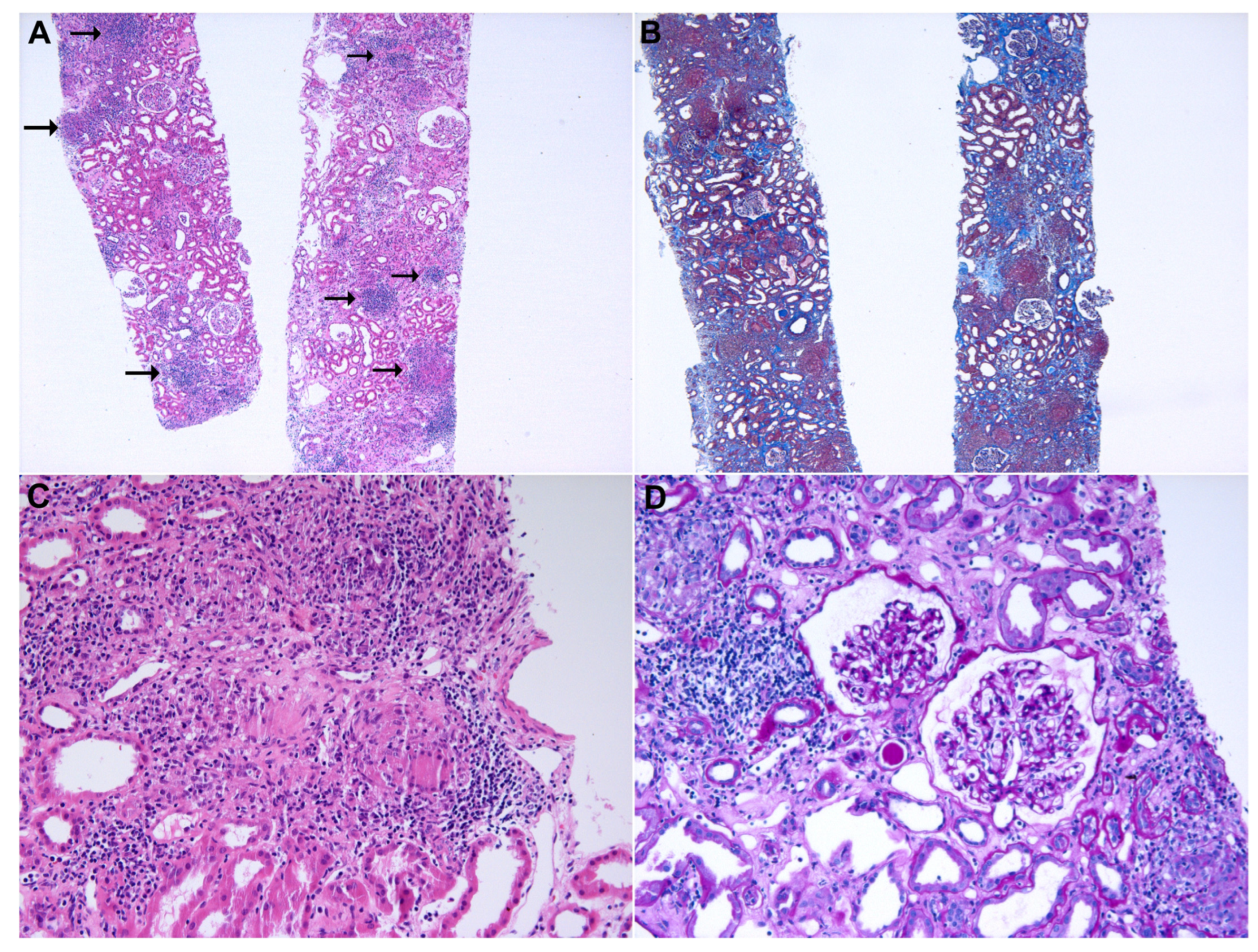
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, E.M.; Golay, H.G.; Liu, Y.; Koyama, S.; Wong, K.; Taylor-Weiner, A.; Giannakis, M.; Harden, M.; Rojas-Rudilla, V.; Chevalier, A.; et al. Long-term Benefit of PD-L1 Blockade in Lung Cancer Associated with JAK3 Activation. Cancer Immunol. Res. 2015, 3, 855–863. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Gupta, S.; Cortazar, F.B.; Riella, L.V.; Leaf, D.E. Immune Checkpoint Inhibitor Nephrotoxicity: Update 2020. Kidney360 2020, 1, 130–140. [Google Scholar] [CrossRef]
- Callahan, M.K.; Kluger, H.; Postow, M.A.; Segal, N.H.; Lesokhin, A.; Atkins, M.B.; Kirkwood, J.M.; Krishnan, S.; Bhore, R.; Horak, C.; et al. Nivolumab Plus Ipilimumab in Patients With Advanced Melanoma: Updated Survival, Response, and Safety Data in a Phase I Dose-Escalation Study. J. Clin. Oncol. 2018, 36, 391–398. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2016, 27, 559–574. [Google Scholar] [CrossRef]
- Perazella, M.A.; Shirali, A.C. Nephrotoxicity of Cancer Immunotherapies: Past, Present and Future. J. Am. Soc. Nephrol. 2018, 29, 2039–2052. [Google Scholar] [CrossRef]
- Gkiozos, I.; Kopitopoulou, A.; Kalkanis, A.; Vamvakaris, I.N.; Judson, M.A.; Syrigos, K.N. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J. Thorac. Oncol. 2018, 13, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Rambhia, P.H.; Reichert, B.; Scott, J.F.; Feneran, A.N.; Kazakov, J.A.; Honda, K.; Koon, H.; Gerstenblith, M.R. Immune checkpoint inhibitor-induced sarcoidosis-like granulomas. Int. J. Clin. Oncol. 2019, 24, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.Y.; Yuan, J.; Page, D.B.; Schroeder, S.E.; Panageas, K.S.; Carvajal, R.D.; Chapman, P.B.; Schwartz, G.K.; Allison, J.P.; Wolchok, J.D. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: Lymphocyte count after 2 doses correlates with survival. Cancer 2010, 116, 1767–1775. [Google Scholar] [CrossRef]
- Ji, R.R.; Chasalow, S.D.; Wang, L.; Hamid, O.; Schmidt, H.; Cogswell, J.; Alaparthy, S.; Berman, D.; Jure-Kunkel, M.; Siemers, N.O.; et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immunother. 2012, 61, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.R. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 1999, 16, 24–31. [Google Scholar]
- von Euw, E.; Chodon, T.; Attar, N.; Jalil, J.; Koya, R.C.; Comin-Anduix, B.; Ribas, A. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J. Transl. Med. 2009, 7, 35. [Google Scholar] [CrossRef]
- Facco, M.; Cabrelle, A.; Teramo, A.; Olivieri, V.; Gnoato, M.; Teolato, S.; Ave, E.; Gattazzo, C.; Fadini, G.P.; Calabrese, F.; et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2011, 66, 144–150. [Google Scholar] [CrossRef]
- Huang, H.; Lu, Z.; Jiang, C.; Liu, J.; Wang, Y.; Xu, Z. Imbalance between Th17 and regulatory T-Cells in sarcoidosis. Int. J. Mol. Sci. 2013, 14, 21463–21473. [Google Scholar] [CrossRef]
- Chanson, N.; Ramos-Casals, M.; Pundole, X.; Suijkerbuijk, K.; de Barros e Silva, M.J.; Lidar, M.; Benesova, K.; Leipe, J.; Acar-Denizli, N.; Pradère, P.; et al. Immune checkpoint inhibitor-associated sarcoidosis: A usually benign disease that does not require immunotherapy discontinuation. Eur. J. Cancer 2021, 158, 208–216. [Google Scholar] [CrossRef]
- Kidd, J.M.; Gizaw, A.B. Ipilimumab-associated minimal-change disease. Kidney Int. 2016, 89, 720. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kawakami, H.; Ichikawa, M.; Yamamoto, S.; Otsuka, Y.; Mashiko, A.; Takashima, Y.; Ito, A.; Nakagawa, K.; Arima, S. Nivolumab-induced acute granulomatous tubulointerstitial nephritis in a patient with gastric cancer. Investig. New Drugs 2018, 36, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Person, F.; Chahoud-Schriefer, T.; Fehrle, W.; Janneck, M.; Huber, T.B.; Wiech, T. Severe Acute Kidney Injury Due to Nivolumab/Ipilimumab-induced Granulomatosis and Fibrinoid Vascular Necrosis. J. Immunother. 2020, 43, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Thajudeen, B.; Madhrira, M.; Bracamonte, E.; Cranmer, L.D. Ipilimumab granulomatous interstitial nephritis. Am. J. Ther. 2015, 22, e84–e87. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Gueutin, V.; Gharbi, C.; Mateus, C.; Robert, C.; Routier, E.; Thomas, M.; Baumelou, A.; Rouvier, P. Kidney injuries related to ipilimumab. Investig. New Drugs 2014, 32, 769–773. [Google Scholar] [CrossRef]
- Charkviani, M.; Herrmann, S.M. Immune Checkpoint Inhibitor-Associated Sarcoidosis Reaction in the Kidney: Case Report. Kidney Med. 2023, 5, 100626. [Google Scholar] [CrossRef]
| Study | Age, Years | Sex | Immune Checkpoint Inhibitor | Primary Cancer | Onset (after ICI Cycle) | Histopathological Findings | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Nakatani et al. [24] | 68 | F | Nivolumab (PD-1 inhibitor) | Gastric cancer | 30th cycle | Granulomatous AIN | Steroid (mPd) 1.0 mg/kg/day (40 mg/day) for 7 days, 30 mg/day for 7 days, 20 mg/day for 7 days, then slowly tapered off | Resolved |
| Person et al. [25] | 55 | M | Nivolumab/Ipilimumab (PD-1/CTLA-4 inhibitor) | Melanoma | 2nd cycle | Granulomatous AIN and thrombotic microangiopathy-like lesion | Steroid (mPd 200 mg once) Mycophenolic acid TNF-α blockade (infliximab) | Progression to ESKD |
| Thajudeen et al. [26] | 74 | M | Ipilimumab (CTLA-4 inhibitor) | Melanoma | 3rd cycle | Granulomatous AIN | Steroid (Pd) 60 mg/day for 4 weeks, then tapered off for two weeks | Resolved |
| Izzedine et al. [27] | 60 | F | Ipilimumab (CTLA-4 inhibitor) | Melanoma | 3rd cycle | Granulomatous AIN | Steroid (Pd) 1.0 mg/kg/day for four weeks, then tapered off quickly | Resolved |
| Charkviani et al. [28] | Early 60s | M | Nivolumab/Ipilimumab (PD-1/CTLA-4 inhibitor) | RCC | 10 weeks | Granulomatous AIN | Steroid (mPd) 500 mg once, 80 mg/day for seven days, then tapered for eight weeks | Resolved |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-D.; Kim, M.-S.; Han, M.-H.; Kim, Y.-J.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; et al. Renal Sarcoidosis-like Reaction Induced by PD-1 Inhibitor Treatment in Non-Small Cell Lung Cancer: A Case Report and Literature Review. Medicina 2023, 59, 991. https://doi.org/10.3390/medicina59050991
Park S-D, Kim M-S, Han M-H, Kim Y-J, Jung H-Y, Choi J-Y, Cho J-H, Park S-H, Kim C-D, Kim Y-L, et al. Renal Sarcoidosis-like Reaction Induced by PD-1 Inhibitor Treatment in Non-Small Cell Lung Cancer: A Case Report and Literature Review. Medicina. 2023; 59(5):991. https://doi.org/10.3390/medicina59050991
Chicago/Turabian StylePark, Sang-Don, Mee-Seon Kim, Man-Hoon Han, Yong-Jin Kim, Hee-Yeon Jung, Ji-Young Choi, Jang-Hee Cho, Sun-Hee Park, Chan-Duck Kim, Yong-Lim Kim, and et al. 2023. "Renal Sarcoidosis-like Reaction Induced by PD-1 Inhibitor Treatment in Non-Small Cell Lung Cancer: A Case Report and Literature Review" Medicina 59, no. 5: 991. https://doi.org/10.3390/medicina59050991
APA StylePark, S.-D., Kim, M.-S., Han, M.-H., Kim, Y.-J., Jung, H.-Y., Choi, J.-Y., Cho, J.-H., Park, S.-H., Kim, C.-D., Kim, Y.-L., & Lim, J.-H. (2023). Renal Sarcoidosis-like Reaction Induced by PD-1 Inhibitor Treatment in Non-Small Cell Lung Cancer: A Case Report and Literature Review. Medicina, 59(5), 991. https://doi.org/10.3390/medicina59050991









