Adult-Onset Linear Morphea (en coupe de sabre) of the Face Successfully Treated with Photoactivated Low-Temperature Platelet-Rich Plasma: A Valid Therapeutic Option
Abstract
1. Introduction
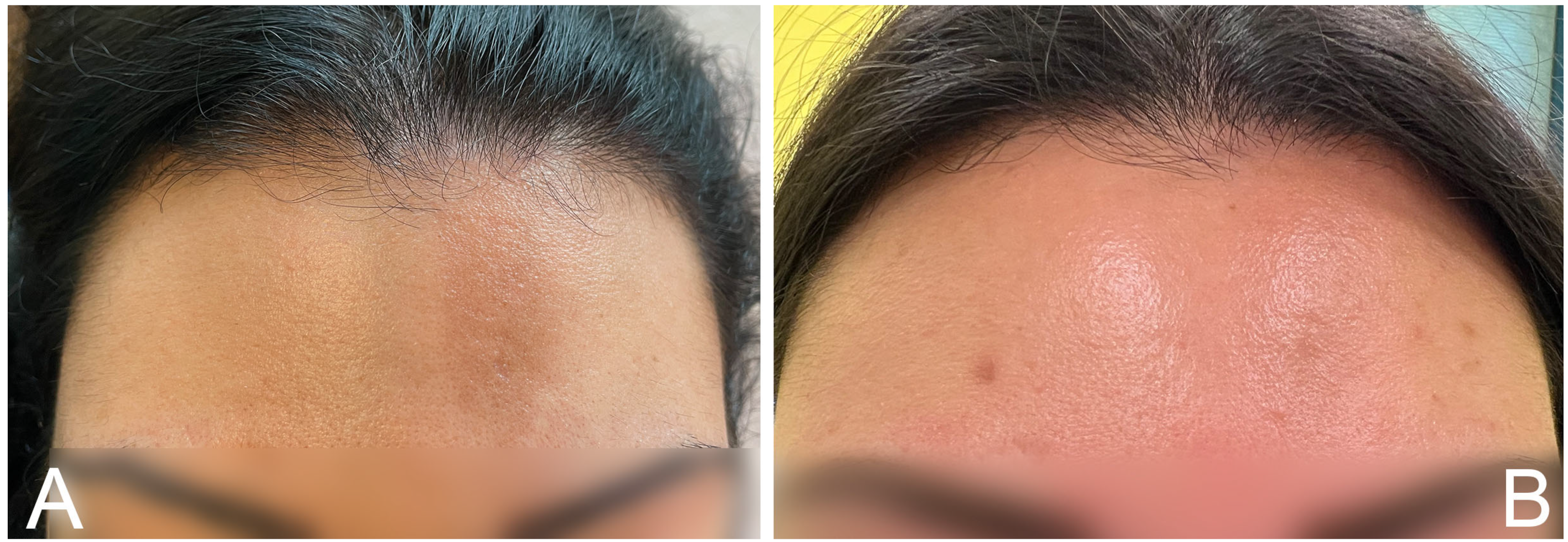
2. Case Report
3. Discussion
- (a)
- Pure platelet-rich plasma: a PRP poor in leukocytes, with a low-density fibrin network after activation;
- (b)
- Leukocyte-rich platelet-rich plasma: a PRP rich in leukocytes with a low-density fibrin network after activation;
- (c)
- Pure platelet-rich fibrin: a PRP poor in leukocytes, with a high-density fibrin network (unlike the previous ones, this cannot be injected and exists in an activated gel form);
- (d)
- Leukocyte- and platelet-rich fibrin: a PRP rich in leukocytes with a high-density fibrin network.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belgaumkar, V.A.; Deshmukh, N.S.; Doshi, B.R.; Mhaske, C.B. En Coup de Sabre Treated with Platelet-Rich Plasma. Indian J. Drugs Dermatol. 2015, 1, 27. [Google Scholar]
- Kunzler, E.; Florez-Pollack, S.; Teske, N.; O’Brien, J.; Prasad, S.; Jacobe, H. Linear Morphea: Clinical Characteristics, Disease Course, and Treatment of the Morphea in Adults and Children Cohort. J. Am. Acad. Dermatol. 2019, 80, 1664–1670.e1. [Google Scholar] [CrossRef] [PubMed]
- Saracino, A.M.; George, C.; Nihtyanova, S.I.; Denton, C.P. Comparing Paediatric- and Adult-Onset Linear Morphoea in a Large Tertiary-Referral Scleroderma Centre. J. Scleroderma Relat. Disord. 2021, 6, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, S.R.; Vollono, L.; Paolino, G. The Usefulness of Platelet-Rich Plasma (PRP) for the Treatment of Vitiligo: State of the Art and Review. Drug Des Devel Ther. 2020, 14, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Matteucci, E.; Dogliotti, G.; Corsi, M.M.; Banfi, G.; Maroni, P.; Desiderio, M.A. Molecular Basis of Anti-Inflammatory Action of Platelet-Rich Plasma on Human Chondrocytes: Mechanisms of NF-ΚB Inhibition via HGF. J. Cell. Physiol. 2010, 225, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, S.R.; Di Nicola, M.R.; Brianti, P.; Bianchi, V.G.; Paolino, G. Pilot Study on the Use of the “Monocyte-Rich” Platelet-Rich Plasma in Combination with 1927 Nm Fractional and 308 Nm Excimer Lasers for the Treatment of Vitiligo. Medicina 2021, 57, 904. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Barnard, A.; Rotstein, A. Photoactivated Platelet-Rich Plasma Therapy for a Traumatic Knee Chondral Lesion. Case Rep. 2012, 2012, bcr2012006858. [Google Scholar] [CrossRef] [PubMed]
- Fallahnezhad, S.; Jajarmi, V.; Shahnavaz, S.; Amini, A.; Ghoreishi, S.K.; Kazemi, M.; Chien, S.; Bayat, M. Improvement in Viability and Mineralization of Osteoporotic Bone Marrow Mesenchymal Stem Cell through Combined Application of Photobiomodulation Therapy and Oxytocin. Lasers Med. Sci. 2020, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Irmak, G.; Demirtaş, T.T.; Gümüşderelioğlu, M. Sustained Release of Growth Factors from Photoactivated Platelet Rich Plasma (PRP). Eur. J. Pharm. Biopharm. 2020, 148, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, S.; Lin, W.; Yang, D. Autologous Concentrated Growth Factor Used to Treat Linear Scleroderma En Coup de Sabre: A Case Report. CCID 2022, 15, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Virzì, F.; Bianca, P.; Giammona, A.; Apuzzo, T.; Di Franco, S.; Mangiapane, L.R.; Colorito, M.L.; Catalano, D.; Scavo, E.; Nicotra, A.; et al. Combined Platelet-Rich Plasma and Lipofilling Treatment Provides Great Improvement in Facial Skin-Induced Lesion Regeneration for Scleroderma Patients. Stem Cell Res. Ther. 2017, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Pirrello, R.; Verro, B.; Grasso, G.; Ruscitti, P.; Cordova, A.; Giacomelli, R.; Ciccia, F.; Guggino, G. Hyaluronic Acid and Platelet-Rich Plasma, a New Therapeutic Alternative for Scleroderma Patients: A Prospective Open-Label Study. Arthritis Res. Ther. 2019, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Khashaba, S.; Elhanfi, A. Morphea Patients Treated with Platelet Rich Plasma:A Pilot Study. Zagazig Univ. Med. J. 2019, 28, 40–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercuri, S.R.; Di Nicola, M.R.; Bianchi, V.G.; Paolino, G. Adult-Onset Linear Morphea (en coupe de sabre) of the Face Successfully Treated with Photoactivated Low-Temperature Platelet-Rich Plasma: A Valid Therapeutic Option. Medicina 2023, 59, 1114. https://doi.org/10.3390/medicina59061114
Mercuri SR, Di Nicola MR, Bianchi VG, Paolino G. Adult-Onset Linear Morphea (en coupe de sabre) of the Face Successfully Treated with Photoactivated Low-Temperature Platelet-Rich Plasma: A Valid Therapeutic Option. Medicina. 2023; 59(6):1114. https://doi.org/10.3390/medicina59061114
Chicago/Turabian StyleMercuri, Santo Raffaele, Matteo Riccardo Di Nicola, Vittoria Giulia Bianchi, and Giovanni Paolino. 2023. "Adult-Onset Linear Morphea (en coupe de sabre) of the Face Successfully Treated with Photoactivated Low-Temperature Platelet-Rich Plasma: A Valid Therapeutic Option" Medicina 59, no. 6: 1114. https://doi.org/10.3390/medicina59061114
APA StyleMercuri, S. R., Di Nicola, M. R., Bianchi, V. G., & Paolino, G. (2023). Adult-Onset Linear Morphea (en coupe de sabre) of the Face Successfully Treated with Photoactivated Low-Temperature Platelet-Rich Plasma: A Valid Therapeutic Option. Medicina, 59(6), 1114. https://doi.org/10.3390/medicina59061114






