Treatment Durations and Whitening Outcomes of Different Tooth Whitening Systems
Abstract
1. Introduction
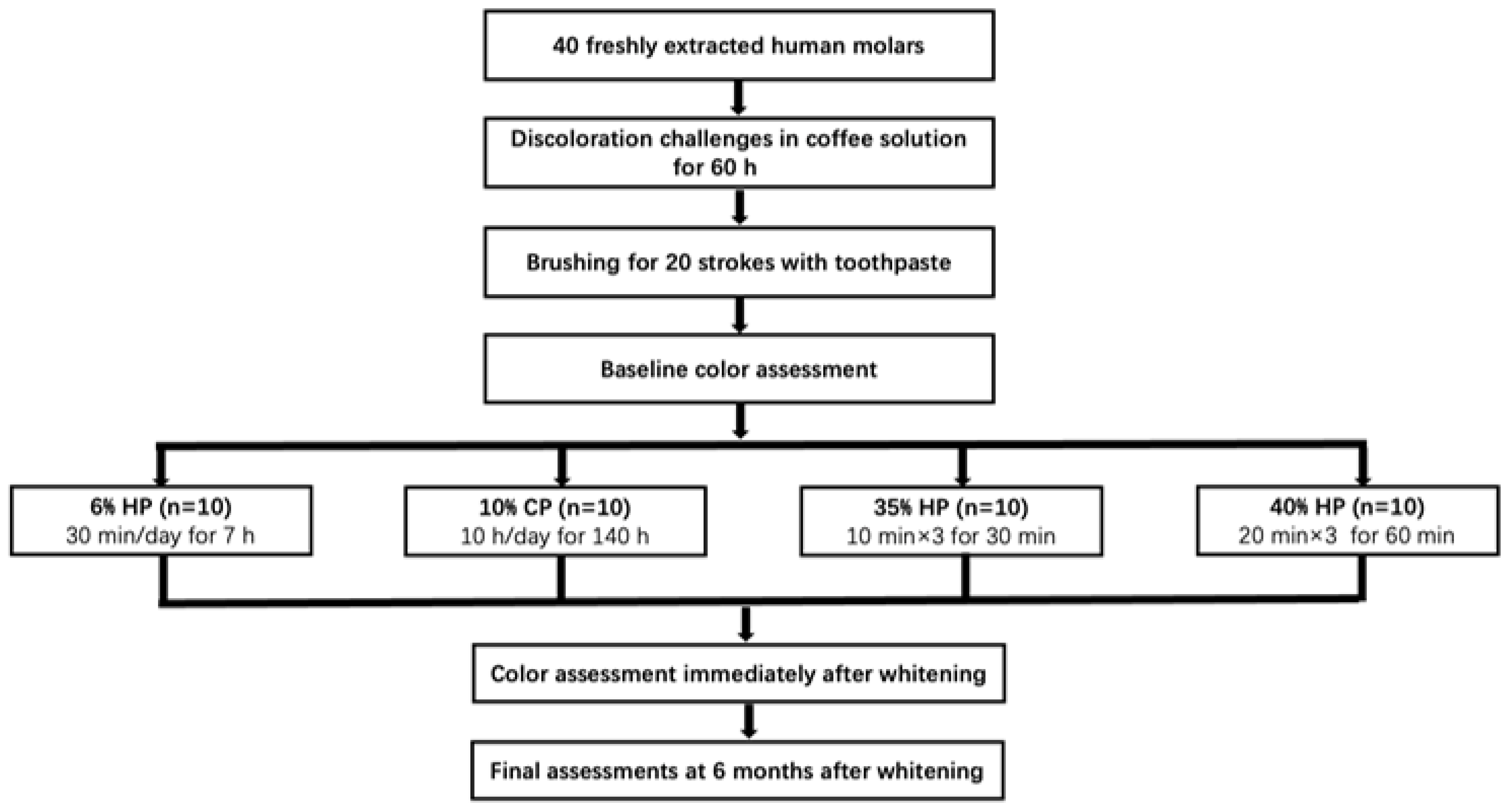
2. Materials and Methods
2.1. Sample Preparation
2.2. Whitening Treatments
2.3. Color Assessments
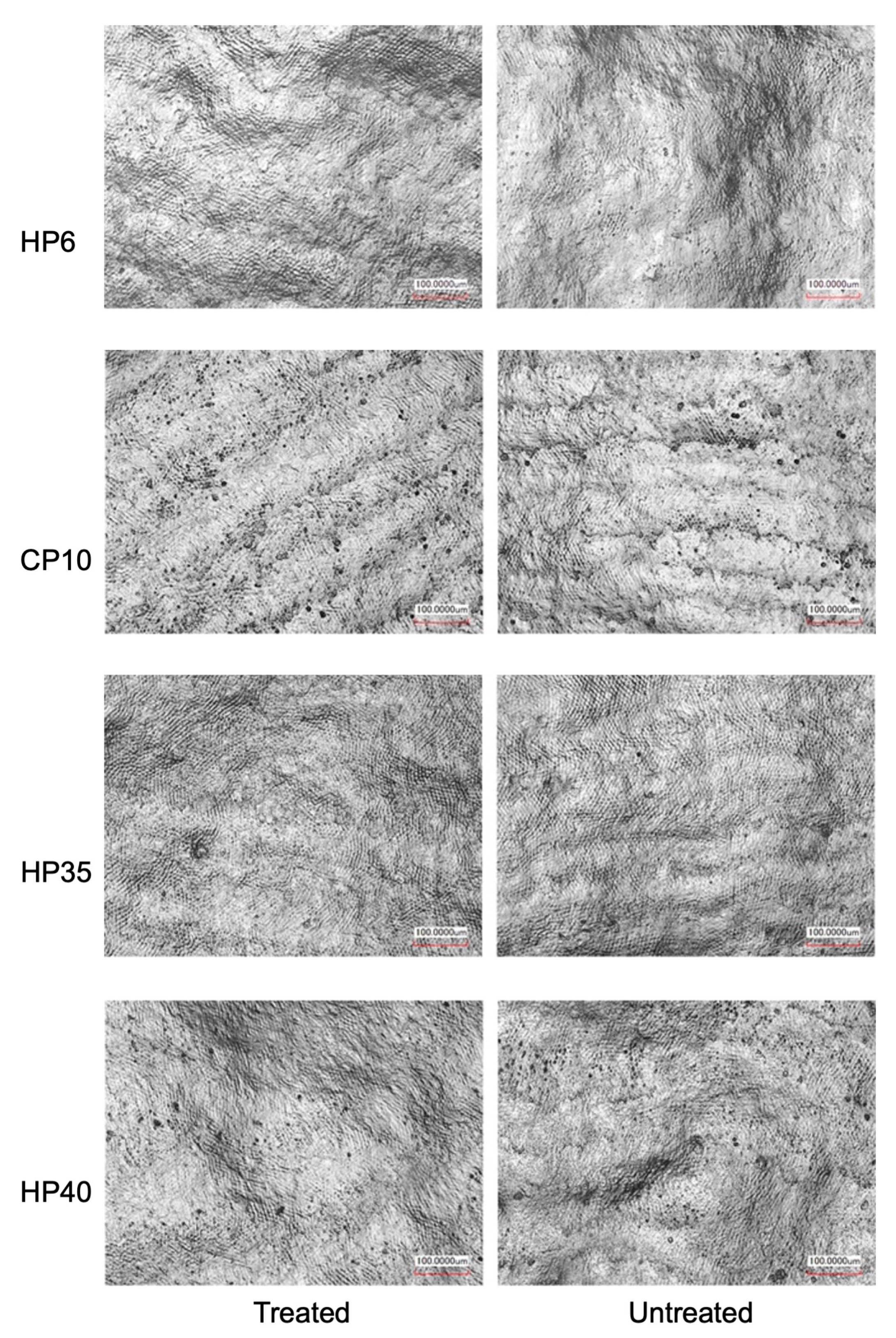
2.4. 3D Scanning for Surface Morphology and Surface Roughness Measurements
2.5. Statistical Analysis
3. Results
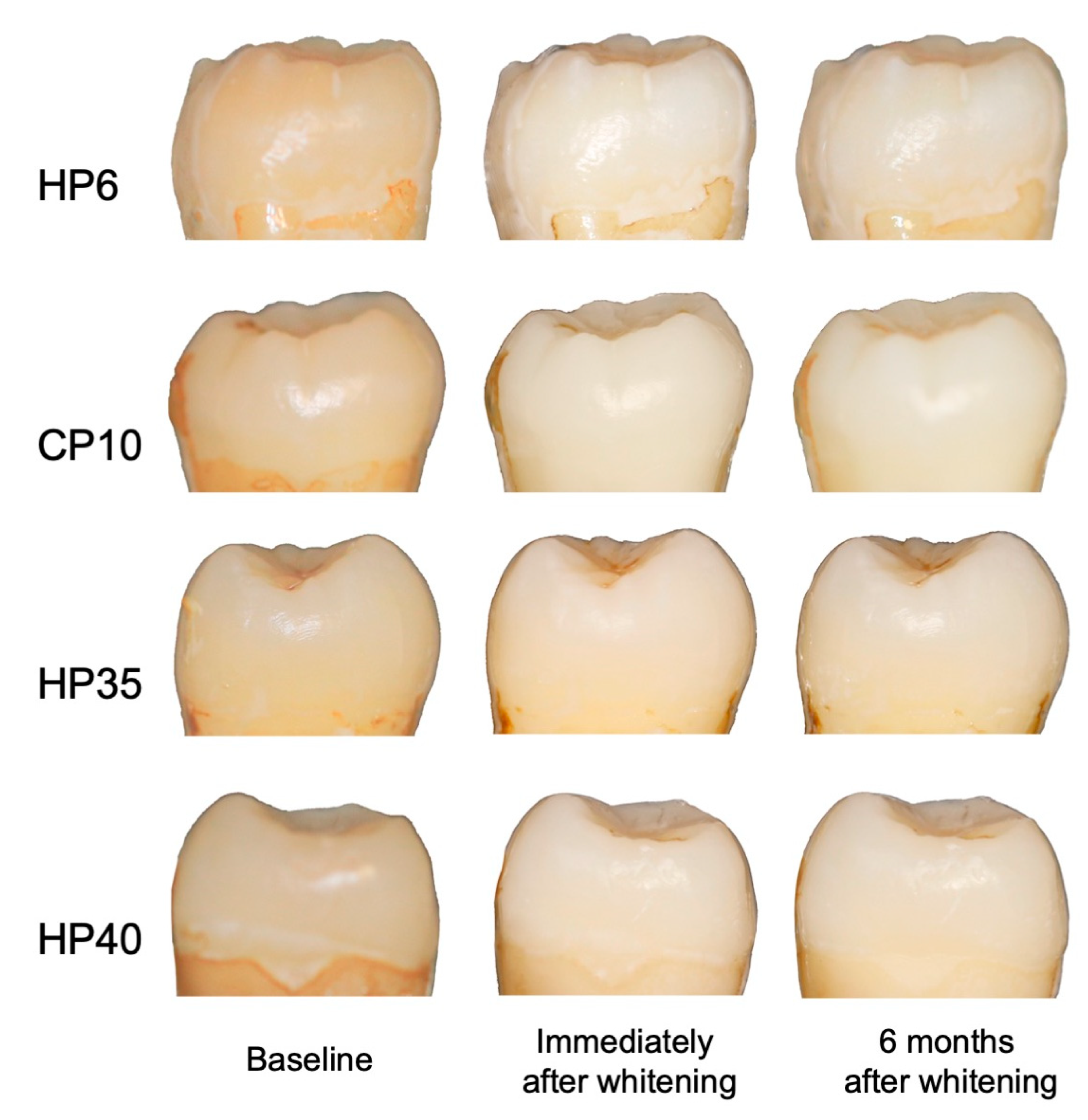
3.1. Color Changes after Whitening Treatments
3.2. Surface Roughness of Treated and Untreated Enamel Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Angari, S.S.; Lippert, F.; Platt, J.A.; Eckert, G.J.; González-Cabezas, C.; Li, Y.; Hara, A.T. Dental bleaching efficacy and impact on demineralization susceptibility of simulated stained-remineralized caries lesions. J. Dent. 2019, 81, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.R.; Wertz, P.W. Review of the Mechanism of Tooth Whitening. J. Esthet. Restor. Dent. 2015, 27, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Mailart, M.C.; Sakassegawa, P.A.; Santos, K.C.; Torres, C.R.G.; Palo, R.M.; Borges, A.B. One-year follow-up comparing at-home bleaching systems outcomes and the impact on patient’s satisfaction: Randomized clinical trial. J. Esthet. Restor. Dent. 2021, 33, 1175–1185. [Google Scholar]
- Rodríguez-Martínez, J.; Valiente, M.; Sánchez-Martín, M.J. Tooth whitening: From the established treatments to novel approaches to prevent side effects. J. Esthet. Restor. Dent. 2019, 31, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Aka, B.; Celik, E.U. Evaluation of the Efficacy and Color Stability of Two Different At-Home Bleaching Systems on Teeth of Different Shades: A Randomized Controlled Clinical Trial. J. Esthet. Restor. Dent. 2017, 29, 325–338. [Google Scholar]
- da Costa, J.B.; McPharlin, R.; Paravina, R.D.; Ferracane, J.L. Comparison of at-home and in-office tooth whitening using a novel shade guide. Oper. Dent. 2010, 35, 381–388. [Google Scholar] [CrossRef]
- Vieira, I.; Vieira-Junior, W.F.; Pauli, M.C.; Theobaldo, J.D.; Aguiar, F.H.; Lima, D.A.; Leonardi, G.R. Effect of in-office bleaching gels with calcium or fluoride on color, roughness, and enamel microhardness. J. Clin. Exp. Dent. 2020, 12, e116–e122. [Google Scholar] [CrossRef]
- SoutoMaior, J.R.; de Moraes, S.; Lemos, C.; Vasconcelos, B.D.E.; Montes, M.; Pellizzer, E.P. Effectiveness of Light Sources on In-Office Dental Bleaching: A Systematic Review and Meta-Analyses. Oper. Dent. 2019, 44, e105–e117. [Google Scholar] [CrossRef]
- Matis, B.A.; Cochran, M.A.; Franco, M.; Al-Ammar, W.; Eckert, G.J.; Stropes, M. Eight in-office tooth whitening systems evaluated in vivo: A pilot study. Oper. Dent. 2007, 32, 322–327. [Google Scholar] [CrossRef]
- Lilaj, B.; Dauti, R.; Agis, H.; Schmid-Schwap, M.; Franz, A.; Kanz, F.; Moritz, A.; Schedle, A.; Cvikl, B. Comparison of Bleaching Products with Up to 6% and With More Than 6% Hydrogen Peroxide: Whitening Efficacy Using BI and WI (D) and Side Effects—An in vitro Study. Front. Physiol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Ozdemir, Z.M.; Surmelioglu, D. Effects of different bleaching application time on tooth color and mineral alteration. Ann. Anat.-Anat. Anz. 2021, 233, 151590. [Google Scholar] [CrossRef]
- Burrows, S. A review of the efficacy of tooth bleaching. Dent. Update 2009, 36, 537–551. [Google Scholar] [CrossRef]
- Li, Q.; Xu, B.T.; Li, R.; Yu, H.; Wang, Y.N. Quantitative evaluation of colour regression and mineral content change of bleached teeth. J. Dent. 2010, 38, 253–260. [Google Scholar] [CrossRef]
- Mondelli, R.F.; Azevedo, J.F.; Francisconi, A.C.; Almeida, C.M.; Ishikiriama, S.K. Comparative clinical study of the effectiveness of different dental bleaching methods—Two year follow-up. J. Appl. Oral. Sci. 2012, 20, 435–443. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Jurado, C.A.; Sayed, M.E.; Fischer, N.G.; Takamizawa, T.; Latta, M.A.; Miyazaki, M.; Garcia-Godoy, F. Influence of light irradiation for in-office tooth whitening: A randomized clinical study. Am. J. Dent. 2021, 34, 201–204. [Google Scholar]
- Nie, J.; Tian, F.C.; Wang, Z.H.; Yap, A.U.; Wang, X.Y. Comparison of efficacy and outcome satisfaction between in-office and home teeth bleaching in Chinese patients. J. Oral Sci. 2017, 59, 527–532. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Pan, J.; Malmstrom, H.; Ren, Y.F. Effects of desensitizing dentifrices on dentin tubule occlusion and resistance to erosive challenges. BMC Oral Health 2021, 21, 610. [Google Scholar] [CrossRef]
- Da Silva, J.D.; Park, S.E.; Weber, H.-P.; Ishikawa-Nagai, S. Clinical performance of a newly developed spectrophotometric system on tooth color reproduction. J. Prosthet. Dent. 2008, 99, 361–368. [Google Scholar] [CrossRef]
- Ishikawa-Nagai, S.; Yoshida, A.; Sakai, M.; Kristiansen, J.; Da Silva, J.D. Clinical evaluation of perceptibility of color differences between natural teeth and all-ceramic crowns. J. Dent. 2009, 37 (Suppl. 1), e57–e63. [Google Scholar] [CrossRef]
- Peixoto, A.C.; Vaez, S.C.; Pereira, N.A.R.; Santana, C.; Soares, K.D.A.; Romão, A.; Ferreira, L.F.; Martins-Filho, P.R.S.; Faria, E.S.A.L. High-concentration carbamide peroxide can reduce the sensitivity caused by in-office tooth bleaching: A single-blinded randomized controlled trial. J. Appl. Oral Sci. 2018, 26, e20170573. [Google Scholar] [CrossRef]
- Mayer-Santos, E.; Maravic, T.; Comba, A.; Freitas, P.M.; Marinho, G.B.; Mazzitelli, C.; Mancuso, E.; Scotti, N.; Florenzano, F.; Breschi, L.; et al. The Influence of Different Bleaching Protocols on Dentinal Enzymatic Activity: An In Vitro Study. Molecules 2022, 27, 1684. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B. Present status and future directions—Managing discoloured teeth. Int. Endod. J. 2022, 55 (Suppl. S4), 922–950. [Google Scholar] [CrossRef] [PubMed]
- Maran, B.M.; Ziegelmann, P.K.; Burey, A.; de Paris Matos, T.; Loguercio, A.D.; Reis, A. Different light-activation systems associated with dental bleaching: A systematic review and a network meta-analysis. Clin. Oral Investig. 2019, 23, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Maran, B.M.; Burey, A.; de Paris Matos, T.; Loguercio, A.D.; Reis, A. In-office dental bleaching with light vs. without light: A systematic review and meta-analysis. J. Dent. 2018, 70, 1–13. [Google Scholar] [CrossRef]
- De Freitas, P.M.; Menezes, A.N.; da Mota, A.C.; Simões, A.; Mendes, F.M.; Lago, A.D.; Ferreira, L.S.; Ramos-Oliveira, T.M. Does the hybrid light source (LED/laser) influence temperature variation on the enamel surface during 35% hydrogen peroxide bleaching? A randomized clinical trial. Quintessence Int. 2016, 47, 61–73. [Google Scholar]
- Vargas-Koudriavtsev, T.; Fonseca-Jiménez, P.; Barrantes-Delgado, P.; Ruiz-Delgado, B.; Conejo-Barboza, G.; Herrera-Sancho, Ó.-A. Effects of Bleaching Gels on Dental Enamel Crystallography. Oral Health Prev. Dent. 2021, 19, 7–14. [Google Scholar]
- Eimar, H.; Marelli, B.; Nazhat, S.N.; Abi Nader, S.; Amin, W.M.; Torres, J.; de Albuquerque, R.F., Jr.; Tamimi, F. The role of enamel crystallography on tooth shade. J. Dent. 2011, 39 (Suppl. S3), e3–e10. [Google Scholar] [CrossRef]
- Polydorou, O.; Scheitza, S.; Spraul, M.; Vach, K.; Hellwig, E. The effect of long-term use of tooth bleaching products on the human enamel surface. Odontology 2018, 106, 64–72. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Batista, A.L.; Neto, J.A.; Simões, T.M.; da Silva, M.G.; de Barros, D.D.; Catão, J.S.; de Oliveira, T.A.; Catão, M.V. Evaluation of dental enamel microproperties after bleaching with 35% hydrogen peroxide and different light sources: An in vitro study. J. Clin. Exp. Dent. 2021, 13, e969–e974. [Google Scholar] [CrossRef]
- Scribante, A.; Poggio, C.; Gallo, S.; Riva, P.; Cuocci, A.; Carbone, M.; Arciola, C.R.; Colombo, M. In Vitro Re-Hardening of Bleached Enamel Using Mineralizing Pastes: Toward Preventing Bacterial Colonization. Materials 2020, 13, 818. [Google Scholar] [CrossRef]
| Groups | Whitening Systems | Compositions | Manufacturer Directions * | Treatment Duration |
|---|---|---|---|---|
| HP6 | Beyond Corewhite (Beyond International Inc. Stafford, TX, USA) | An amount of 6% hydrogen peroxide, de-ionized water, propylene glycon, stabilizers, carbomer, trolamine, sodium hydroxide, flavor, and sodium saccharin | Load the whitening gel to facial surface inside a custom tray, and wear the tray for 30 min per day | 30 min/d, 14 d (total 7 h) |
| CP10 | Opalescence TM PF 10% (Ultradent Products Inc, South Jordan, UT, USA) | An amount of 10% carbamide peroxide, glycerin, water, xylitol, carbomer, PEG-300, sodium hydroxide, EDTA, potassium nitrate, and sodium fluoride | Load the whitening gel into the deepest and outmost portion of custom tray, and wear the tray 8–10 h overnight | 10 h/d, 14 d (total 140 h) |
| HP35 | Beyond Max 5 (Beyond International Inc. Stafford, TX, USA) | An amount of 35% hydrogen peroxide, potassium nitrate, hydroxyapatite, propylene glycon, sodium hydroxide, and carbomer | Apply a 2–3 mm layer of to the dry surface. Position the Whitening Accelerator lamp head at a 90° angle to the teeth to begin the first 10 min cycle. Repeat for a total of 3 cycles. | 10 min × 3 (total 30 min) |
| HP40 | Opalescence Boost (Ultradent Products Inc, South Jordan, UT, USA) | An amount of 40% hydrogen peroxide, potassium nitrate, sodium fluoride, potassium hydroxide, carbopol, glycerin, and distilled water | Apply a 0.5–1.0 mm thick layer of gel to the labial surface of the tooth and slightly onto the incisal surface. Allow gel to remain on the teeth 20 min. Repeat for a total of 3 cycles. | 20 min × 3 (total 60 min) |
| HP6 | CP10 | HP35 | HP40 | ||
|---|---|---|---|---|---|
| Baseline | L* | 68.89 ± 2.49 | 70.24 ± 1.80 | 69.59 ± 1.65 | 69.98 ± 1.21 |
| a* | 3.51 ± 0.95 | 3.30 ± 0.53 | 3.20 ± 0.68 | 3.32 ± 0.60 | |
| b* | 18.83 ± 1.98 | 21.07 ± 3.30 | 19.41 ± 2.02 | 20.70 ± 3.59 | |
| After whitening | L* | 76.80 ± 2.57 | 77.50 ± 1.81 | 73.75 ± 1.35 | 73.66 ± 0.81 |
| a* | 0.86 ± 0.72 | 0.16 ± 0.53 | 2.61 ± 0.84 | 2.65 ± 0.47 | |
| b* | 12.54 ± 1.70 | 13.01 ± 2.70 | 15.29 ± 2.45 | 17.28 ± 2.47 | |
| After six months | L* | 75.17 ± 3.02 | 74.35 ± 2.62 | 74.66 ± 1.37 | 75.48 ± 1.12 |
| a* | 1.13 ± 0.90 | 0.06 ± 0.46 | 1.52 ± 0.71 | 1.13 ± 0.54 | |
| b* | 12.98 ± 1.62 | 13.73 ± 2.57 | 14.79 ± 2.72 | 16.04 ± 2.59 |
| HP6 | CP10 | HP35 | HP40 | |
|---|---|---|---|---|
| After whitening | 10.60 ± 1.62 aA | 11.38 ± 1.69 aA | 5.92 ± 1.19 bA | 5.28 ± 1.74 bA |
| After 6 months | 9.04 ± 1.86 aB | 9.19 ± 2.52 abB | 7.20 ± 1.61 bB | 7.68 ± 1.30 abB |
| HP6 | CP10 | HP35 | HP40 | |
|---|---|---|---|---|
| Treated | 7.76 ± 1.13 aA | 6.44 ± 2.88 aA | 6.93 ± 1.27 aA | 8.72 ± 0.99 aA |
| Untreated | 6.01 ± 1.67 aA | 7.55 ± 2.29 aA | 7.62 ± 1.49 aA | 7.12 ± 2.31 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Pan, J.; Malmstrom, H.; Ren, Y. Treatment Durations and Whitening Outcomes of Different Tooth Whitening Systems. Medicina 2023, 59, 1130. https://doi.org/10.3390/medicina59061130
Zhao X, Pan J, Malmstrom H, Ren Y. Treatment Durations and Whitening Outcomes of Different Tooth Whitening Systems. Medicina. 2023; 59(6):1130. https://doi.org/10.3390/medicina59061130
Chicago/Turabian StyleZhao, Xiaoyi, Jie Pan, Hans Malmstrom, and Yanfang Ren. 2023. "Treatment Durations and Whitening Outcomes of Different Tooth Whitening Systems" Medicina 59, no. 6: 1130. https://doi.org/10.3390/medicina59061130
APA StyleZhao, X., Pan, J., Malmstrom, H., & Ren, Y. (2023). Treatment Durations and Whitening Outcomes of Different Tooth Whitening Systems. Medicina, 59(6), 1130. https://doi.org/10.3390/medicina59061130








