Subglottotracheal Adenoid Cystic Carcinoma in a 16-Year-Old Female—A Case Report
Abstract
:1. Introduction
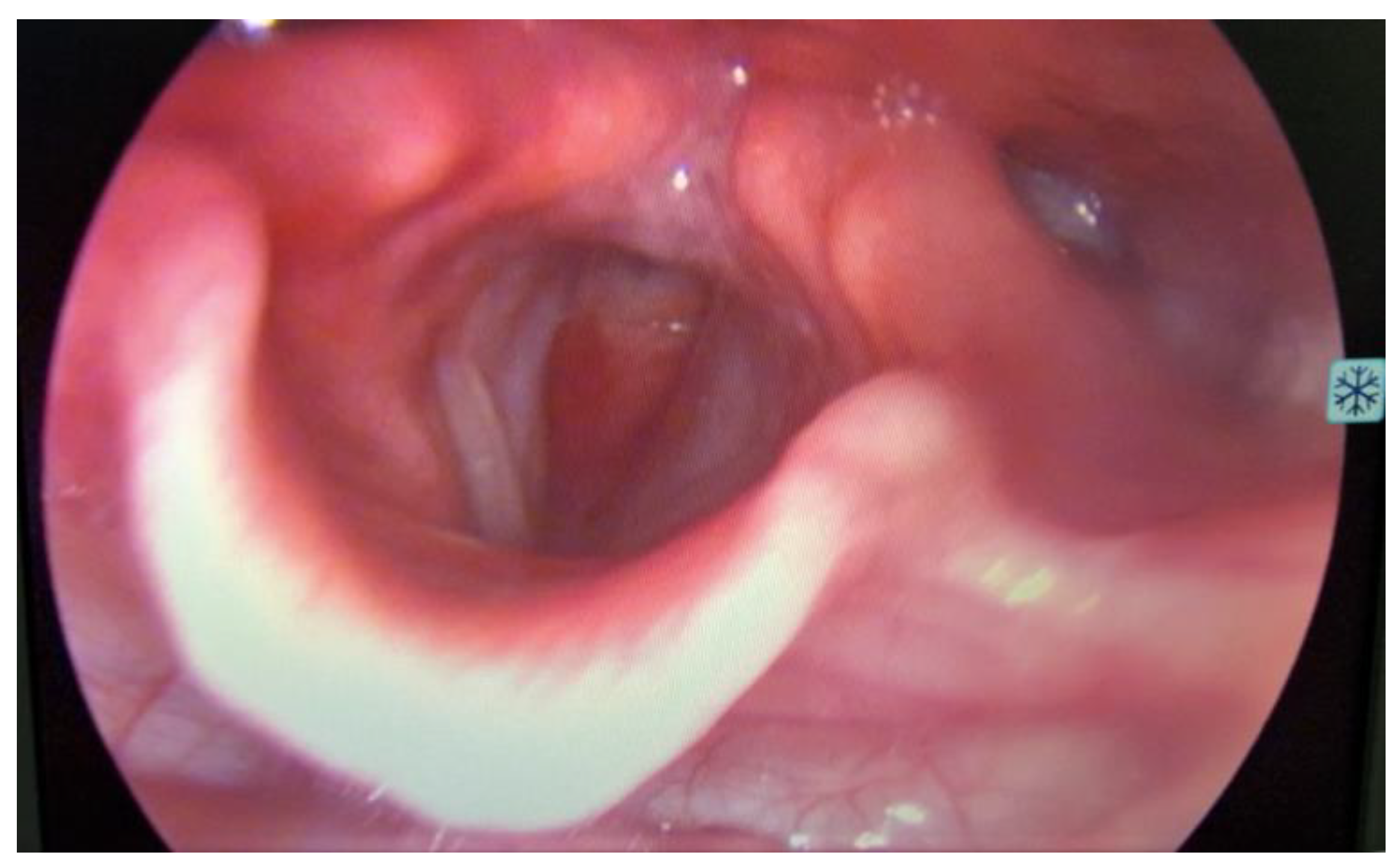
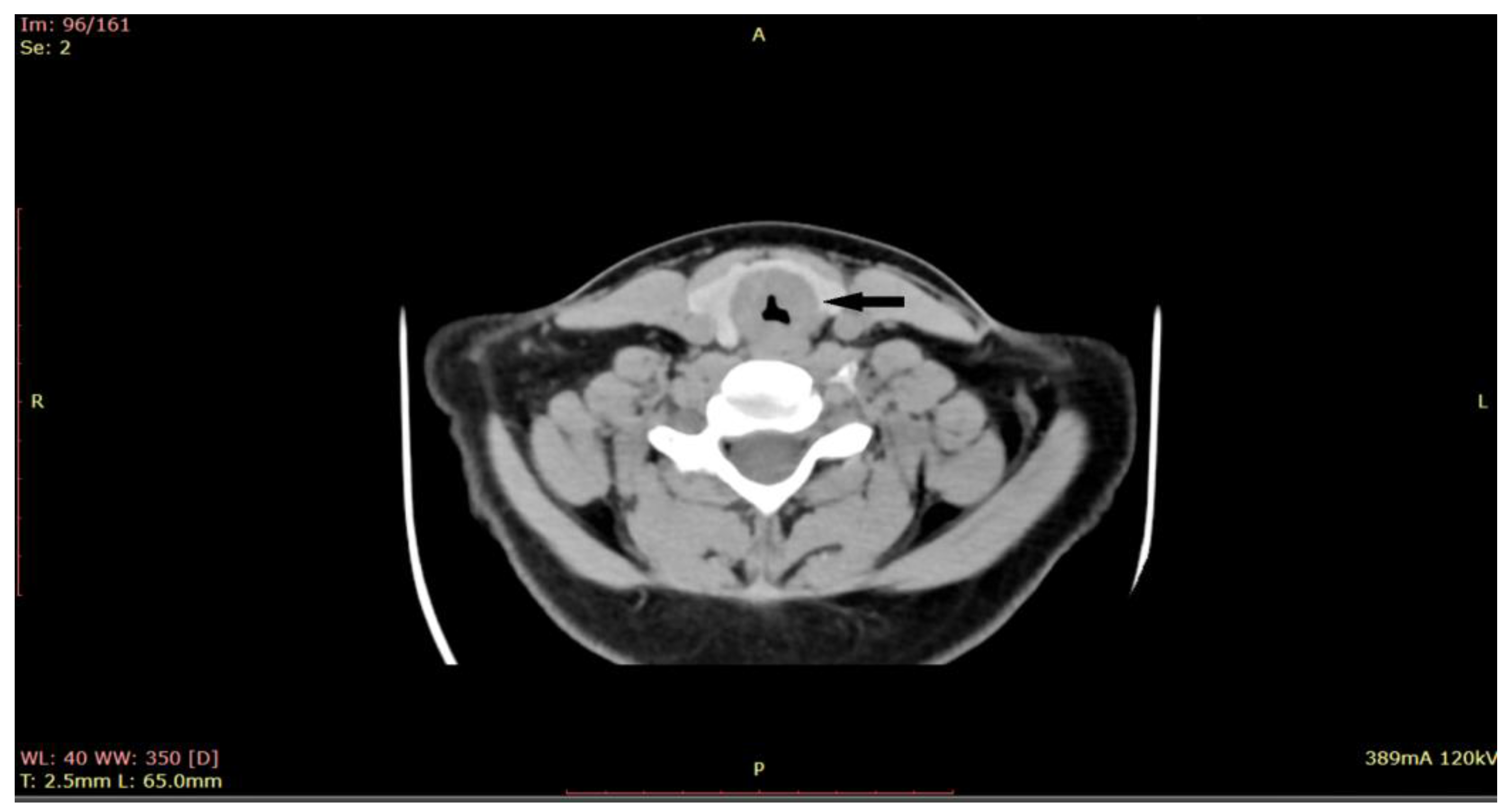
2. Materials and Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, E.J.; Barbara, B.; René, L.C.; Vivian, W.Y.L.; Julie, E.B.; Jennifer, R.G. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 1. [Google Scholar]
- Kawakita, D.; Oze, I.; Iwasaki, S.; Matsuda, T.; Matsuo, K.; Ito, H. Trends in the incidence of head and neck cancer by subsite between 1993 and 2015 in Japan. Cancer Med. 2022, 11, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Andren, Y.; Mark, J.; Horlings, H.M.; Persson, F.; Stenman, G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl. Acad. Sci. USA 2009, 106, 18740–18744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskaluk, C.A. Adenoid Cystic Carcinoma: Clinical and Molecular Features. Head Neck Pathol. 2013, 7, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Owosho, A.A.; Adesina, O.M.; Odujoko, O.; Soyele, O.O.; Komolafe, A.; Bauer, R.; Holte, K.; Summersgill, K.F. MYB-NFIB Translocation by FISH in Adenoid Cystic Carcinoma of the Head and Neck in Nigerian Patients: A Preliminary Report. Head Neck Pathol. 2021, 15, 433–437. [Google Scholar] [CrossRef]
- Bhaijee, F.; Pepper, D.J.; Pitman, K.T.; Bell, D. New developments in the molecular pathogenesis of head and neck tumors: A review of tumor-specific fusion oncogenes in mucoepidermoid carcinoma, adenoid cystic carcinoma, and NUT midline carcinoma. Ann. Diagn. Pathol. 2011, 15, 69–77. [Google Scholar] [CrossRef]
- Dewenter, I.; Otto, S.; Kakoschke, T.K.; Smolka, W.; Obermeier, K.T. Recent Advances, Systemic Therapy, and Molecular Targets in Adenoid Cystic Carcinoma of the Head and Neck. J. Clin. Med. 2023, 12, 1463. [Google Scholar] [CrossRef]
- de Morais, E.F.; da Silva, L.P.; Moreira, D.G.L.; Mafra, R.P.; Rolim, L.S.A.; Santos, E.D.M.; de Souza, L.B.; Freitas, R.D.A. Prognostic Factors and Survival in Adenoid Cystic Carcinoma of the Head and Neck: A Retrospective Clinical and Histopathological Analysis of Patients Seen at a Cancer Center. Head Neck Pathol. 2021, 15, 416–424. [Google Scholar] [CrossRef]
- Andrés, C.P.; Juan, P.R.; Patrick, J.B.; Vincent, V.P.; Asterios, T.; Jennifer, L.H.; Primoz, S.; Alessandra, R.; Missak, H.; Robert, P.T.; et al. Cystic carcinoma of the salivary glands: Emerging molecular targets. Curr. Oncol. Rep. 2018, 20, 100. [Google Scholar]
- Dillon, P.M.; Chakraborty, S.; Moskaluk, C.A.; Joshi, P.J.; Thomas, C.Y. Adenoid cystic carcinoma: A review of recent advances, molecular targets, and clinical trials. Head Neck 2016, 38, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Song, M.; Xie, J.; Huang, X.-Y.; Hu, X.-M.; Gan, R.-H.; Zhao, Y.; Lin, L.-S.; Chen, J.; Lin, X.; et al. Notch2 signaling contributes to cell growth, invasion, and migration in salivary adenoid cystic carcinoma. Mol. Cell. Biochem. 2016, 411, 135–141. [Google Scholar] [CrossRef]
- Ettl, T.; Schwarz-Furlan, S.; Haubner, F.; Müller, S.; Zenk, J.; Gosau, M.; Reichert, T.E.; Zeitler, K. The PI3K/AKT/mTOR signalling pathway is active in salivary gland cancer and implies different functions and prognoses depending on cell localisation. Oral Oncol. 2012, 48, 822–830. [Google Scholar] [CrossRef]
- Bell, D.; Hanna, E.Y. Salivary Gland Cancers: Biology and Molecular Targets for Therapy. Curr. Oncol. Rep. 2012, 14, 166–174. [Google Scholar] [CrossRef]
- Roby, B.B.; Drehner, D.; Sidman, J.D. Pediatric tracheal and endobronchial tumors: An institutional experience. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Domenico., T.; Germano., G.; Giovanni., C.; Michele, N.; D′Errico, G.; Maria, S.; Gennaro, I.; Mario, V.; Francesco, R.; Gaetano, M. Glottic-SubGlottic adenoid cystic carcinoma. A case report and review of the literature. BMC Surg. 2013, 13, S48. [Google Scholar]
- Högerle, B.A.; Lasitschka, F.; Muley, T.; Bougatf, N.; Herfarth, K.; Adeberg, S.; Eichhorn, M.; Debus, J.; Winter, H.; Rieken, S.; et al. Primary adenoid cystic carcinoma of the trachea: Clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat. Oncol. 2019, 14, 1177. [Google Scholar] [CrossRef] [Green Version]
- Zocchi, J.; Campa, M.; Bianchi, G.; Iocca, O.; Di Maio, P.; Petruzzi, G.; Moretto, S.; Campo, F.; De Virgilio, A.; Poorten, V.V.; et al. Occult Neck Metastases in Head and Neck Adenoid Cystic Carcinoma: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4924. [Google Scholar] [CrossRef]
- Moukarbel, R.V.; Goldstein, D.P.; O’Sullivan, B.; Gullane, P.J.; Brown, D.H.; Wang, L.; Irish, J.C. Adenoid cystic carcinoma of the larynx: A 40-year experience. Head Neck 2008, 30, 919–924. [Google Scholar] [CrossRef]
- Kokemueller, H.; Eckardt, A.; Brachvogel, P.; Hausamen, J.-E. Adenoid cystic carcinoma of the head and neck—A 20 years experience. Int. J. Oral Maxillofac. Surg. 2004, 33, 25–31. [Google Scholar] [CrossRef]
- Persson, M.; Andersson, M.K.; Mitani, Y.; Brandwein-Weber, M.S.; Frierson, H.F.; Moskaluk, C.; Fonseca, I.; Ferrarotto, R.; Boecker, W.; Loening, T.; et al. Rearrangements, Expression, and Clinical Significance of MYB and MYBL1 in Adenoid Cystic Carcinoma: A Multi-Institutional Study. Cancers 2022, 14, 3691. [Google Scholar] [CrossRef]
- Ko, J.; Siever, J.; Hao, D.; Simpson, R.; Lau, H. Adenoid Cystic Carcinoma of Head and Neck: Clinical Predictors of Outcome from a Canadian Centre. Curr. Oncol. 2016, 23, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, S.; Yu, J.B.; Wilson, L.D.; Decker, R.H. Determinants and Patterns of Survival in Adenoid Cystic Carcinoma of the Head and Neck, Including an Analysis of Adjuvant Radiation Therapy. Am. J. Clin. Oncol. 2011, 34, 76–81. [Google Scholar] [CrossRef]
- Coates, J.M.; Martinez, S.R.; Bold, R.J.; Chen, S.L. Adjuvant radiation therapy is associated with improved survival for adenoid cystic carcinoma of the breast. J. Surg. Oncol. 2010, 102, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.G.; Amaral, A.L.P.; Prado, L.A.F.; Kligerman, J.; Silveira, T.R.P. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer 1986, 57, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Olgun, Y.; Erdag, T.K.; Aydin, B.; Mutafoglu, K.; Ozer, E.; Ikiz, A.O.; Akman, F. Pediatric laryngeal cancer with 5-year follow up: Case report. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kato, T.; Nakamura, T.; Azami, Y.; Ono, T.; Suzuki, M.; Takada, A.; Yamaguchi, H.; Seto, I.; Nakasato, T.; et al. Proton Beam Therapy Combined with Intra-Arterial Infusion Chemotherapy for Stage IV Adenoid Cystic Carcinoma of the Base of the Tongue. Cancers 2019, 11, 1413. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dai, J.; Jiang, Y.; Ji, Z.; Jiang, P.; Sun, H.; Xu, F.; Wang, J. Long-Term Outcomes of Personalized Stereotactic Ablative Brachytherapy for Recurrent Head and Neck Adenoid Cystic Carcinoma after Surgery or External Beam Radiotherapy: A 9-Year Study. J. Pers. Med. 2021, 11, 839. [Google Scholar] [CrossRef]
- Verma, V.; Lin, L.; Simone, C.B. Proton Beam Therapy for Bronchogenic Adenoid Cystic Carcinoma: Dosimetry, Toxicities, and Outcomes. Int. J. Part. Ther. 2018, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Amit, M.; Na’Ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef]
- Ricciardiello, F.; Addeo, R.; Di Lullo, A.M.; Abate, T.; Mazzone, S.; Oliva, F.; Motta, G.; Caraglia, M.; Mesolella, M. Adenoid cystic carcinoma of the larynx in a 70-year-old patient: A case report. Oncol. Lett. 2018, 16, 2783–2788. [Google Scholar] [CrossRef] [Green Version]
- Zvrko, E.; Golubović, M. Laryngeal adenoid cystic carcinoma. Acta Otorhinolaryngol. Ital. 2009, 29, 279–282. [Google Scholar]
- Żurek, M.; Jasak, K.; Jaros, K.; Daniel, P.; Niemczyk, K.; Rzepakowska, A. Clinico-Epidemiological Analysis of Most Prevalent Parotid Gland Carcinomas in Poland over a 20-Year Period. Int. J. Environ. Res. Public Health 2022, 19, 10247. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, C.Ș.; Balica, N.C. Subglottotracheal Adenoid Cystic Carcinoma in a 16-Year-Old Female—A Case Report. Medicina 2023, 59, 1140. https://doi.org/10.3390/medicina59061140
Dumitru CȘ, Balica NC. Subglottotracheal Adenoid Cystic Carcinoma in a 16-Year-Old Female—A Case Report. Medicina. 2023; 59(6):1140. https://doi.org/10.3390/medicina59061140
Chicago/Turabian StyleDumitru, Cristina Ștefania, and Nicolae Constantin Balica. 2023. "Subglottotracheal Adenoid Cystic Carcinoma in a 16-Year-Old Female—A Case Report" Medicina 59, no. 6: 1140. https://doi.org/10.3390/medicina59061140






