Abstract
Angiotensin II-converting enzyme inhibitors (ACEIs) and selective angiotensin II receptor antagonists (ARAIIs) are widely used antihypertensive agents. Their use has generated controversy due to their possible influence on the health status of chronic patients infected with COVID-19. The objective of this work is to analyze the influence of COVID-19 on chronic hypertensive patients treated with ACEI and ARAII inhibitors. A systematic review and meta-analysis in the databases Pubmed, Pro-Quest and Scopus were carried out. The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The search equation descriptors were obtained from the Medical Subject Headings (MeSH) thesaurus. The search equation was: “Older AND hypertension AND (COVID-19 OR coronavirus) AND primary care” and its equivalent in Spanish. Nineteen articles were obtained, with n = 10,806,159 subjects. Several studies describe the COVID-19 association with ACEI or ARAII treatment in hypertension patients as a protective factor, some as a risk factor, and others without a risk association. In the case of ACEI vs. ARAII, the risk described for the former has an odds ratio (OR) of 0.55, and for ARAII, an OR of 0.59. Some authors talk about mortality associated with COVID-19 and ACEI with a half ratio (HR) of 0.97, and also associated ARAIIs with an HR of 0.98. It is recommended to maintain the use of the renin–angiotensin–aldosterone axis in the context of the COVID-19 disease.
1. Introduction
A chronic patient is defined as one who has suffered from a non-complicated disease for at least six months. These are the main causes of death and disabilities around the world. The most common chronic diseases are cardiovascular diseases, cancer, chronic obstructive pulmonary disease and diabetes [1]. In the period 2000–2019, 35 million people died, on average, per year due to chronic diseases. Half were under 70 years old and women [2]. For example, deaths from diabetes increased by 70% worldwide between 2000 and 2019, with 80% due to this cause among men [3]. However, despite the high prevalence of all these diseases, heart disease stands out for its high morbidity and mortality [4,5].
Heart disease has been the leading cause of death worldwide for 20 years. The number of deaths due to heart disease has increased since 2000 by more than 2 million people, reaching almost 9 million people in 2019. Heart disease currently accounts for 16% of all deaths among all causes [5,6]. Arterial hypertension remains the most prevalent cause of cardiovascular morbidity and mortality [7]. Several authors confirm AHT as a risk factor. In fact, they have observed in their research that reducing systolic and diastolic blood pressure in hypertensive patients by 5 mmHg reduces cardiovascular events by 10%, cerebrovascular events by 15% and mortality by 5% [8,9].
Notably the clinical data published to date indicate that the most common comorbidity of coronavirus disease 2019 (COVID-19) is hypertension (31.2%), and this is even higher (58.3%) in severe COVID-19 [10]. As guidelines have assessed, renin–angiotensin system blockers (angiotensin-converting enzyme or ACEI/angiotensin II receptor antagonists such as, ARAII) are recommended in the first step of pharmacological treatment. This is supported by the strong evidence of cardiovascular and renal protection with these drug classes [11]. Therefore, these antihypertensives have a theoretical increase in COVID-19 mortality because of their inducible effect on the expression of angiotensin-converting enzyme II (ACEII), the known functional receptor utilized by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [12].
The COVID-19 disease appeared at the end of 2019 in Wuhan (China), caused by a new coronavirus called SARS-CoV-2, generating the declaration of a sanitary alert and pandemic in the first quarter of 2020 [13]. This virus has caused 553 million cases and 6.3 million deaths worldwide by July 2022 [14]. SARS-CoV-2 is a ribonucleic acid (RNA) virus, of which the activity depends on its cellular introduction for the replication of its genetic material, which is produced using angiotensin-converting enzyme II (ACEII) as a receptor by interacting with the spike protein (S) of Coronavirus SARS-CoV-2 mediated by the transmembrane protease serine 2 (TMPRSS2) [15,16]. Some research speculated that drugs inhibiting the renin–angiotensin–aldosterone system might upregulate SARS-CoV-2 cell receptors, thereby aiding viral replication [17].
The clinical picture is variable, and it is estimated that up to 33% of patients are asymptomatic [18]. Most patients have a controlled infection that can occur with or without pneumonia, but responds well to home treatment. However, 14% present with severe dyspnea and hypoxia and/or pneumonia that affects more than 50% of the lung field in 24–48 h, with a severe course and hospital admission. Multi-organ failure mediated by a cytokine storm is developed by 5%, that requires admission to intensive care units (ICUs) [19]. Of the cases, 2.3% die from complications arising during the COVID-19 process [20]. This is a common occurrence, as several studies have revealed that individuals with heightened cardiovascular risk (e.g., high blood pressure) have a greater tendency to experience severe outcomes due to COVID-19 [21].
COVID-19 has a higher morbidity and mortality in patients with chronic diseases, including hypertensive patients, among many others [22]. There is evidence of greater severity and mortality with older age, being more accentuated in those older than 65 years [23]. All these patients are more likely to have sequels, defined as persistent COVID, where the patient has symptoms of the disease for more than two months after the end of the infection, and of which the appearance is more likely the more severe the infection has been [24]. In fact, the risk of adverse cardiovascular events in post-COVID patients is increased up to one year after infection, acting as another risk factor [25].
ACEIs/ARAIIs are the most widely used antihypertensive drugs in clinical practice. There are articles with different points of view, variably supporting their use in patients affected by COVID-19. The review of the subject proposed in this manuscript serves to check their safety in patients and evaluate if other treatment alternatives are also safe.
Therefore, the aim of this study was to analyze the relationship between SASR-COV2 and antihypertensive treatment with ACEI or ARAII in the older adults using a protocoled systematic review methodology. Different antihypertensive drugs were used to observe existing data and their possible comparison.
2. Materials and Methods
2.1. Design
This work was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. The study was registered (ID: 354488) in the PROSPERO database (International Prospective Register of Systematic Reviews).
2.2. Information Sources and Search Strategy
The following databases were consulted: Pubmed, Pro-Quest and Scopus. The MeSH terms employed in the search strategy were “Older AND hypertension AND (COVID-19 OR coronavirus) AND primary care”, and their equivalent in Spanish. The search was conducted in August 2022.
2.3. Eligibility Criteria
Inclusion criteria: quantitative primary studies analyzing the relationship between ACEI and ARAII, and the pathology of COVID respiratory syndrome in patients over 60 years of age; published in English and/or Spanish; without restriction by year of publication.
Exclusion criteria: doctoral theses, articles without statistical information, duplicate studies, those not carried out in adults, or of which the main objective was not to investigate the relationship between ACEI/ARAII drugs and their relationship with the disease developed by the SARS-CoV-2 coronavirus.
2.4. Study Selection Process
Two members of the team (M.Q.-C. and C.M.-J) performed the search and study selection independently. In case of a disagreement, a third researcher (A.C.-G.) was consulted. The selection of articles was carried out in four steps: (1) reading the title and abstract, (2) discarding those that did not meet the inclusion criteria, (3) reading the full text, and (4) a reverse search.
2.5. Data Collection and Synthesis
To extract the data from each study, a data collection notebook was created that includes the first author, year of publication, country of the study, design, sample, intervention on which the article has based its study, means with their standard deviation, main results, and level of evidence and grade of the recommendation.
2.6. Risk of Bias Assessment and Level of Evidence
Risk of bias was performed using a Cochrane risk of bias assessment for clinical trials and the elimination questions from the CASP (Critical Apprais-al Skills Program) for cohort studies. The quality of the studies included in this review was assessed following the levels of evidence and grades of recommendation stipulated by the Oxford Center for Evidence-Based Medicine (OCEBM) [27].
2.7. Data Analysis
A descriptive analysis of the information from the included studies was performed for the systematic review. A fixed effects meta-analysis using Review Manager 5.4 was performed. The effect size (odds ratio) for all-cause mortality with the continuation or discontinuation in the ACE inhibitor treatment was calculated. I2 was calculated for heterogeneity.
3. Results
3.1. Study Selection and Study Characteristics of the Studies Included
This review has 18 selected articles from 13 nationalities, including 10,802,367 patients.
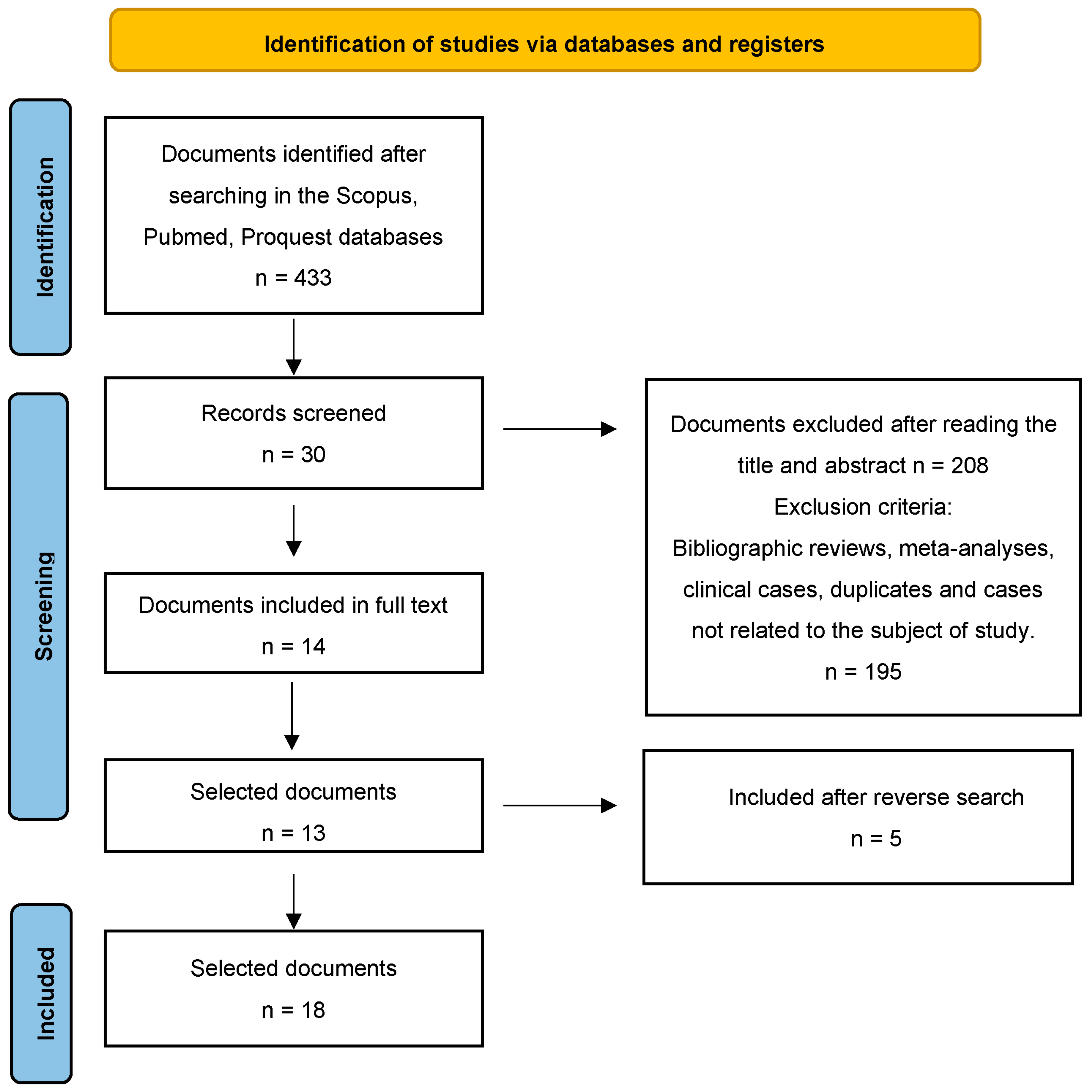
After conducting the search, 433 studies were found. After reading the title and abstract, 209 articles were selected. Of these manuscripts, 195 were discarded because they were duplicates, were not quantitative studies or were not related to the subject. Of the remaining 14 articles, one was excluded as it was not a full text. Finally, five articles were included after a reverse search. Therefore, the final sample was n = 18 definitive articles for analysis (Figure 1).
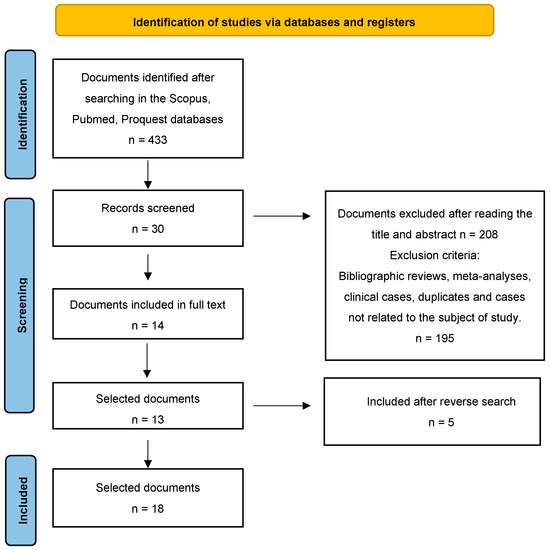
Figure 1.
Flow diagram of the publication search process [26].
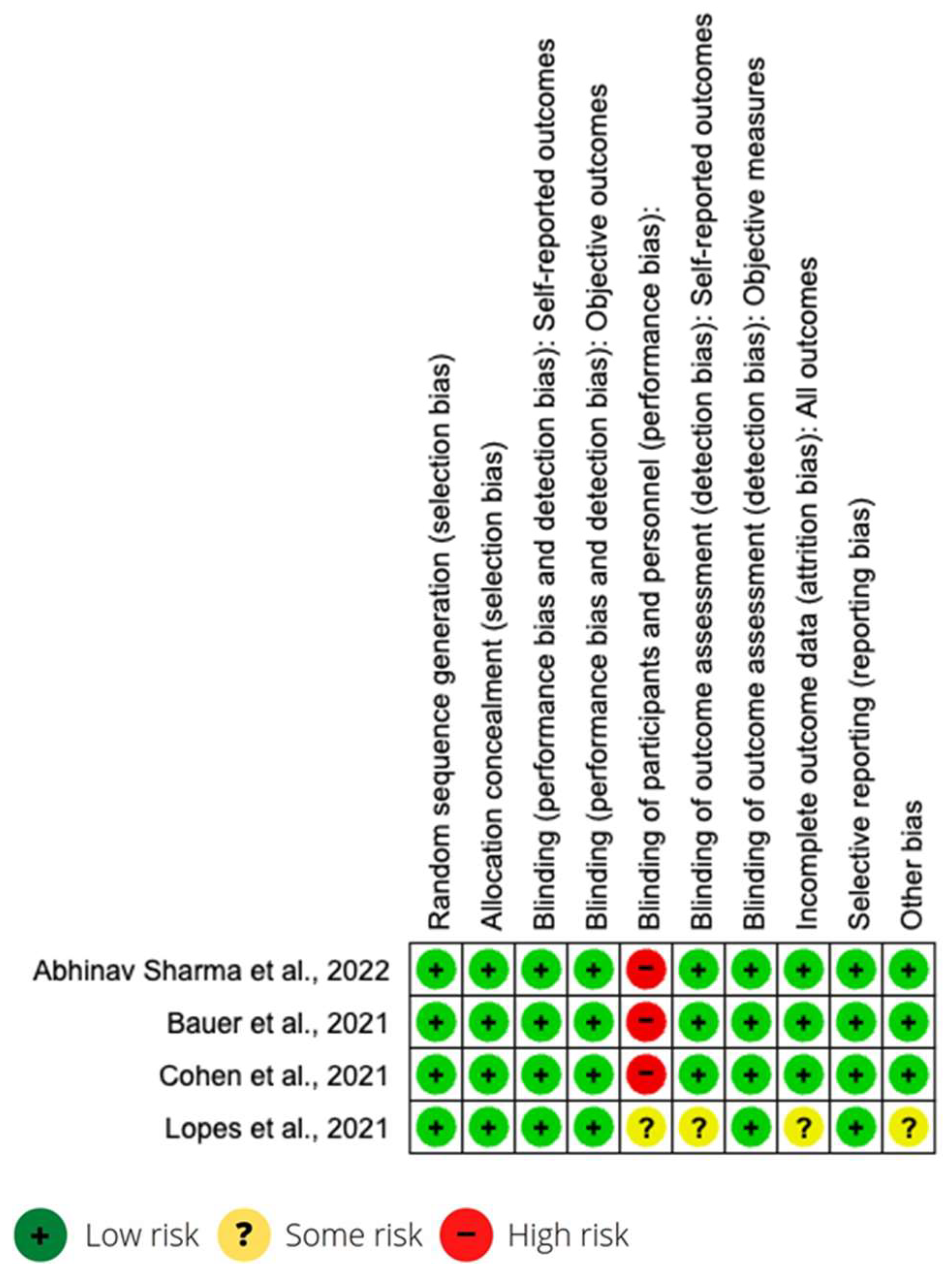
All studies were longitudinal, and the sample selection was randomized. Most of the studies were carried out in other countries (Belgium, USA, Canada, Mexico, Sweden, Peru, Bolivia, Argentina, Germany, Austria, England, Kuwait, Italy, Brazil and Canada). Only one was carried out in Spain, with a mixed Spain–USA study. The articles correspond to 14 cohort studies, 4 clinical trials and 1 case-control study. Information on the characteristics of each study is found in the first two columns of Table 1. The risk of bias in studies is presented in Figure 2 and Table 2.


Table 1.
Characteristics of each study with associations of existing antihypertensive treatment in the articles.
Table 1.
Characteristics of each study with associations of existing antihypertensive treatment in the articles.
| Author, Year, Country | Design | Sample/Age/Prevalence | Intervention | Mean ± SD | Measures | Main Results/ ICU Admission | LE/GR |
|---|---|---|---|---|---|---|---|
| Bauer et al., 2021 (Austria and Germany) [28] | Randomized controlled clinical trial (RCT) | n = 204 outpatients; Age = 75 Continuation Group (1) = 100; Discontinuation Group (2) = 104 Dyslipidaemia = 86 (42%); DMT2 = 67 (33%); Atrial Fibrilation = 35 (17%); Kidney Disease = 37 (18%); IMC = 27.5; HBP = 199 (98%) Vital Parameters Beats/mi: (1) 76–(2) 76; Respiratory/min: (1) 18–(2) 18; Temperature: (1) 37–(2) 37; Blood pressure: (1) 130/77–(2) 130/75; Oxygen Saturation: (1) 94%–(2) 95% Respiratory Therapy Oxygen substitution: (1) 45–(2) 41 Oxygen mask therapy: (1) 43–(2) 37 Non-invasive ventilation: (1) 5–(2) 0 Radiological Signs Bipulmonar infiltrates: (1) 36–(2) 38 Other opacities: (1) 29–(2) 26 C reactive protein: (1) 5.1–(2) 4.5 | Comparison ACEI/ARAII Versus not taking them regardless of whether they are hospitalized | N1 = 104 withdraws ACEI/ARA II N2 = 100 continues ACEI/ARA II Max SOFA score 0 (0–2) vs. 1 (0–3) p = 0.12 Lower AUCsofa 0 (0–9.25) vs. 3.5 (0–23.5) p = 0.040 Mean SOFA score 0 (0–31) vs. 0.12 (0–0.78) p = 0.040 30 days SOFA score 0 (0–1.20) vs. 0 (0–24) Patients with severe involvement with mechanical ventilation (10% N1 vs. 8% N2 p = 0.87) or in ICU (19% N1 vs. 18% N2 p = 0.96) At 30 days, 11% of the withdrawal group and 23% of the drug continuation group had signs of organ dysfunction or have passed away p = 0.017. | 1—Max SOFA score 2—Lower AUCSOFA 3—Mean SOFA 4—30 days SOFA score Prevalence Severity ICU Mechanical ventilation | Withdrawing ACEI/ARAII in COVID-19 patients had no effect in severely affected patients with mechanical ventilation or in ICU, but the results of this trial indicate that withdrawal of ACEI and ARAII may allow a faster and better recovery. / YES | 1a/A |
| Bean et al., 2020 (England) [29] | Cohorts | n = 1200 patients hospitalized in London Age = 63 +/− 20 SD Heart Disease = 107 (8%); DMT2 = 418 (35%); Kidney Disease = 206 (17%); Obesity = 182 (15%); Stroke = 235 (20%); Chronic Obstructive Lung Disease = 121 (10%); HBP = 645 (53%) | Comparison of ACEI/ARAII versus not taking them in hospitalized patients | N1 (ACEI/ARAII) = 399 N2 (no ACEI/ARAII) = 801 COVID-19 severity results OR 0.63 IC 0.47–0.84 (p = 0.01) | Odds Ratio Prevalence Severity | There is no evidence of an increase in the severity of COVID-19 in patients treated with ACEIs and ARBs in the study, and a possible protective factor is appreciated that must be corroborated in subsequent clinical trials. / NO | 2a/B |
| Cohen et al., 2021 (USA, Canada, Mexico, Sweden, Peru, Bolivia, Argentina) [30] | Randomized controlled clinical trial (RCT) | n = 152 hospitalized patients Age = 62 male (52%) and 68 female (48%) Hispanic 82 (54%) DMT2 79 (52%) with insuline 36 (23.7%) HBP = 152 | They compare ACEI/ARAII versus not taking them in hospitalized patients | N = 75 patients continue with ACEI/ARAII y N = 77 withdraws. Mean Rank 73 (IQR 40–100) for continuation group and 81 (IQR 38–117) for the group that withdraws. X2 for adverse events without differences between both groups p = 0.77 | Mean Rank X2 | No differences were found in the GLOBAL RANK SCORE, nor in adverse events, blood pressure, nor in serum levels of potassium or creatinine between both groups. / YES | 1a/A |
| De Spiegeleer et al., 2020 (Belgium) [31] | Cohorts | n = 154 patients with COVID-19 in home care Age = 86 +/− 7 SD COVID symtoms = 113 Asymptoms = 41 DMT2 = 28 (18%) HBP = 39 (25%) | They compare ACEI/ARAII and statins versus not taking them in non-hospitalized patients | Statins and the absence of COVID-19 symptoms (OR 2.91; CI 1.27–6.71) Statins and COVID-19 clinical improvement (OR 0.75; CI 0.24–1.87) ACEI/ARAII and COVID-19 clinical improvement (OR 0.48; CI 0.1–1.97). | Odds Ratio Prevalence | Finds significantly statistical results in taking statins and the absence of symptoms during COVID-19 infection. / NO | 2a/B |
| Elabd et al., 2021 (Kuwait) [32] | Cohorts | n = 4019 hospitalized in Kuwait City Age = 43.49 Heart Disease = 168 (4%); DMT2 = 634 (15%); Chronic Obstructive Lung Disease = 17 (0.4%); HBP = 782 (20%) | They compare ACEI/ARAII versus not taking them in hospitalized patients | N1 (ACEI/ARAII) = 325 N2 (no ACEI/ARAII) = 3694 N1 is inversely associated with ICU admission OR 0.57 CI 0.34–0.88 (p = 0.01) and inversely associated with mortality OR 0.56 IC 0.33–0.95 (p = 0.032). | Odds Ratio Prevalence Mortality | The results recommend continuing ACEI/ARAII in patients who acquire COVID-19. The protective effects of the study support this hypothesis. / YES | 2a/B |
| Fosbϕl et al., 2020 (Denmark) [33] | Cohorts retrospectives | n = 4480 patients without discriminating hospitalized or not. Age = 54.6 Heart Disease = 243 (5%); Atrial Fibrillation = 317 (7%); DMT2 = 411 (9%); Kidney Disease = 172 (4%); Stroke = 402 (9%); Chronic Obstructive Lung Disease = 634 (14%); HBP = 843 (18%) | They compare ACEI and ARAII versus not taking them regardless of being hospitalized. | N1 (ACEI/ARAII) = 895 N2 (no ACEI/ARAII) = 3585 Mortality 0.83 HR IC 0.67–1.03 p = 0.05 Death in severe COVID-19 HR 1.04 IC 0.89–1.29 p = 0.05 Incidence of COVID-19 in N1 HR 1.05 IC 0.80–1.36 p = 0.05 | Hazard Ratio Incidence Mortality Severity | ACEI/ARAII is not significantly associated with the diagnosis of COVID-19, nor with the severity of the infection nor with increased mortality. / NO | 2a/B |
| Hippisley-Cox et al., 2020 (England) [34] | Cohorts retrospectives | n = 8,275,949 Age = 48.47 PV = 19,486 (0.2%) COVID patients without discriminating hospitalization (1286 ICU of them) Heart Disease = 433,631 (5%); Atrial Fibrillation = 201,911 (2.4%); DMT2 = 536,516 (6%); Kidney Disease = 338,693 (4%); Obesity = 1,709,529 (20%); Chronic Obstructive Lung Disease = 195,115 (2%); HBP = 1,414,021 (17%) | They compare ACEI and ARAII versus not taking them regardless of being hospitalized. | N1 = ACEI = 2864 N2 = ARAII = 1417 N2 = no ACEI/ARAII = 3304 N1 vs. N3 COVID-19 risk HR 0.71 IC 0.67–0.74 p = 0.05 N2 vs. N3 COVID-19 risk HR 0.63 IC 0.59–0.67 p = 0.05 N1 vs. N3 increase ICU risk HR 0.89 IC 0.75–1.06 p = 0.05 N2 vs. N3 increase ICU risk HR 1.02 IC 0.83–1.25 p = 0.05 | Hazard Ratio Prevalence Severity | ACEI and ARAII are significantly associated with a reduced risk of COVID-19 in this study. / YES | 2a/B |
| Jeffery et al., 2022 (USA) [35] | Cohorts retrospectives | n = 1,059,474 Age = 72,623 Heart Disease = 254,773 (24%); DMT2 = 422,780 (40%); Kidney Disease = 212,362 (20%); Obesity = 116,557 (11%); Stroke = 73,361 (7%); Chronic Obstructive Lung Disease = 372,735 (35%); HBP = 1,059,474 (100%) | Reported association between ACEI/ARAII use and respiratory viral diseases without discriminating in-hospital and out-of-hospital patients. | N1 = 653,797 IECA/ARAII N2 = 405,677 No IECA/ARAII ICU risk 1.5 pp IC (1.2–1.9) (p = 0.05) Dyspnea Risk 0.7 pp (0.1–1.2 IC) (p = 0.05) AVDS Risk 0.9 pp (0.4–1.3 IC) (p = 0.05) | Percentage Point | Patients with AVRIs using ACEi/ARAII for HTN had a greater increase in poor outcomes during the COVID-19 pandemic than those using other HTN drugs. / YES | 2a/B |
| Lopes et al. 2021 (Brazil) [36] | Randomized controlled clinical trial (RCT) | n = 659 Age = 55.5 (46.1–66.1) Heart Disease = 39 (5%); DMT2 = 210 (32%); Kidney Disease = 9 (1%); Obesity = 341 (52%); HBP = 659 (100%) | Determine whether discontinuation compared with continuation of ACEI or ARAII changed the number of days alive and out of hospital within 30 days. | N1 Discontinue IECA/ARAII = 334 N2 Continue IECA/ARAII = 325 Not statistical association for Death Cardiovascular Death Evolution Days alive Day salive out of hospital p = 0.3 | Odds Ratio | In patients hospitalized with mild-to-moderate COVID-19 and who were taking ACE inhibitors or ARBs prior to hospital admission, there was no significant difference in the mean number of days alive and out of hospital for those assigned to Discontinue vs. continue these medications. / NO | 1a/A |
| Mancia et al., 2020 (Italy) [37] | Control cases | n = 37,031 Age = 68 +/− 13 SD VP = 6272 (16.93%) C = 30,759 Heart Disease = 8570 (23%); Kidney Disease = 1129 (3%); Chronic Obstructive Lung Disease = 521 (1%); Cancer = 5729 (15%) | They compare ACEI and ARAII versus not taking it and with other antihypertensive regardless of being hospitalized or not | N1 = ACEI 1508 N2 = ARAII 1394 N3 = ACC 1446 N4 = DIURETICS 1902 OR N1 COVID-19 = 0.95 (0.86–1.05) p = 0.05 OR N2 COVID-19 = 0.96 (0.87–1.07) p = 0.05 OR N1 Severity/Lethality 0.83 (0.63–1.10) p = 0.05 OR N2 0.91 (0.69–1.21) p = 0.05 | Odds Ratio Severity Mortality | There is no evidence of an association between ACEI and ARA II and the risk of COVID-19 or increased severity or lethality / NO | 2b/B |
| Mazzoni et al., 2022 (Italy) [38] | Cohorts retrospectives | n = 615 Age = 70.9 Atrial Fibrillation = 54 (9%); DMT2 = 107 (17%); Kidney Disease = 15 (2%); Obesity = 38 (6%); Chronic Obstructive Lung Disease = 25 (4%); Heart Disease = 59 (10%); ICU = 96 (15,6%); HBP = 86 (14%) | Analyzes hospitalized patients in one area of Italy and compares deaths who have taken ACEI/ARAII with Haven taken other hypertensive drug to observe a possible association. | N1 IECA/ARAII Death 86 N2 no IECA/ARAII Death 78 30 days alive 94.9% N1 91.8 N2 Mortality 2.8% N1 2.7% N2 IC (0.3–2.52) (p = 0.03) | Odds Ratio | The apparent increase in morbidity in patients with COVID-19 who received long-term treatment with ACE inhibitors or ARBs is not due to the drugs themselves, but to the conditions associated with their use. / NO | 2a/B |
| Morales et al., 2021 (Spain and USA) [39] | Cohorts | n = 1,355,349 hypertensive patients using ACE inhibitors/ARBs (363,785 monotherapy) Age = 67.20 Data from: SIDIAP 37796 (System for Research in Primary care) (1) VA-OMOP 320450 (Veterans Affair Observational Medicals outcomes) (2) CUIMC 5539 (Columbia University Irving medical Center) (3) Adjusted dates for Analysis Dyslipidemia: (1) 27%–(2) 44.7%–(3) 35.5%; DMT2: (1) 20.7%–(2) 29.5%–(3) 16.7%; Heart Disease: (1) 21.8%–(2) 13.3%–(3) 26.4%; Atrial Fibrillation: (1) 4.1%–(2) 2.7%–(3) 5%; Kidney disease: (1) 9.4%–(2) 6.7%–(3) 7.8%; Obesity: (1) 35.5%–(2) 11.9%–(3) 9.3%; Stroke/Cerebrovascular: (1) 2.2%–(2) 2.3%–(3) 5.2%; Chronic Obstructive Lung: (1) 6.2%–(2) 8.4%–(3) 2.9%; HBP: (1) 99.2%–(2) 68%–(3) 61% | They compare ACEI and ARAII versus not taking them regardless of being hospitalized. | N1 (ACEI/ARAII in monotherapy) = 363,785 N2 (ACC/HCLTZ in monotherapy) = 248,915 N3 (ACEI/ARAII in combination) = 711,799 N4 (ACC/HCLTZ in combination) = 473,076 Risk N1 vs. Risk N2 HR 0.98 IC 0.84–1.14 p < 0.05 Risk N3 vs. Risk N4 HR 1.01 IC 0.9–1.15 p < 0.05 Risk ACEI vs. Risk N2 HR 0.91 IC 0.68–1.21 p < 0.05 Risk ACEI vs. Risk N4 HR 0.95 IC 0.83–1.07 p < 0.05 Risk ACEI vs. Risk ARAII (both in combination) HR 0.88 IC 0.79–0.99 p < 0.05 Risk ACEI vs. risk ARAII (monotherapy) (HR 0.85 IC 0.69–1.05) | Relative risk | There is no significant increase in the risk of diagnosis of COVID-19 or in the results associated with ACEI/ARAII. / NO | 2a/B |
| Palazzuoli et al., 2020 (Italy) [40] | Cohorts retrospetives | n = 781 patients hospitalized for COVID-19 Age = 69 Heart Disease = 171 (21%); DMT2 = 143 (18%); Chronic Obstructive Lung Disease = 84 (11%); BP = 451 (58%); ICU care = 225 (29%) | They compare ACEI/ARAII versus not in hospital for patients over 50 years old. | N1 (ARAII) = 131 N2 (ACEI) = 171 N3 (no ACEI/ARAII) = 477 Mortality N1 OR 0.58 IC 0.35–1.07 p = 0.0796 Mortality N2 OR 0.55 IC 0.3–0.98 p = 0.0436 | Odds Ratio Prevalence Mortality | In patients over 50 years of age hospitalized for COVID-19, the use of ACEIs significantly reduces the risk of death. / NO | 2a/B |
| Peñalvo et al., 2021 (Belgium) [41] | Cohorts | n = 10,866 hospitalized patients from 119 Belgian hospitals Age = 67.82 Heart Disease (CVD) = 3984 (37%); DMT2 = 2522 (23%); Kidney Disease = 1513 (14%); Obesity = 782 (7%); Chronic obstructive Lung Disease = 1731 (16%); HBP = 4593 (42%); Cognitive issues = 1320 (12%); ICU care: (1) 425–(2) 990; Length of hospital stay: (1) 13.9–(2) 12.1 | They compare ACEI/ARAII versus not taking them in hospitalized patients | ACEI/ARAII in non-ICU patients are associated with a slight increase in recovery HR 1.07 IC 1.01–1.13 (p = 0.027) and mortality reduction HR 0.83 IC 0.75–0.93 (p = 0.001) not so in ICU patients in recovery HR 1.16 IC 0.97–1.38 (p = 0.098) nor in reduction of ICU mortality HR 0.91 IC 0.73–1.12 (p = 0.381) | Hazard Ratio Prevalence Recovery Mortality | The use of ACEI/ARAII in hospitalized patients, according to the results observed, protects the patient not admitted to the ICU, being associated with a discreet reduction in mortality. / YES | 2a/B |
| Sha et al., 2020 (USA) [42] | Cohorts retrospetives | n = 531 Afro-american patients hospitalized for COVID-19 Age = 60.01 +/− 15 Congestive Heart Disease = 79 (14%); DMT2 = 228 (42%); Kidney disease = 77 (14%); Chronic Obstructive Lung Disease = 36 (6%); BMI = 35 +/− 8.1; HBP = 425 (80%) | Comparison of ACEI/ARAII versus not taking them in hospitalized Afro-Americans | N1 (ACEI/ARAII) = 207 N2 (no ACEI/ARAII) =324 Hospital Mortality 18,4% N1 vs. 14,8% N2 (p = 0.28) Mechanical ventilation 22.2% N1 vs. 16% N2 (p = 0.07) Hospital length of stay 10 days N1 vs. 8.8 days N2 (p = 0.14) | Relative risk | The use of ACEI/ARAII in hospitalized Afro-Americans in the study carried out does not show differences with respect to withdrawing them. / YES | 2a/B |
| Sharma et al., 2022 (Canada) [43] | Randomized controlled clinical trial (RCT) | n = 46 Age = 69 Dislypidaemia = 27 (59%); Heart disease = 15 (32%); Atrial Fibrillation = 7 (15%); DMT2 = 20 (43%); Kidney Disease = 9 (20%); HBP = 46 (100%); Stroke = 3 (6%); Chronic Obstructive Lung Disease = 2 (4%) | Compares continue ACEI/ARAII treatment versus Discontinuation ACEI/ARAII. Hospital Study | N1 continue IECA/ARA II = 21 N2 discontinue IECA/ARAII = 25 Results p greater than 0.05 not statistically significant for BNP increase, heart failure and risk of adverse outcome. | Standard deviation | The continuation of RAASi in hospitalized participants with COVID-19 appears safe. It cannot be associated with the data increased risk of COVID-19 disease or morbidity and mortality to ACEi/ARAII. / YES | 1a/A |
| Trifiró et al., 2020 (Italy) [44] | Cohorts retrospectives | n = 42,926 hospitalized patients Age = 69 Heart Disease = 10,019 (23%); Atrial Fibrillation = 2899 (7%); DMT2 = 7710 (18%); Kidney Disease = 1046 (2%); Stroke = 3441 (8%); Chronic Obstructive Lung Disease = 1521 (4%); HBP = 5610 (13%); Death = 11,205 (26%) | They compare ACEI/ARAII against calcium antagonists and against no antihypertensive in hospitalized patients | N1 = ACEI 4663 N2 = ARA II 4859 N3 = ACC 2178 N4 no ATH treatment = 21,974 N5 Other antihypertensive = 4068 Death risk N1 HR 0.97 IC 0.89–1.06 p = 0.05 Death risk N2 HR 0.98 IC 0.89–1.06 p = 0.05 Compared both with ACC | Hazard Ratio Mortality | ACEI and ARA II are not associated with an increased or decreased risk of mortality compared to ACC. / YES | 2a/B |
| Zhang et al., 2020 (China) [45] | Cohorts retrospectives | n = 3611; PV = 1128 (31.23%); HBP = 525 (47%); Age = 64; HBP = 1128 (31%); Heart Disease in HBP = 131 (12%); DMT2 in HBP = 200 (18%); Stroke in HBP = 41 (3%); Chronic Obstructive Lung in HBP = 6 (0.5%) | They compare ACEI and ARAII versus not taking them regardless of being hospitalized | N1 = ACEI/ARAII = 188 N2 = no ACEI/ARAII = 940 Mortality N1 vs. N2 HR = 0.42 (0.19–0.92) p = 0.03 COVID-19 Mortality Risk N1 vs. N2 HR = 0.37 (0.15–0.89) p = 0.03. Comparison of other antihypertensive drugs Mortality N1 HR = 0.3 (0.12–0.70) p = 0.01 | Hazard Ratio Mortality | ACEI/ARAII in patients hospitalized for COVID-19 with hypertension is associated with lower mortality than all causes and from COVID-19. / NO | 2a/B |
Remark: ACEI: angiotensin-converting enzyme inhibitors; ARAII: angiotensin II receptor antagonist; ARBII: angiotensin II receptor blocker; ARDS: acute respiratory distress syndrome; BB: beta-blocker; BMI: Body Mass Index; CCB: calcium channel blocker; CID: coagulation intravascular disseminate; CVD: cardiovascular disease; DMT2: diabetes mellitus type 2; GR: grade of recommendation; HBP: high blood pressure; IQRs: interquartile ranges; ICU: intensive care unit; LE: level of evidence; RAASi: renin angiotensin aldosterone inhibitors; RCT: randomized controlled clinical trial; SD: standard deviation; SOFA: sequential organ failure assessment score.
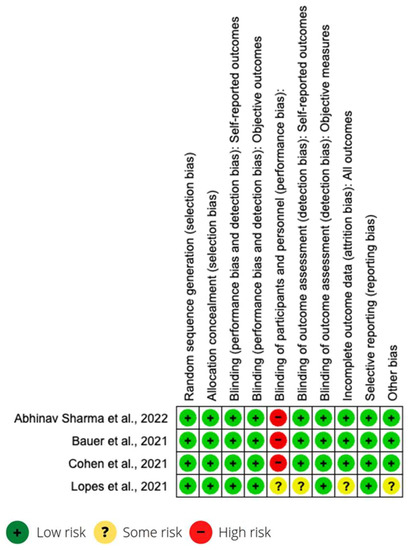
Figure 2.
Risk of bias of clinical trials [28,30,36,43].

Table 2.
Risk of bias of cohort studies using CASP critical reading guidelines.
The number of patients studied (N) is 10,802,367, of which 8,280,000 correspond to the study of Hippisley-Cox et al. [34], 1,355,349 to that of Morales et al. [39] and 1,059,474 to Jeffery MM. et al. 2022, that are the three articles that exceed one million patients [25,26,27,28,29,30,31,32,33,34,37,39,40,41,42,44,45].
The mean age of the articles ranged from 48.41 to 86 +/− 7 years. The prevalence of diabetes, hypertension and obesity as the main comorbidities in COVID-19 patients was collected. Data were also collected on renal failure, COPD, heart failure and atrial fibrillation, as well as stroke, as the diseases that most frequently alter blood pressure figures. These figures and the associations of existing antihypertensive treatment in the articles are shown in Table 1.
3.2. Risk Association to the Use of ACEI/ARAII
There are seven studies discussing protective factors, one discussing risk factors and nine without risk associations.
According to the criteria for defining the risk associated with ACEI/ARAII, the articles studied are classified into three groups. First, the papers [29,32,34,39,40,41,45] establish the drugs studied as a protective factor or decrease of severity and fatality conditions. Zhang et al. [45], however, found a decrease in mortality caused by all causes in COVID patients. Elabd et al. [32] observed decreased ICU admission and increased recovery in ACEI and ARAII patients. Pallazuoli et al. [40] show decrease of COVID-19 risk in patients with ACEI treatment. Morales et al. [39] analyzed less COVID-19 risk in ACEI patients, and Hippisley-Cox et al. [34] found less COVID-19 risks in ACEI/ARAII patients. Peñalvo et al. [41], as well as Bean et al. [29] deduced no increasing severity and a slight protective factor that requires more evaluation. The second group [30,35,36,37,43,45,46] did not establish a risk or protection association with respect to the consumption of ACEI/ARAII. Finally, conversely to the studies above, Bauer et al. [28] reported that when studying patients with severe involvement, an increase or reduction in risk cannot be observed. However, they indicate that the results point to a faster and better recovery of patients if ACEI/ARAII is withdrawn from COVID patients [31]. They also advise of a worsening outcome of COVID-19 respiratory syndrome in patients using ACEI/ARAII than in the rest of the hypertensive patients treated with other medication. Nevertheless, there are slight differences that do not allow recommending the withdrawal of treatment in these patients [33].
3.3. Risk Association to the Use of ACEI vs. ARAII
Six studies show the comparation of ACEI to ARAII.
Four of the articles addressed a statistical comparison of the results between these drugs. In Palazzuoli et al. [40], a statistical study of mortality was carried out where an association with ACEI (OR 0.55) and ARAII (OR 0.59) was established. They found a significant protective factor on the part of both. Trifiró et al. [44] study the mortality risk of COVID associated with ACEI (HR 0.97) and ARAII (HR 0.98), not observing significant data. Mancia et al. [37] postulate an association between the risk of COVID and the lethality/severity of the disease associated with the consumption of ACEI/ARAII, with an OR of 0.95 for risk associated with ACEI and 0.96 for ARAII. An OR of 0.83 was found in lethality/severity associated with ACE inhibitors and OR 0.91 in severity/lethality associated with ARAII. Hippisley-Cox et al. [34] associated the use of ACEI and ARAII with the risk of COVID and the risk of admission to the ICU: HR 0.71 (0.67–0.74) for ACEI and the risk of COVID, HR 0.63 (0.54–0.67) for ARAII and the risk of COVID, HR 0.89 (0.75–1.06) for ACEI and ICU risk, and HR 1.02 (0.83–1.25) for ARAII and ICU risk.
Morales et al. [39] observed an ACEI-associated significantly lower risk of COVID-19 in combination use (HR 0.88 (0.79–0.99)).
3.4. Risk Association to the Use of ACEI/ARAII vs. Calcium Antagonists (CA) in COVID
Four articles analyze ACEI/ARAII vs. CA in COVID.
Morales et al. [39] did not observe a torpid COVID-19 evolution or COVID-19 hospital admission increase. Pneumonic risk results with CA, ACEIs and ARAII were similar in monotherapy (HR 0.98) or in combination (HR 1.01). Trifiró et al. [44] did not appreciate an increase or decrease in mortality among patients using ACEI (HR 0.97) or ARAII (HR 0.98) compared to CA treatment (HR 1.02 + ACEI) (HR 1.05 + ARAII) or in monotherapy (HR 1.11). Mancia et al. [37] associated CA as a risk factor (OR 1.03) versus ACEI (OR 0.95) ARAs (OR 0.96) as a COVID-19 infection and mortality protection factor. In addition, they reported a combination of antihypertensive drugs with ACEI (OR 0.99) or ARAII (OR 0.98) compared to monotherapy (OR 1.03). Hippisley-Cox et al. [34] found statistical association of COVID-19 risk (HR 0.92) and the risk of developing severe COVID-19 and ICU admission (HR 1.33). ACEI decreased both risks (HR 0.71) (HR 0.89) and ARA II decreased only the COVID-19 infection risk (HR 0.63).
3.5. Risk Association to the Use of ACEI/ARAII vs. Diuretics in COVID
Three studies were conducted with ACEI/ARAII vs. diuretics in COVID.
Mancia et al. [37] described ASA diuretics as a risk COVID-19 factor (OR 1.46) and did not observe association in treatments with thiazide diuretics (OR 1.03). Hippisley-Cox et al. [34] did not find an association between potassium-sparing diuretics and COVID-19 disease (HR 1.03), and reported thiazides as a COVID-19 protecting factor (HR 0.70). Neither of the two diuretics obtained significant data associated with ICU admission (HR 0.6) (HR 0.9).
Morales et al. [39] did not observe an association between COVID-19 diagnosis and exposure to ACEI/ARAII versus thiazide diuretics monotherapy (HR 0.98 (0.84–1.14)) and combination (HR 1.01).
3.6. ACEI/ARAII Complications in COVID
The data of high mortality in ACE + ARAII are also used in COVID.
According to several authors, the combined use (ACEI + ARAII) has an excessive risk of hyperkalemia, hypotension and renal failure, and a high mortality rate compared to the use of monotherapy. These data are applicable to COVID patients [36]. Bauer et al. [28] indicated a possibly faster and more complete recovery for suspended ACEI/ARAII therapy, contrary to Peñalvo et al. who affirmed a faster recovery by continuing ACEI/ARAII [28].
3.7. Meta-Analysis of ACEI
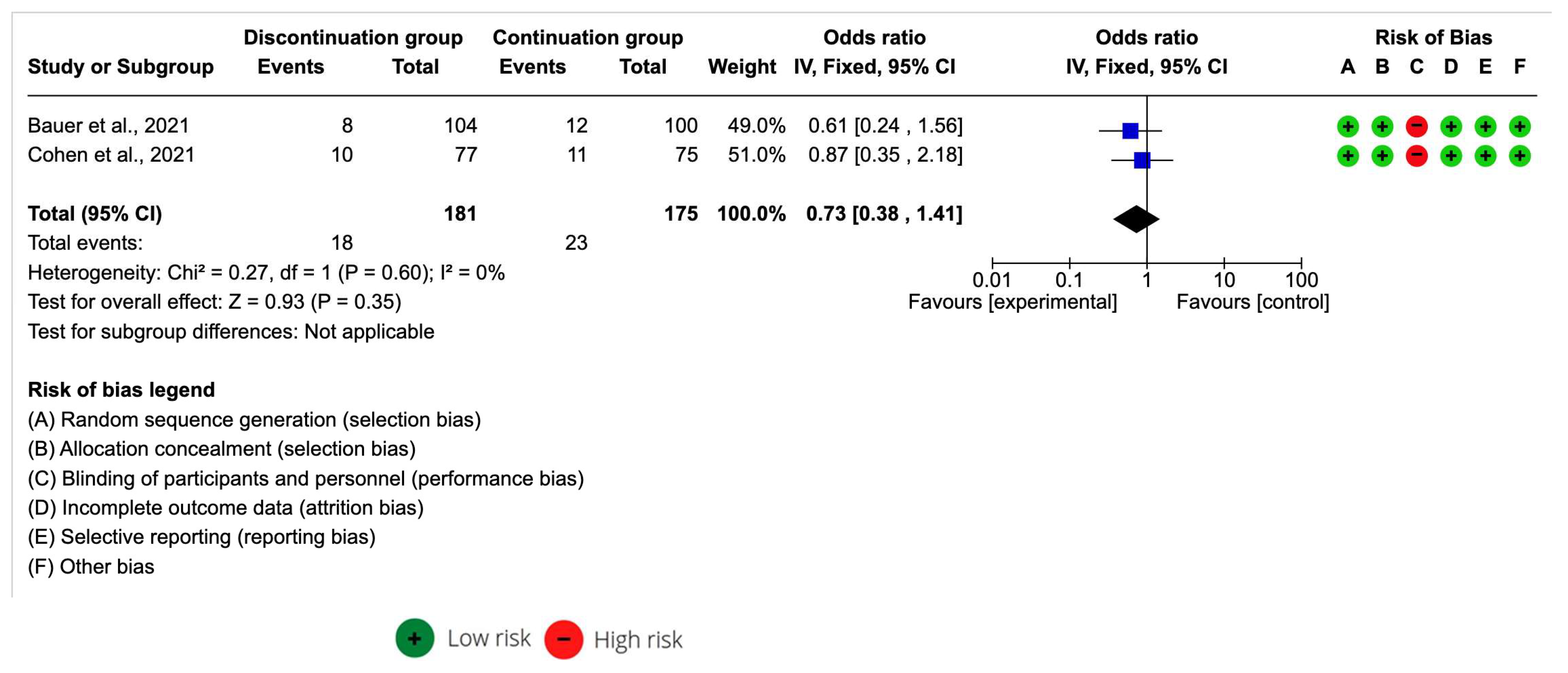
Of the studies included in the systematic review, only two included the data necessary for a meta-analysis of the effect size.
The ACEI discontinuation group sample was n = 181 and the ACEI continuation group was n = 175. The meta-analysis estimation for an all-cause mortality odds ratio was 0.73 [95% CI 0.38–1.41] with p > 0.05. I2 was 0% (Figure 3). A decrease in all-cause mortality in COVID in Bauer et al. [28] and Cohen et al. [30] was associated with discontinued ACEI.
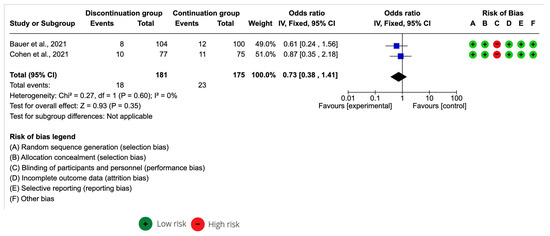
Figure 3.
Forest plot of all-cause mortality after the continuation and discontinuation of ACEI [28,30].
4. Discussion
The role of ACEI/ARAII in the framework of COVID-19 disease has been questioned since the beginning of the pandemic. The possibility that they were harmful or beneficial has been raised and is currently being evaluated for the benefit of the patient [36,46].
Angiotensinogen is discharged by the liver. Renin facilitates the conversion of angiotensinogen I into angiotensinogen II, while the angiotensin-converting enzyme (ACE) converts to angiotensinogen II, which exhibits potent vasoconstrictive effects. Angiotensinogen II stimulates the release of aldosterone, which plays a role in retaining water and sodium in the human body. SARS-CoV-2 enters cells by binding to ACE, potentially modifying its function. In fact, studies indicate that there is an elevated level of ACE in older patients with hypertension, which may increase their susceptibility to infection [47].
The data that advocate a beneficial role of ACEI and ARAII are based on the existence of lower levels of cytokines, decreasing inflammatory levels and IL6 expression in patients with severe COVID-19, and lower associated mortality data [48,49,50], as well as a possible protective factors for severe cases [51,52]. However, this has not been demonstrated in clinical practice [53]. A recent clinical trial has not been able to show improvement in lung function in patients with losartan (ARAII) [54] or provide statistically significant data that may have a clinical impact [55]. Heart congestive failure and atrial fibrillation can be an adverse evolution in hypertensive patients, but actually, in the COVID era, hypertensive patients cannot be associated ACEI/ARAII use [56,57]. Recent studies have found that pre-treatment with ACEI/ARAII could be continued, as it was associated with lower hospital mortality, ICU admission and IMV in patients with COVID-19 [58]. For this reason, this medication should not be discontinued, especially ARAIIs [59,60,61].
Initial uncertainty about the role of hypertension and its treatment led to heterogeneous management and major changes in antihypertensive treatment, mainly at the cost of discontinuation of ACE inhibitors or ARAIIs. However, there is now consistent evidence that the use of antihypertensive drugs is not associated with the risk and severity of COVID-19 [17]. Survival with withdrawal of ACEI/ARAII in patients hospitalized in the ward does not improve [43]. In critical patients with hemodynamic repercussion and low blood pressure, its withdrawal can be assessed, but in the rest of the hypertensive patients with ACEI/ARAII and COVID disease, its use continues to be considered in a hospital setting [62]. In fact, changes in antihypertensive treatment are still made in older hospitalized patients due to their comorbidities [63].
Regarding calcium antagonists, the data from our study have not been able to find an association with COVID-19. Other studies claim that any first-line antihypertensive treatment decreases had a significantly lower risk of in-hospital death [64], although it is true that the literature consulted shows a higher risk of calcium antagonists compared to ACEI/ARAII for severity, mortality and risk of intubation. Therefore, the use of ACEI/ARAII would be recommended as the first choice against calcium antagonists in COVID patients [65].
Regarding the use of diuretics in COVID-19, some studies claim that in-hospital use and initiation of diuretics during hospitalization, but not pre-hospital exposure, were associated with increased mortality in patients with COVID-19 [66]. Conversely, other studies have identified a protective association with thiazide diuretics, while ASA diuretics have been identified as a risk factor. In the analyzed literature, worse results in intubation, severity and mortality are observed in monotherapy than patients treated with ACEI/ARAII [57], and an improvement in patient data was observed when associating ACEI/ARAII with diuretics [66].
This study had some limitations. First, although all the selected studies analyzed the effect of antihypertensive drugs in patients with COVID-19, the great variability in study designs and characteristics results in heterogeneity of the results. In addition, the duration of the intervention and the different times at which the different parameters were measured can influence the results. A meta-analysis was performed, although it was not possible to include all the RCTs as there was great variability in the intervention programs. In addition, the follow-up of effects maintained over time was not analyzed. Therefore, it is necessary to conduct more RCTs with larger samples and examine the effects maintained over time.
Our results do not include some of the medium- and long-term benefits of appropriate use of antihypertensive drugs in patients who have suffered from COVID-19. The analysis of the expenses incurred per hospital stay, morbidity and mortality of these patients are interesting issues as recurrence of coronary events are sensitive problems that should be analyzed in future lines of research.
5. Conclusions
The findings suggest that the use of renin–angiotensin–aldosterone axis hypotensors should be maintained in the context of COVID-19 disease. In fact, a heterogeneous management of treatments other than ACEI and ARAII seems to worsen the prognosis in hospitalized patients.
Although there is no evidence of a clear role for the use of angiotensin blockers as antihypertensive treatment, their use does not harm patients with COVID-19. Furthermore, the use of other antihypertensive has not shown better results compared to ACEII and ARAII.
Because of this, it is advisable to follow the clinical guidelines and statements of International Scientific Societies that advise not to modify the use of ACEI/ARAII in patients with COVID-19.
Author Contributions
Conceptualization, M.Q.-C. and G.A.C.-D.l.F.; methodology, M.Q.-C., A.C.-G. and L.A.-G.; software, A.C.-G.; validation, C.M.-J., G.A.C.-D.l.F. and J.L.R.-B.; formal analysis, J.L.R.-B.; resources, S.C.-P. and C.M.-J.; data curation, M.Q.-C., C.M.-J., S.C.-P. and A.C.-G.; writing—original draft preparation, M.Q.-C. and G.A.C.-D.l.F.; writing—review and editing, J.L.R.-B. and M.Q.-C.; visualization, L.A.-G., S.C.-P. and M.Q.-C.; supervision, J.L.R.-B. and G.A.C.-D.l.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study forms part of the Doctoral Thesis of the first-named author within the Clinical Medicine and Public Health Doctoral Program from the University of Granada.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Campbell, N.R.; Burnens, M.P.; Whelton, P.K.; Angell, S.Y.; Jaffe, M.G.; Cohn, J.; Brito, A.E.; Irazola, V.; Brettler, J.W.; Roccella, E.J.; et al. 2021 World Health Organization guideline on pharmacological treatment of hypertension: Policy implications for the Region of the Americas [Directrices de la Organización Mundial de la Salud del 2021 sobre el tratamiento farmacológico de la hipertensión: Implicaciones de política para la Región de las Américas]. Rev. Pan Am. Salud Publica 2022, 46, 1. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Solomon, B.; Singleton, R.F.K.; Iurilli, M.L.C.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Lisy, K.; Campbell, J.M.; Tufanaru, C.; Moola, S.; Lockwood, C. The prevalence of disability among people with cancer, cardiovascular disease, chronic respiratory disease and/or diabetes: A systematic review. Int. J. Evid. Based Healthc. 2018, 16, 154–166. [Google Scholar] [CrossRef]
- Bueno, H.; Moura, B.; Lancellotti, P.; Bauersachs, J. The year in cardiovascular medicine 2020: Heart failure and cardiomyopathies. Eur. Heart J. 2021, 42, 657–670. [Google Scholar] [CrossRef]
- Yang, Y.S.; Pei, Y.H.; Gu, Y.Y.; Zhu, J.F.; Yu, P.; Chen, X.H. Association between short-term exposure to ambient air pollution and heart failure: An updated systematic review and meta-analysis of more than 7 million participants. Front. Public Health 2023, 10, 948765. [Google Scholar] [CrossRef]
- Gordon, J.; Miller, G.C.; Britt, H. What are chronic conditions that contribute to multimorbidity? Aust. J. Gen. Pract. 2018, 47, 21–22. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Ott, C.; Schmieder, R.E. Diagnosis and treatment of arterial hypertension 2021. Kidney Int. 2022, 101, 36–46. [Google Scholar] [CrossRef]
- Adler, A.; Agodoa, L.; Algra, A.; Asselbergs, F.W.; Beckett, N.S.; Berge, E.; Black, H.; Brouwers, F.P.J.; Brown, M.; Bulpitt, C.J.; et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: An individual participant-level data meta-analysis. Lancet 2021, 397, 1625–1636. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069, Erratum in: JAMA 2021, 325, 1113. [Google Scholar] [CrossRef]
- Kollias, A.; Foukarakis, E.; Karakousis, K.; HYPEDIA Study Group; Stergiou, G.S. Implementation of the 2018 ESC/ESH Guidelines for the management of hypertension in primary care: The HYPEDIA study. J. Hum. Hypertens. 2022; in press. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Guidance on Routine Immunization Services during COVID-19 Pandemic in the WHO European Region, 20 March 2020. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2020-1059-40805-55114 (accessed on 13 July 2022).
- Weekly Epidemiological Update on COVID-19—13 July 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---13-july-2022 (accessed on 13 July 2022).
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 1422. [Google Scholar] [CrossRef]
- Águila Gordo, D.; Martínez del Rio, J.; Piqueras Flores, J. Changes in antihypertensive treatment in surviving patients SARS-CoV-2 respiratory infection and its cardiovascular impact after one year of follow-up. Med. Clin. 2022, 158, 196–197, (In English, Spanish). [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A Systematic Review. Ann. Intern. Med. 2021, 174, 655–662. [Google Scholar] [CrossRef]
- Luis, J.; Rubio, C.; Millán, I.A.; Moreno Higueras, M.; Mu, L.M.; Medina, M.; López López, M.; Ceballos Torres, A. Revista Española de Geriatría y Gerontología. Tratamiento y evolución del síndrome de tormenta de citoquinas asociados a infección por SARS-CoV-2 en pacientes octogenarios. Rev. Esp. Geriatr. Gerontol. 2020, 55, 286–288. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Davidson, J.A.; Strongman, H.; Herrett, E.; Smeeth, L.; Breuer, J.; Banerjee, A. Severe COVID-19 outcomes by cardiovascular risk profile in England in 2020: A population-based cohort study. Lancet Reg. Health Eur. 2023, 27, 100604. [Google Scholar] [CrossRef]
- Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 14 July 2022).
- COVID-19 Forecasting Team. Variation in the COVID-19 infection-fatality ratio by age, time, and geography during the pre-vaccine era: A systematic analysis. Lancet 2022, 399, 1469–1488. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 30 October 2021).
- Bauer, A.; Schreinlechner, M.; Sappler, N.; Dolejsi, T.; Tilg, H.; Aulinger, B.A.; Weiss, G.; Bellmann-Weiler, R.; Adolf, C.; Wolf, D.; et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): A prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir. Med. 2021, 9, 863–872. [Google Scholar] [CrossRef]
- Bean, D.M.; Kraljevic, Z.; Searle, T.; Bendayan, R.; Kevin, O.; Pickles, A.; Folarin, A.; Roguski, L.; Noor, K.; Shek, A.; et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur. J. Heart Fail. 2020, 22, 967–974. [Google Scholar] [CrossRef]
- Cohen, J.B.; Hanff, T.C.; William, P.; Sweitzer, N.; Rosado-Santander, N.R.; Medina, C.; Andrade-Villanueva, J.F.; Barbagelata, A.; Cristodulo-Cortez, R.; Díaz Cucho, O.; et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: A prospective, randomised, open-label trial. Lancet Respir. Med. 2021, 9, 275–284. [Google Scholar] [CrossRef]
- De Spiegeleer, A.; Bronselaer, A.; Teo, J.T.; Byttebier, G.; De Tré, G.; Belmans, L.; Dobson, R.; Wynendaele, E.; Van De Wiele, C.; Vandaele, F.; et al. The Effects of ARBs, ACEis, and Statins on Clinical Outcomes of COVID-19 Infection Among Nursing Home Residents. J. Am. Med. Dir. Assoc. 2020, 21, 909–914.e2. [Google Scholar] [CrossRef]
- ElAbd, R.; AlTarrah, D.; AlYouha, S.; Bastaki, H.; Almazeedi, S.; Al-Haddad, M.; Jamal, M.; AlSabah, S. Angiotensin-Converting Enzyme (ACE) Inhibitors and Angiotensin Receptor Blockers (ARB) Are Protective against ICU Admission and Mortality for Patients with COVID-19 Disease. Front. Med. 2021, 8, 600385. [Google Scholar] [CrossRef]
- Fosbϕl, E.L.; Butt, J.H.; Østergaard, L.; Andersson, C.; Selmer, C.; Kragholm, K.; Schou, M.; Phelps, M.; Gislason, G.H.; Gerds, T.A.; et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use with COVID-19 Diagnosis and Mortality. JAMA 2020, 324, 168–177. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Young, D.; Coupland, C.; Channon, K.M.; Tan, P.S.; Harrison, D.A.; Rowan, K.; Aveyard, P.; Pavord, I.D.; Watkinson, P.J. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people Special populations. Heart 2020, 106, 1503–1511. [Google Scholar] [CrossRef]
- Jeffery, M.M.; Oliveira, J.; Silva, L.; Bellolio, F.; Garovic, V.D.; Dempsey, T.M.; Limper, A.; Cummins, N.W. Association of outpatient use of renin–angiotensin–aldosterone system blockers on outcomes of acute respiratory illness during the COVID-19 pandemic: A cohort study. BMJ Open 2022, 12, e060305. [Google Scholar] [CrossRef]
- Lopes, R.D.; Macedo, A.V.S.; De Barros, E.; Silva, P.G.M.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; D’Andréa Saba Arruda, G.; De Albuquerque, D.C.; et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254–264. [Google Scholar] [CrossRef]
- Mancia, G.; Rea, F.; Ludergnani, M.; Apolone, G.; Corrao, G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2431–2440. [Google Scholar] [CrossRef]
- Mazzoni, T.; Maraia, Z.; Ruggeri, B.; Polidori, C.; Micioni di Bonaventura, M.V.; Armillei, L.; Pomilio, I.; Mazzoni, I. Sartans and ACE Inhibitors: Mortality in Patients Hospitalized with COVID-19. Retrospective Study in Patients on Long-Term Treatment Who Died in the Italian Hospitals of Area Vasta n.5—Marche Region. J. Clin. Med. 2022, 11, 2580. [Google Scholar] [CrossRef]
- Morales, D.R.; Conover, M.M.; You, S.C.; Pratt, N.; Kostka, K.; Duarte-Salles, T.; Fernández-Bertolín, S.; Aragón, M.; DuVall, S.L.; Lynch, K.; et al. Renin–angiotensin system blockers and susceptibility to COVID-19: An international, open science, cohort analysis. Lancet Digit. Health 2021, 3, e98–e114. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Mancone, M.; De Ferrari, G.M.; Forleo, G.; Secco, G.G.; Ruocco, G.M.; D’Ascenzo, F.; Monticone, S.; Paggi, A.; Vicenzi, M.; et al. Antecedent Administration of Angiotensin-Converting Enzyme Inhibitors or Angiotensin II Receptor Antagonists and Survival After Hospitalization for COVID-19 Syndrome. J. Am. Heart Assoc. 2020, 9, e017364. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Genbrugge, E.; Mertens, E.; Sagastume, D.; Van der Sande, M.A.B.; Widdowson, M.A.; Van Beckhoven, D.; Belgian Collaborative Group on COVID-19 Hospital Surveillance. Insights into the association of ACEIs/ARBs use and COVID-19 prognosis: A multistate modelling study of nationwide hospital surveillance data from Belgium. BMJ Open 2021, 11, e053393. [Google Scholar] [CrossRef]
- Shah, P.; Owens, J.; Franklin, J.; Jani, Y.; Kumar, A.; Doshi, R. Baseline use of angiotensin-converting enzyme inhibitor/AT1blocker and outcomes in hospitalized coronavirus disease 2019 African-American patients. J. Hypertens. 2020, 38, 2537–2541. [Google Scholar] [CrossRef]
- Sharma, A.; Elharram, M.; Afilalo, J.; Flannery, A.; Afilalo, M.; Tselios, C.; Ni, J.; Ezekowitz, J.A.; Cheng, M.P.; Ambrosy, A.P.; et al. A randomized controlled trial of renin-angiotensin-aldosterone system inhibitor management in patients admitted in hospital with COVID-19. Am. Heart J. 2022, 247, 76–89. [Google Scholar] [CrossRef]
- Trifirò, G.; Massari, M.; Da Cas, R.; Menniti Ippolito, F.; Sultana, J.; Crisafulli, S.; Giorgi Rossi, P.; Marino, M.; Zorzi, M.; Bovo, E.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Death in Patients Hospitalised with COVID-19: A Retrospective Italian Cohort Study of 43,000 Patients. Drug Saf. 2020, 43, 1297–1308. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.M.; Zhao, Y.C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality among Patients with Hypertension Hospitalized with COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Makani, H.; Bangalore, S.; Desouza, K.A.; Shah, A.; Messerli, F.H. Efficacy and safety of dual blockade of the renin-angiotensin system: Meta-analysis of randomised trials. BMJ 2013, 346, f360. [Google Scholar] [CrossRef]
- Reindl-Schwaighofer, R.; Hödlmoser, S.; Domenig, O.; Krenn, K.; Eskandary, F.; Krenn, S.; Schörgenhofer, C.; Rumpf, B.; Karolyi, M.; Traugott, M.T.; et al. The systemic renin-angiotensin system in COVID-19. Sci. Rep. 2022, 12, 20117. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Yang, B.; Li, R.; Luo, J.; Huang, J.; Long, Y.; Huang, Y.; Zhou, J.; Zha, Y.; et al. Clinical characteristics of 134 convalescent patients with COVID-19 in Guizhou, China. Respir. Res. 2020, 21, 314. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, G.; Zhang, J.; He, X.; Ou, M.; Bi, J.; Yang, R.; Di, W.; Wang, Z.; Li, Z.; et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020, 9, 757–760. [Google Scholar] [CrossRef]
- Golpe, R.; Pérez-de-Llano, L.A.; Dacal, D.; Guerrero-Sande, H.; Pombo-Vide, B.; Ventura-Valcárcel, P. Risk of severe COVID-19 in hypertensive patients treated with renin-angiotensin-aldosterone system inhibitors. Med. Clin. 2020, 155, 488–490. [Google Scholar] [CrossRef]
- De Abajo, F.J.; Rodríguez-Martín, S.; Lerma, V.; Mejía-Abril, G.; Aguilar, M.; García-Luque, A.; Laredo, L.; Laosa, O.; Centeno-Soto, G.A.; Ángeles Gálvez, M.; et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: A case-population study. Lancet 2020, 395, 1705–1714. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Yoshihara, F.; Ohtsu, H.; Nakai, M.; Tsuzuki, S.; Hayakawa, K.; Terada, M.; Matsunaga, N.; Yasuda, S.; Ogawa, H.; Ohmagari, N. Renin-angiotensin system blocker and the COVID-19 aggravation in patients with hypertension, diabetes, renal failure, Cerebro-cardiovascular disease, or pulmonary disease: Report by the COVID-19 Registry Japan. J. Cardiol. 2022, 80, 292–297. [Google Scholar] [CrossRef]
- Puskarich, M.A.; Ingraham, N.E.; Merck, L.H.; Driver, B.E.; Wacker, D.A.; Black, L.P.; Jones, A.E.; Fletcher, C.V.; South, A.M.; Murray, T.A.; et al. Efficacy of Losartan in Hospitalized Patients With COVID-19-Induced Lung Injury: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e222735. [Google Scholar] [CrossRef]
- Loader, J.; Taylor, F.C.; Lampa, E.; Sundström, J. Renin-Angiotensin Aldosterone System Inhibitors and COVID-19: A Systematic Review and Meta-Analysis Revealing Critical Bias Across a Body of Observational Research. J. Am. Heart Assoc. 2022, 11, 25289. [Google Scholar] [CrossRef]
- Kumar, S.; Nikravesh, M.; Chukwuemeka, U.; Randazzo, M.; Flores, P.; Choday, P.; Raja, A.; Aseri, M.; Shivang, S.; Chaudhuri, S.; et al. Safety of ACEi and ARB in COVID-19 management: A retrospective analysis. Clin. Cardiol. 2022, 45, 759–766. [Google Scholar] [CrossRef]
- Job, R.; Abdul Qader, M.; Torres, P.; Al Abbasi, B.; Dewaswala, N.; Abdallah, A.; Chen, K.; Pino, J.E.; Chait, R.D. Renin-Angiotensin System Blocker in COVID-19. A Single Center Study. J. Cardiovasc. Pharmacol. 2022, 79, 311–314. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, W.; Zhu, C.J.; Ding, Z.H.; Dong, B.B.; Sun, B.Q.; Chen, R.C. Effect of continuing the use of renin-angiotensin system inhibitors on mortality in patients hospitalized for coronavirus disease 2019: A systematic review, meta-analysis, and meta-regression analysis. BMC Infect. Dis. 2023, 23, 53. [Google Scholar] [CrossRef]
- Yin, J.; Wang, C.; Song, X.; Li, X.; Miao, M. Effects of Renin-Angiotensin System Inhibitors on Mortality and Disease Severity of COVID-19 Patients: A Meta-analysis of Randomized Controlled Trials. Am. J. Hypertens. 2022, 35, 462–469. [Google Scholar] [CrossRef]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef]
- Al-Makki, A.; DiPette, D.; Whelton, P.K.; Murad, M.H.; Mustafa, R.A.; Acharya, S.; Beheiry, H.M.; Champagne, B.; Connell, K.; Cooney, M.T.; et al. Hypertension Pharmacological Treatment in Adults: A World Health Organization Guideline Executive Summary. Hypertension 2022, 79, 293–301. [Google Scholar] [CrossRef]
- Chan, C.K.; Huang, Y.S.; Liao, H.W.; Tsai, I.J.; Sun, C.Y.; Pan, H.C.; Chueh, J.S.; Wang, J.T.; Wu, V.C.; Chu, T.S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risks of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Systematic Review and Meta-Analysis. Hypertension 2020, 76, 1563–1571. [Google Scholar] [CrossRef]
- Xiong, S.; Liu, L.; Lin, F.; Shi, J.; Han, L.; Liu, H.; He, L.; Jiang, Q.; Wang, Z.; Fu, W.; et al. Clinical characteristics of 116 hospitalized patients with COVID-19 in Wuhan, China: A single-centered, retrospective, observational study. BMC Infect. Dis. 2020, 20, 787. [Google Scholar] [CrossRef]
- Semenzato, L.; Botton, J.; Drouin, J.; Baricault, B.; Vabre, C.; Cuenot, F.; Penso, L.; Herlemont, P.; Sbidian, E.; Weill, A.; et al. Antihypertensive Drugs and COVID-19 Risk: A Cohort Study of 2 Million Hypertensive Patients. Hypertension 2021, 77, 833–842. [Google Scholar] [CrossRef]
- Wojciechowska, W.; Terleck, M.; Klocek, M.; Pac, A.; Olszanecka, A.; Stolarz-Skrzypek, K.; Jastrzębski, M.; Jankowski, P.; Ostrowska, A.; Drożdż, T.; et al. Impact of Arterial Hypertension and Use of Antihypertensive Pharmacotherapy on Mortality in Patients Hospitalized due to COVID-19: The CRACoV-HHS Study. Hypertension 2022, 79, 2601–2610. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Tecson, K.M.; Vicenzi, M.; D’Ascenzo, F.; De Ferrari, G.M.; Monticone, S.; Secco, G.G.; Tavazzi, G.; Forleo, G.; Severino, P.; et al. Usefulness of Combined Renin-Angiotensin System Inhibitors and Diuretic Treatment In Patients Hospitalized with COVID-19. Am. J. Cardiol. 2022, 167, 133–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).