Antibiotic Susceptibility Surveillance in the Punjab Province of Pakistan: Findings and Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Processing of the Samples and Susceptibility Testing
2.4. Statistical Analysis
2.5. Ethical Consideration and Patient’s Consent
3. Results
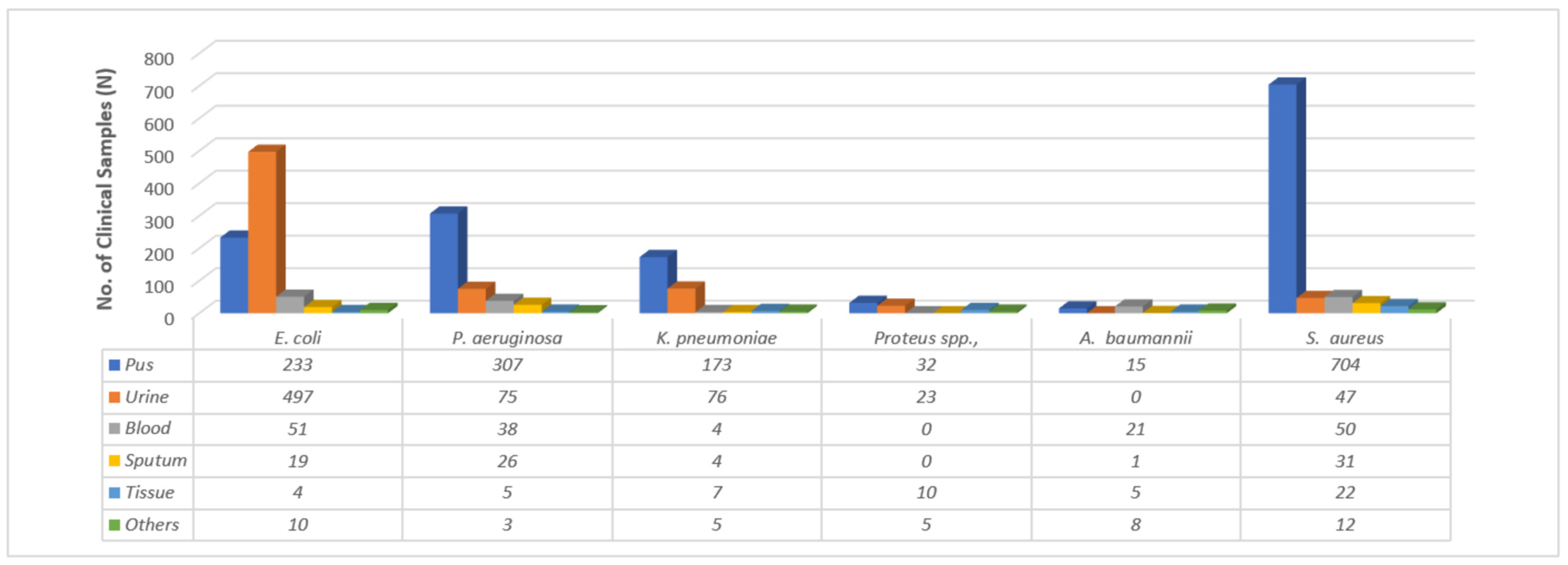
3.1. Identification of Clinical Pathogens
3.2. Antimicrobial Resistance
3.3. Antimicrobial Susceptibility Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Algammal, A.; Hetta, H.F.; Mabrok, M.; Behzadi, P. Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front. Microbiol. 2023, 14, 1135614. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A. Travellers take heed: Outbreak of extensively drug resistant (XDR) typhoid fever in Pakistan and a warning from the US CDC. Travel Med. Infect. Dis. 2019, 27, 127. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Cassini, A.; Diaz Högberg, L.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.; Colomb-Cotinat, M.; Kretzschmar, M.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modeling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, D.K.; Farooqi, J.; Shakoor, S.; Hasan, R. Antimicrobial resistance among GLASS priority pathogens from Pakistan: 2006–2018. BMC Infect. Dis. 2021, 21, 1231. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [Green Version]
- Will, C.M. The problem and the productivity of ignorance: Public health campaigns on antibiotic stewardship. Sociol. Rev. 2020, 68, 55–76. [Google Scholar] [CrossRef]
- Gautam, A. Antimicrobial Resistance: The Next Probable Pandemic. JNMA 2022, 60, 225–228. [Google Scholar] [CrossRef]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Dong, K.; Zhang, Y.; Liu, C.; Chang, Y.-F.; et al. Global antimicrobial resistance: A system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty 2022, 11, 92. [Google Scholar] [CrossRef]
- Almangour, T.A.; Ghonem, L.; Aljabri, A.; Alruwaili, A.; Al Musawa, M.; Damfu, N.; Almalki, M.S.; Alattas, M.; Abed, H.; Naeem, D.; et al. Ceftazidime-avibactam versus colistin for the treatment of infections due to carbapenem-resistant Enterobacterales: A multicenter cohort study. Infect. Drug Resist. 2022, 15, 211. [Google Scholar] [CrossRef]
- Almangour, T.A.; Alenazi, B.; Ghonem, L.; Alhifany, A.A.; Aldakheel, B.A.; Alruwaili, A. Inhaled colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria: A real-life experience in tertiary care hospitals in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1009–1013. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Sartelli, M.; Hardcastle, T.C.; Catena, F.; Chichom-Mefire, A.; Coccolini, F.; Dhingra, S.; Haque, M.; Hodonou, A.; Iskandar, K.; Labricciosa, F.M.; et al. Antibiotic Use in Low and Middle-Income Countries and the Challenges of Antimicrobial Resistance in Surgery. Antibiotics 2020, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.; Alotaibi, F.; Ahmed, H.; Alharbi, F.; Bukhari, O.; Youssef, A.-R. Effect of medical education on the knowledge, attitude and compliance regarding infection control measures among dental students in Makkah. J. Umm Al-Qura Univ. Med. Sci. 2021, 7, 14–17. [Google Scholar] [CrossRef]
- Almeleebia, T.M.; Alhifany, A.A.; Almutairi, F.; Alshibani, M.; Alhossan, A.M. Regulating antimicrobial sales in Saudi Arabia: Achievements and challenges. Int. J. Clin. Pract. 2021, 75, e13833. [Google Scholar] [CrossRef]
- Khonsari, M.S.; Behzadi, P.; Foroohi, F. The prevalence of type 3 fimbriae in Uropathogenic Escherichia coli isolated from clinical urine samples. Meta Gene 2021, 28, 100881. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Hashmi, F.K.; Saleem, F. Antimicrobial prescribing and determinants of antimicrobial resistance: A qualitative study among physicians in Pakistan. Int. J. Clin. Pharm. 2019, 41, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-resistant Klebsiella pneumoniae: Virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef]
- Njoga, E.O.; Ogugua, A.J.; Nwankwo, I.O.; Awoyomi, O.J.; Okoli, C.E.; Buba, D.M.; Oyeleye, F.A.; Ajibo, F.E.; Azor, N.; Ogunniran, T.M.; et al. Antimicrobial drug usage pattern in poultry farms in Nigeria: Implications for food safety, public health and poultry disease management. Vet. Ital. 2021, 57, 5–12. [Google Scholar] [PubMed]
- Gajdács, M.; Paulik, E.; Szabó, A. Knowledge, attitude and practice of community pharmacists regarding antibiotic use and infectious diseases: A cross-sectional survey in Hungary (KAPPhA-HU). Antibiotics 2020, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life 2021, 11, 528. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsin, M.; Van Boeckel, T.P.; Saleemi, M.K.; Umair, M.; Naseem, M.N.; He, C.; Khan, A.; Laxminarayan, R. Excessive use of medically important antimicrobials in food animals in Pakistan: A five-year surveillance survey. Glob. Health Action 2019, 12 (Suppl. S1), 1697541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, M.A.; Karami, N.A.; Al-Muwalid, A.S.; Al-Otabi, A.; Al-Subahi, E.; Bamomen, A.; Mohamed, M.M.A.; Elrggal, M.E. Community pharmacists’ knowledge, attitude, and practices towards dispensing antibiotics without prescription (DAwP): A cross-sectional survey in Makkah Province, Saudi Arabia. Int. J. Infect. Dis. 2016, 47, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elton, L.; Thomason, M.J.; Tembo, J.; Velavan, T.P.; Pallerla, S.R.; Arruda, L.B.; Vairo, F.; Montaldo, C.; Ntoumi, F.; Abdel Hamid, M.M.; et al. Antimicrobial resistance preparedness in sub-Saharan African countries. Antimicrob. Resist. Infect. Control 2020, 9, 145. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 27 March 2023).
- Inoue, H. Strategic approach for combating antimicrobial resistance (AMR). Glob. Health Med. 2019, 1, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Harant, A. Assessing transparency and accountability of national action plans on antimicrobial resistance in 15 African countries. Antimicrob. Resist. Infect. Control 2022, 11, 15. [Google Scholar] [CrossRef]
- WHO Implementation Handbook for National Action Plans on Antimicrobial Resistance: Guidance for the Human Health Sector. 2022. Available online: https://www.who.int/publications/i/item/9789240041981 (accessed on 27 March 2023).
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Ministry of National Health Services Regulations & Coordination Government of Pakistan. National AMR Action Plan for Pakistan. 2017. Available online: https://www.nih.org.pk/wp-content/uploads/2018/08/AMR-National-Action-Plan-Pakistan.pdf (accessed on 29 March 2023).
- Mendelson, M.; Matsoso, M.P. The World Health Organization global action plan for antimicrobial resistance. SAMJ S. Afr. Med. J. 2015, 105, 325. [Google Scholar] [CrossRef] [Green Version]
- Munkholm, L.; Rubin, O. The global governance of antimicrobial resistance: A cross-country study of alignment between the global action plan and national action plans. Glob. Health 2020, 16, 1–11. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Yakob, L. Filling the gaps in global antimicrobial resistance research/surveillance. BMC Infect. Dis. 2020, 20, 39. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2021. Available online: https://www.who.int/publications/i/item/9789240027336 (accessed on 21 March 2023).
- Tornimbene, B.; Eremin, S.; Abednego, R.; Abualas, E.O.; Boutiba, I.; Egwuenu, A.; Fuller, W.; Gahimbare, L.; Githii, S.; Kasambara, W.; et al. Global Antimicrobial Resistance and Use Surveillance System on the African continent: Early implementation 2017–2019. Afr. J. Lab. Med. 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.C.; Gordon, N.C.; Islam, J.; Peacock, S.J.; Scott, J.A.G. AMR Surveillance in low and middle-income settings—A roadmap for participation in the Global Antimicrobial Surveillance System (GLASS). Wellcome Open Res. 2017, 2, 92. [Google Scholar] [CrossRef]
- Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017–2018; WHO: Geneva, Switzerland, 2018.
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M.; et al. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti-Infect. Ther. 2022, 20, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.C.; Hutchison, C.; Fernandes, S.; Stoesser, N.; Kelly, H.; Lowe, B.; Turner, P.; Hanson, K.; Chandler, C.I.R.; Goodman, C.; et al. Supporting surveillance capacity for antimicrobial resistance: Laboratory capacity strengthening for drug resistant infections in low and middle income countries. Wellcome Open Res. 2017, 2, 91. [Google Scholar] [CrossRef] [Green Version]
- Saleem, Z.; Hassali, M.A.; Hashmi, F.K. Pakistan’s national action plan for antimicrobial resistance: Translating ideas into reality. Lancet Infect. Dis. 2018, 18, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Khan, M.N.; Rehman, T.; Hameed, M.F.; Yang, X. Antibiotic resistance in Pakistan: A systematic review of past decade. BMC Infect. Dis. 2021, 21, 244. [Google Scholar] [CrossRef]
- Jabeen, F.; Khan, Z.; Sohail, M.; Tahir, A.; Tipu, I.; Murtaza Saleem, H.G. Antibiotic Resistance Pattern Of Acinetobacter Baumannii Isolated From Bacteremia Patients In Pakistan. J. Ayub Med. Coll. Abbottabad 2022, 34, 95–100. [Google Scholar]
- Shaikh, Q.; Sarfaraz, S.; Rahim, A.; Hussain, A.; Behram, S.; Kazi, A.S.; Hussain, M.; Salahuddin, N. WHO Point Prevalence Survey to Describe the Use of Antimicrobials at a Tertiary Care Center in Pakistan: A Situation Analysis for Establishing an Antimicrobial Stewardship Program. Antibiotics 2022, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Vernet, G.; Mary, C.; Altmann, D.M.; Doumbo, O.; Morpeth, S.; Bhutta, Z.A.; Klugman, K.P. Surveillance for antimicrobial drug resistance in under-resourced countries. Emerg. Infect. Dis. 2014, 20, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atif, M.; Azeem, M.; Saqib, A.; Scahill, S. Investigation of antimicrobial use at a tertiary care hospital in Southern Punjab, Pakistan using WHO methodology. Antimicrob. Resist. Infect. Control 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, Z.; Hassali, M.A.; Versporten, A.; Godman, B.; Hashmi, F.K.; Goossens, H.; Saleem, F. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: Findings and implications. Expert Rev. Anti Infect. Ther. 2019, 17, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, Z.; Haseeb, A.; Godman, B.; Batool, N.; Altaf, U.; Ahsan, U.; Mustafa, Z.U.; Nadeem, M.U.; Farrukh, M.J.; Mugheera, M.; et al. Point Prevalence Survey of Antimicrobial Use during the COVID-19 Pandemic among Different Hospitals in Pakistan: Findings and Implications. Antibiotics 2023, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Saeed, H.; Hassali, M.A.; Godman, B.; Asif, U.; Yousaf, M.; Ahmed, Z.; Riaz, H.; Raza, S.A. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: A longitudinal surveillance and implications. Antimicrob. Resist. Infect. Control 2019, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, H.; Akhtar, S.; Rahman, F.U.; Afridi, M.; Khalid, S.; Ali, S.; Akhtar, N.; Khader, Y.S.; Ahmad, H.; Khan, M.M. An overview of the treatment options used for the management of COVID-19 in Pakistan: Retrospective observational study. JMIR Public Health Surveill. 2021, 7, e28594. [Google Scholar] [CrossRef]
- Arif, S.; Sadeeqa, S.; Saleem, Z. Patterns of antimicrobial use in hospitalized children: A repeated point prevalence survey from Pakistan. J. Pediatr. Infect. Dis. Soc. 2021, 10, 970–974. [Google Scholar] [CrossRef]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Hashmi, F.K.; Godman, B.; Ahmed, Z. Snapshot of antimicrobial stewardship programs in the hospitals of Pakistan: Findings and implications. Heliyon 2019, 5, e02159. [Google Scholar] [CrossRef] [Green Version]
- Hayat, K.; Rosenthal, M.; Gillani, A.H.; Chang, J.; Ji, W.; Yang, C.; Jiang, M.; Zhao, M.; Fang, Y. Perspective of Key Healthcare Professionals on Antimicrobial Resistance and Stewardship Programs: A Multicenter Cross-Sectional Study From Pakistan. Front. Pharmacol. 2019, 10, 1520. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, N.; Khan, A.S.; Zahid, T.; Ijaz, U.E.B.; Aziz, M.M.; Khan, R.; Mahmood, K.; Saif-ur-Rehman, N.; Zin, C.S. Assessment of Adherence to the Core Elements of Hospital Antibiotic Stewardship Programs: A Survey of the Tertiary Care Hospitals in Punjab, Pakistan. Antibiotics 2021, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Zia, R.; Malik, I.; Ahmad, N.; Sarwar, S. Treatment outcomes, antibiotic use and its resistance pattern among neonatal sepsis patients attending Bahawal Victoria Hospital, Pakistan. PLoS ONE 2021, 16, e0244866. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.S.Z.; Altaf, U.; Batool, N.; Godman, B.; Ahsan, U.; Ashiq, M.; Razzaq, M.; Hanif, R.; E-Huma, Z.; Amir, A.; et al. Impact of Positive Culture Reports of E. coli or MSSA on De-Escalation of Antibiotic Use in a Teaching Hospital in Pakistan and the Implications. Infect. Drug Resist. 2023, 16, 77–86. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Levy Hara, G. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Hindler, J.F.; Stelling, J. Analysis and presentation of cumulative antibiograms: A new consensus guideline from the Clinical and Laboratory Standards Institute. Clin. Infect. Dis. 2007, 44, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guma, S.P.; Godman, B.; Campbell, S.M.; Mahomed, O. Determinants of the Empiric Use of Antibiotics by General practitioners in South Africa: Observational, Analytic, Cross-Sectional Study. Antibiotics 2022, 11, 1423. [Google Scholar] [CrossRef]
- Godman, B.; Haque, M.; McKimm, J.; Abu Bakar, M.; Sneddon, J.; Wale, J.; Campbell, S.; Martin, A.P.; Hoxha, I.; Abilova, V.; et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: Findings and implications for the future. Curr. Med. Res. Opin. 2020, 36, 301–327. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Hashmi, F.K.; Saleem, F. A multicenter point prevalence survey of healthcare-associated infections in Pakistan: Findings and implications. Am. J. Infect. Control 2019, 47, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Saleem, Z.; Hassali, M.A.; Hashmi, F.K.; Godman, B.; Saleem, F. Antimicrobial dispensing practices and determinants of antimicrobial resistance: A qualitative study among community pharmacists in Pakistan. Fam. Med. Community Health 2019, 7, e000138. [Google Scholar] [CrossRef]
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. 2021. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 27 March 2023).
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M.; et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 2023, 101, 290–296. [Google Scholar] [CrossRef]
- Hsia, Y.; Lee, B.R.; Versporten, A.; Yang, Y.; Bielicki, J.; Jackson, C.; Newland, J.; Goossens, H.; Magrini, N.; Sharland, M. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health 2019, 7, e861–e871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latif, S.; Anwar, M.S.; Ahmad, I. Bacterial pathogens responsible for blood stream infection (BSI) and pattern of drug resistance in a tertiary care hospital of Lahore. Biomedica 2009, 25, 101–105. [Google Scholar]
- Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.; Ahmed, N.; et al. The usage of antibiotics by COVID-19 patients with comorbidities: The risk of increased antimicrobial resistance. Antibiotics 2021, 11, 35. [Google Scholar] [CrossRef]
- Senbayrak, S.; Boz, E.S.; Cevan, S.; Inan, A.; Engin, D.O.; Dosoglu, N.; Cobanoglu, N.; Dagli, O.; Davarci, I.; Aksaray, S. Antibiotic Resistance Trends and The ESBL Prevalence of Escherichia coli and Klebsiella spp. Urinary Isolates in In-and Outpatients in a Tertiary Care Hospital in Istanbul, 2004–2012. Jundishapur J. Microbiol. 2017, 10, e13098. [Google Scholar] [CrossRef] [Green Version]
- Córdoba, G.; Holm, A.; Hansen, F.; Hammerum, A.M.; Bjerrum, L. Prevalence of antimicrobial resistant Escherichia coli from patients with suspected urinary tract infection in primary care, Denmark. BMC Infect. Dis. 2017, 17, 670. [Google Scholar] [CrossRef]
- Sakran, W.; Smolkin, V.; Odetalla, A.; Halevy, R.; Koren, A. Community-acquired urinary tract infection in hospitalized children: Etiology and antimicrobial resistance. A comparison between first episode and recurrent infection. Clin. Pediatr. 2015, 54, 479–483. [Google Scholar] [CrossRef]
- Arslan, H.; Azap, Ö.K.; Ergönül, Ö.; Timurkaynak, F. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J. Antimicrob. Chemother. 2005, 56, 914–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Fatima, M.; Ahmad, Z.; Sajid, A.; Rehman, I.U.; Nadeem, M.U.; Javaid, Z.; Malik, M.; et al. Sale of WHO AWaRe groups antibiotics without a prescription in Pakistan: A simulated client study. J. Pharm. Policy Pract. 2020, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, R.; Rubin, J.; Thys, E.; Friedman, C.R.; Riley, L.W. Persistent Pandemic Lineages of Uropathogenic Escherichia coli in a College Community from 1999 to 2017. J. Clin. Microbiol. 2018, 56, e01834-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, T.I.; Ismail, H.A.; Elgamal, S.A.; Gad, A.H. The Occurrence of Multidrug Resistant E. coli which Produce ESBL and Cause Urinary Tract Infections. J. Appl. Microbiol. Biochem. 2017, 1, 8. [Google Scholar] [CrossRef]
- Cheema, S.; Cheema, S.U.R. Prevalence of Antibiotic Resistance among Patients with Escherichia Coli Urinary Tract Infection in a Private Hospital at Lahore-Pakistan. Pak. J. Med. Health Sci. 2016, 10, 364–367. [Google Scholar]
- Bartoloni, A.; Pallecchi, L.; Riccobono, E.; Mantella, A.; Magnelli, D.; Di Maggio, T.; Villagran, A.L.; Lara, Y.; Saavedra, C.; Strohmeyer, M.; et al. Relentless increase of resistance to fluoroquinolones and expanded-spectrum cephalosporins in Escherichia coli: 20 years of surveillance in resource-limited settings from Latin America. Clin. Microbiol. Infect. 2013, 19, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care—Associated bloodstream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Brumfitt, W.; Hamilton-Miller, J. Methicillin-Resistant Staphylococcus aureus. New Engl. J. Med. 1989, 320, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stryjewski, M.E.; Corey, G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014, 58 (Suppl. S1), S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.C.; Shaheduzzaman, M.; Sultana, N.; Jahid, I.K. Comparative Antibiotic Sensitivity Pattern of Hospital and Community Acquired Staphylococcus aureus Isolates of Jessore, Bangladesh. J. Biosci. Med. 2015, 3, 17. [Google Scholar]
- Wilson, P.; Andrews, J.A.; Charlesworth, R.; Walesby, R.; Singer, M.; Farrell, D.J.; Robbins, M. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 2003, 51, 186–188. [Google Scholar] [CrossRef]
- Hafiz, S.; Hafiz, A.; Ali, L.; Chughtai, A.; Memon, B.; Ahmed, A.; Hussain, S.; Sarwar, G.; Mughal, T.; Awan, A.; et al. Methicillin resistant Staphylococcus aureus: A multicentre study. J. Pak. Med. Assoc. 2002, 52, 312–314. [Google Scholar]
- Wong, C.K.M.; Kung, K.; Au-Doung, P.L.W.; Ip, M.; Lee, N.; Fung, A.; Wong, S.Y.S. Antibiotic resistance rates and physician antibiotic prescription patterns of uncomplicated urinary tract infections in southern Chinese primary care. PLoS ONE 2017, 12, e0177266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yayan, J.; Ghebremedhin, B.; Rasche, K. Antibiotic resistance of Pseudomonas aeruginosa in pneumonia at a single university hospital center in Germany over a 10-year period. PLoS ONE 2015, 10, e0139836. [Google Scholar] [CrossRef] [Green Version]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, D.; Reyes, L.; Faverio, P.; Aliberti, S.; Restrepo, M. prevalence and Risk Factors for Enterobacteriaceae (eb) and Multidrug-resistant Eb in Community-acquired Pneumonia. Chest 2017, 152, A156. [Google Scholar] [CrossRef]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Aldisio, E.; Marchese, A.E.; Mattaliano, A.R.; Tsakris, A. Antibiotic trends of Klebsiella pneumoniae and Acinetobacter baumannii resistance indicators in an intensive care unit of Southern Italy, 2008–2013. Antimicrob. Resist. Infect. Control 2015, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Karaaslan, A.; Çağan, E.; Kadayifci, E.K.; Atıcı, S.; Akkoç, G.; Yakut, N.; Öcal Demir, S.; Soysal, A.; Bakır, M. Intravenous Colistin Use for Multidrug-Resistant Gram-Negative Infections in Pediatric Patients. Balk. Med. J. 2016, 33, 627. [Google Scholar] [CrossRef]
- Billington, E.O.; Phang, S.H.; Gregson, D.B.; Pitout, J.D.D.; Ross, T.; Church, D.L.; Laupland, K.B.; Parkins, M.D. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: A population-based study. Int. J. Infect. Dis. 2014, 26, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Monteserin, N.; Larson, E. Temporal trends and risk factors for healthcare-associated vancomycin-resistant enterococci in adults. J. Hosp. Infect. 2016, 94, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Zafar, H.; Lakhnana, N.K.; Tauseef, K.; Zahid, M.; Kazmi, A.; Usman, A.; Ali, A. Antibiotic Susceptibility Pattern of Various Isolates in Urine Specimen at a Tertiary Care Hospital of Islamabad. Br. J. Pharm. Res. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Najjuka, C.F.; Kateete, D.P.; Kajumbula, H.M.; Joloba, M.L.; Essack, S.Y. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res. Notes 2016, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Núñez, M.; Navarro, M.D.; Palomo, V.; Rajendran, N.B.; del Toro, M.D.; Voss, A.; Sharland, M.; Sifakis, F.; Tacconelli, E.; Rodríguez-Baño, J. The methodology of surveillance for antimicrobial resistance and healthcare-associated infections in Europe (SUSPIRE): A systematic review of publicly available information. Clin. Microbiol. Infect. 2017, 24, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, I.U.; Khan, T.M.; Ali, I.; Bukhsh, A. Antimicrobial susceptibility in a tertiary care hospital in Pakistan. Can. J. Infect. Control 2016, 31, 178–181. [Google Scholar]
- Barlow, R.; Gobius, K. Pilot Survey for Antimicrobial Resistant (AMR) Bacteria in Australian Food; CSIRO: Cannon Hill, Australia, 2008.
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Siachalinga, L.; Mufwambi, W.; Lee, I.H. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: A systematic review and meta-analysis. J. Hosp. Infect. 2022, 129, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Otieno, P.A.; Campbell, S.; Maley, S.; Obinju Arunga, T.; Otieno Okumu, M. A Systematic Review of Pharmacist-Led Antimicrobial Stewardship Programs in Sub-Saharan Africa. Int. J. Clin. Pract. 2022, 2022, 3639943. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Saleem, Z.; Maqadmi, A.F.; Allehyani, R.A.; Mahrous, A.J.; Elrggal, M.E.; Kamran, S.H.; AlGethamy, M.; Naji, A.S.; AlQarni, A.; et al. Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): Findings and Implications. Antibiotics 2023, 12, 827. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug-resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef]
- Samad, A.; Ahmed, T.; Rahim, A.; Khalil, A.; Ali, I. Antimicrobial susceptibility patterns of clinical isolates of Pseudomonas aeruginosa isolated from patients of respiratory tract infections in a Tertiary Care Hospital, Peshawar. Pak. J. Med. Sci. 2017, 33, 670. [Google Scholar] [CrossRef]
- Mansoor, T.; Musani, M.A.; Khalid, G.; Kamal, M. Pseudomonas aeruginosa in chronic suppurative otitis media: Sensitivity spectrum against various antibiotics in Karachi. J. Ayub Med. Coll. Abbottabad 2009, 21, 120–123. [Google Scholar] [PubMed]
- Khan, M.; Siddiqui, S.; Haider, S.; Zafar, A.; Zafar, F.; Khan, R.; Afshan, K.; Jabeen, A.; Khan, M.S.; Hasan, R. Infection control education: Impact on ventilator-associated pneumonia rates in a public sector intensive care unit in Pakistan. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Masood, R.; Aman, A.; Karim, F. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Pattern. Pak. J. Med. Health Sci. 2022, 16, 846. [Google Scholar] [CrossRef]
- Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects; WMA: Ferney-Voltaire, France, 2009.
- Chowdhury, K.; Haque, M.; Nusrat, N.; Adnan, N.; Islam, S.; Lutfor, A.B.; Begum, D.; Rabbany, A.; Karim, E.; Malek, A.; et al. Management of Children Admitted to Hospitals across Bangladesh with Suspected or Confirmed COVID-19 and the Implications for the Future: A Nationwide Cross-Sectional Study. Antibiotics 2022, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Skosana, P.P.; Schellack, N.; Godman, B.; Kurdi, A.; Bennie, M.; Kruger, D.; Meyer, J.C. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev. Anti Infect. Ther. 2021, 19, 1353–1366. [Google Scholar] [CrossRef]
- Kurdi, A.; Hasan, A.J.; Baker, K.I.; Seaton, R.A.; Ramzi, Z.S.; Sneddon, J.; Godman, B. A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan regional government of Northern Iraq: The need for urgent action. Expert Rev. Anti Infect. Ther. 2021, 19, 805–814. [Google Scholar] [CrossRef]

| Isolates | Resistant to 1 Antibiotic Class n (%) | Resistant to 2 Antibiotic Classes n (%) | Resistant to 3 Antibiotic Classes n (%) | Resistant to 4 Antibiotic Classes n (%) | Resistant to 5 Antibiotic Classes n (%) | Resistant to 6 Antibiotic Classes n (%) | Resistant to 7 Antibiotic Classes n (%) | Total No. of Isolates |
|---|---|---|---|---|---|---|---|---|
| E. coli | 81 (9.95) | 108 (13.26) | 221 (27.14) | 174 (21.37) | 127 (15.60) | 61 (7.49) | 6 (0.73) | 814 |
| P. aeruginosa | 42 (9.25) | 76 (16.74) | 122 (26.87) | 86 (18.94) | 57 (12.55) | 23 (5.06) | 6 (1.32) | 454 |
| K. pneumoniae | 30 (11.15) | 38 (14.12) | 72 (26.76) | 51 (18.95) | 39 (14.49) | 23 (8.55) | 5 (1.85) | 269 |
| Proteus spp. | 6 (8.57) | 5 (7.14) | 21 (30) | 16 (22.85) | 12 (17.14) | 6 (8.57) | 0 (0) | 70 |
| A. baumannii | 3 (6) | 6 (12) | 8 (16) | 9 (18) | 8 (16) | 16 (32) | 0 (0) | 50 |
| S. aureus | 180 (20.78) | 202 (23.32) | 190 (21.93) | 115 (13.27) | 50 (5.77) | 40 (4.61) | 7 (0.80) | 866 |
| Total | 342 | 435 | 634 | 451 | 293 | 169 | 24 | 2523 |
| Antibiotics | ATC Classification 1 | AWaRe Classification 2 | A. baumannii | E. coli | K. pneumoniae | Proteus spp. | P. aeruginosa | S. aureus | Total |
|---|---|---|---|---|---|---|---|---|---|
| Penicillins | |||||||||
| Penicillin | J01CE01 | Access | NA * | NA | NA | NA | NA | 153/511 (29.9) | 153/579 (26.39%) |
| Ampicillin | J01CA01 | Access | NA | 54/543 (9.9%) | NA | 5/53 (9.4) | NA | NA | 129/1112 (11.61%) |
| Oxacillin | J01CF04 | Access | NA | NA | NA | NA | NA | 23/53 (43.4) | 23/53 (43.4) |
| Beta-lactam inhibitors | |||||||||
| Amoxicillin-clavulanate | J01CR02 | Access | NA | 51/198 (25.8) | 23/81 (28.4) | 14/44 (31.8) | NA | 27/48 (56.3) | 115/371 (31.00%) |
| Ampicillin–Sulbactam | J01CR01 | Access | NA | 31/93 (33.3) | 4/16 (25.0) | 0/1 (0) | NA | 14/38 (36.8) | 49/148 (33.11%) |
| Piperacillin–Tazobactam | J01CR05 | Watch | 10/28 (35.7) | 415/573 (72.4) | 173/231 (74.9) | 54/60 (90.0) | 241/360 (66.9) | 25/47 (53.2) | 918/1299 (70.70%) |
| Cephalosporins | |||||||||
| Cephalexin | J01DB01 | Access | NA | 7/145 (4.8) | 3/37 (8.1) | 0/3 (0.0) | NA | 60/138 (43.5) | 70/323 (21.67%) |
| Cefazolin | J01DB04 | Access | NA | 3/44 (6.8) | 2/19 (10.5) | 1/2 (50.0) | NA | 35/67 (52.2) | 41/132 (31.06%) |
| Cephradine | J01DB09 | Access | NA | NA | NA | NA | NA | 14/42 (33.3) | 14/42 (33.3) |
| Cefoxitin | J01DC01 | Watch | NA | NA | NA | NA | NA | 243/336 (72.3) | 279/400 (69.75%) |
| Cefuroxime | J01DC02 | Watch | NA | 74/474 (15.6) | 50/201 (24.9) | 10/53 (18.9) | NA | 69/134 (51.4) | 203/862 (23.56%) |
| Cefaclor | J01DC04 | Watch | NA | 31/222 (14) | 32/91 (35.2) | 1/14 (7.1) | NA | 35/85 (41.2) | 99/412 (24.03%) |
| Cefotaxime | J01DD01 | Watch | 0/1 (0.0) | 38/171 (22.2) | 9/34 (26.5) | 0/1 (0.0) | NA | 23/72 (31.9) | 70/279 (25.09%) |
| Ceftazidime | J01DD02 | Watch | 0/22 (0.0) | 122/336 (36.3) | 67/156 (42.9) | 14/25 (56) | 122/271 (45.0) | 28/86 (32.6) | 353/896 (39.42%) |
| Ceftriaxone | J01DD04 | Watch | 1/38 (2.6) | 95/412 (23.1) | 20/86 (23.3) | 9/34 (26.5) | NA | 111/210 (52.9) | 236/780 (30.26%) |
| Cefixime | J01DD08 | Watch | NA | 94/437 (21.5) | 56/169 (33.1) | 11/38 (28.9) | NA | 30/86 (34.9) | 191/730 (26.16%) |
| Cefoprazone | J01DD12 | Watch | NA | NA | NA | NA | NA | 40/64 (62.5) | 40/64 (62.5) |
| Cefoperazone–Sulbactam | J01DD62 | Watch | NA | NA | NA | NA | NA | 12/29 (41.4) | 31/62 (50.0%) |
| Cefepime | J01DE01 | Watch | 4/34 (11.8) | 56/244 (23.0) | 22/56 (39.3) | 8/19 (42.1) | 48/107 (44.9) | 20/69 (29.0) | 37/117 (31.7) |
| Carbapenems | |||||||||
| Imipenem | J01DH51 | Watch | 10/28 (35.7) | 373/427 (87.4) | 92/108 (85.2) | 25/36 (69.4) | 105/149 (70.5) | 98/143 (68.5) | 703/891 (78.9) |
| Meropenem | J01DH02 | Watch | 17/48 (35.4) | 542/639 (84.8) | 142/203 (70) | 48/57 (84.2) | 228/346 (65.9) | 148/244 (60.7) | 1125/1537 (73.12%) |
| Etrapenem | J01DH03 | Watch | NA | 96/123 (78.0) | 27/42 (64.3) | 14/19 (73.7) | NA | 2/4 (50.0) | 156/225 (69.3) |
| Macrolides | |||||||||
| Erythromycin | J01FA01 | Watch | NA | NA | NA | NA | NA | 296/559 (53) | 296/559 (53) |
| Clarithromycin | J01FA09 | Watch | NA | NA | NA | NA | NA | 122/255 (47.8) | 122/255 (47.8) |
| Azithromycin | J01FA10 | Watch | NA | NA | NA | NA | NA | 36/128 (28.1) | 36/128 (28.1) |
| Clindamycin | J01FF01 | Access | NA | NA | NA | NA | NA | 412/665 (62.0) | 412/665 (62.0) |
| Aminoglycosides | |||||||||
| Gentamicin | J01GB03 | Access | 7/38 (18.4) | 291/518 (56.2) | 75/149 (50.3) | 22/48 (45.8) | 120/225 (53.3) | 331/495 (66.9) | 846/1473 (57.4) |
| Amikacin | J01GB06 | Access | 16/41 (39) | 498/631(78.9) | 141/211 (66.8) | 40/58 (69) | 247/356 (69.4) | NA | 942/1297 (72.63%) |
| Tobramycin | J01GB01 | Watch | 8/23 (34.8) | 107/233 (45.9) | 61/111 (55) | 14/23 (60) | 137/227 (60) | NA | 327/617 (52.97%) |
| Fluoroquinolones | |||||||||
| Ciprofloxacin | J01MA02 | Watch | 7/32 (21.9) | 164/555 (29.5) | 81/164 (49.4) | 27/47 (57.4) | 150/288 (52.1) | 250/580 (43.1) | 679/1666 (40.8) |
| Levofloxacin | J01MA12 | Watch | 11/23 (47.8) | 143/374 (38.2) | 59/152 (38.8) | 20/39 (51.3) | 117/215 (54.4) | 134/245 (54.7) | 484/1048 (46.2) |
| Norfloxacin | J01MA06 | Watch | NA | 90/344 (26.2) | 46/118 (39.0) | 11/23 (47.8) | 73/182 (40.1) | 33/116 (28.4) | 253/783 (32.36%) |
| Ofloxacin | J01MA01 | Watch | NA | 62/256 (24.2) | 50/83 (60.2) | 1/9 (11.1) | 56/133 (42.1) | 25/68 (36.8) | 194/549 (35.34%) |
| Moxifloxacin | J01MA14 | Watch | NA | NA | 6/34 (17.6) | NA | NA | 56/107 (52.3) | 62/141 (44.0%) |
| Nalidixic acid | J01MB02 | NA | NA | 48/294 (16.3) | 24/104 (23.1) | 2/20 (10.0) | NA | NA | 74/418 (17.70%) |
| Tetracyclines | |||||||||
| Doxycycline | J01AA02 | Access | 1/11 (9.1) | 82/191 (42.9) | 9/20 (45.0) | 3/5 (60.0) | NA | 69/126 (54.8) | 164/353 (46.45) |
| Tetracycline | J01AA07 | Access | 2/16 (12.5) | 77/258 (29.8) | 57/120 (47.5) | NA | NA | 194/399 (48.6) | 339/825 (36.63) |
| Minocycline | J01AA08 | Watch | 21/30 (70.0) | 25/52 (48.1) | 12/19 (63.2) | 5/12 (41.7) | NA | 228/248 (91.9) | 291/361 (80.61%) |
| Tigecycline | J01AA12 | Reserve | NA | 86/95 (90.5) | NA | NA | NA | 60/64 (93.8) | 146/159 (91.82%) |
| Carb–Monobactams | |||||||||
| Aztreonam | J01DF01 | Reserve | NA | 35/100 (35) | 1/3 (33.3) | NA | 14/42 (33.3) | 0/6 (0.0) | 50/151 (33.1) |
| Glycopeptide | |||||||||
| Vancomycin | A07AA09 | Watch | NA | NA | NA | NA | NA | 513/737 (69.6) | 513/737 (69.6) |
| Tecoplanin | J01XA02 | Watch | NA | NA | NA | NA | NA | 73/125 (58.4) | 73/125 (58.4) |
| Polymixin | |||||||||
| Colistin | A07AA10 | Reserve | 28/35 (80.0) | 177/231 (76.6) | 94/99 (94.9) | NA | 179/209 (85.6) | 489/601 (81.36%) | |
| Polymixin-B | A07AA05 | Reserve | 27/28 (96.4) | 80/87 (92.0) | 48/48 (100.0) | NA | 102/108 (94.4) | NA | 265/288 (92.01%) |
| Phosphonic | |||||||||
| Fosfomycin | J01XX01 | Watch | NA | 255/277 (92.1) | 25/32 (78.1) | NA | NA | NA | 280/309 (90.61%) |
| Nitrofuran | |||||||||
| Nitrofurantoin | J01XE01 | Access | 3/5 (60.0) | 171/205 (83.4) | 14/18 (77.8) | NA | 25/34 (73.5) | 222/283 (78.4) | |
| Oxazolidinones | |||||||||
| Linezolid | J01XX08 | Reserve | NA | NA | NA | NA | NA | 497/575 (86.4) | 497/575 (86.4) |
| Sulphonamide | |||||||||
| Trimethoprim–sulfamethoxazole | J01EE01 | Access | 1/32 (3.1) | 68/359 (18.9) | 28/92 (30.4) | 4/25 (16.0) | NA | 78/327 (23.8) | 179/835 (21.44%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, Z.; Haseeb, A.; Abuhussain, S.S.A.; Moore, C.E.; Kamran, S.H.; Qamar, M.U.; Azmat, A.; Pichierri, G.; Raees, F.; Asghar, S.; et al. Antibiotic Susceptibility Surveillance in the Punjab Province of Pakistan: Findings and Implications. Medicina 2023, 59, 1215. https://doi.org/10.3390/medicina59071215
Saleem Z, Haseeb A, Abuhussain SSA, Moore CE, Kamran SH, Qamar MU, Azmat A, Pichierri G, Raees F, Asghar S, et al. Antibiotic Susceptibility Surveillance in the Punjab Province of Pakistan: Findings and Implications. Medicina. 2023; 59(7):1215. https://doi.org/10.3390/medicina59071215
Chicago/Turabian StyleSaleem, Zikria, Abdul Haseeb, Safa S. Almarzoky Abuhussain, Catrin E. Moore, Sairah Hafeez Kamran, Muhammad Usman Qamar, Aisha Azmat, Giuseppe Pichierri, Fahad Raees, Shahzad Asghar, and et al. 2023. "Antibiotic Susceptibility Surveillance in the Punjab Province of Pakistan: Findings and Implications" Medicina 59, no. 7: 1215. https://doi.org/10.3390/medicina59071215









