Plasma-Rich Fibrin—Regenerative Material in Tympanic Membrane Surgery
Abstract
:1. Introduction
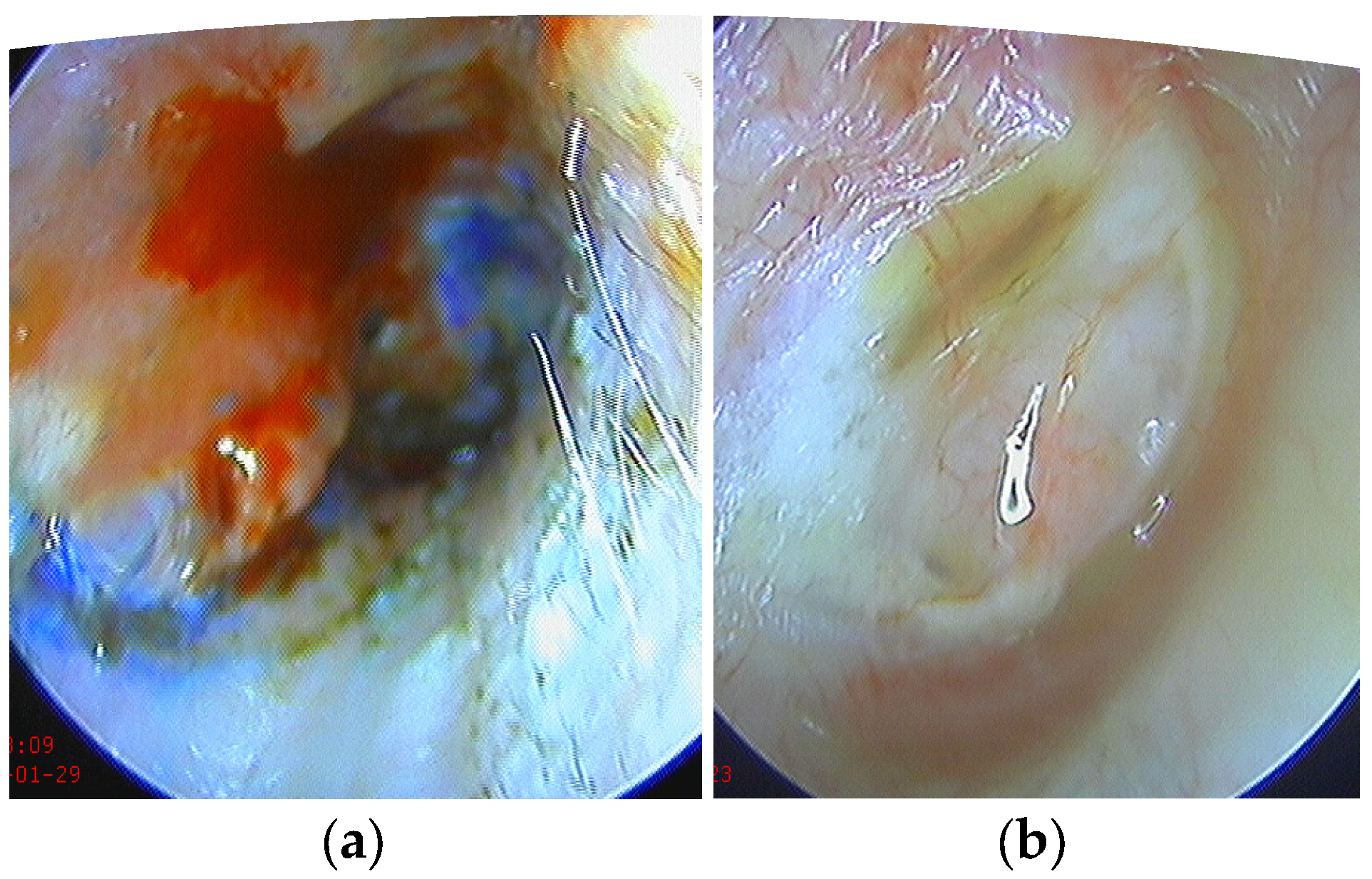
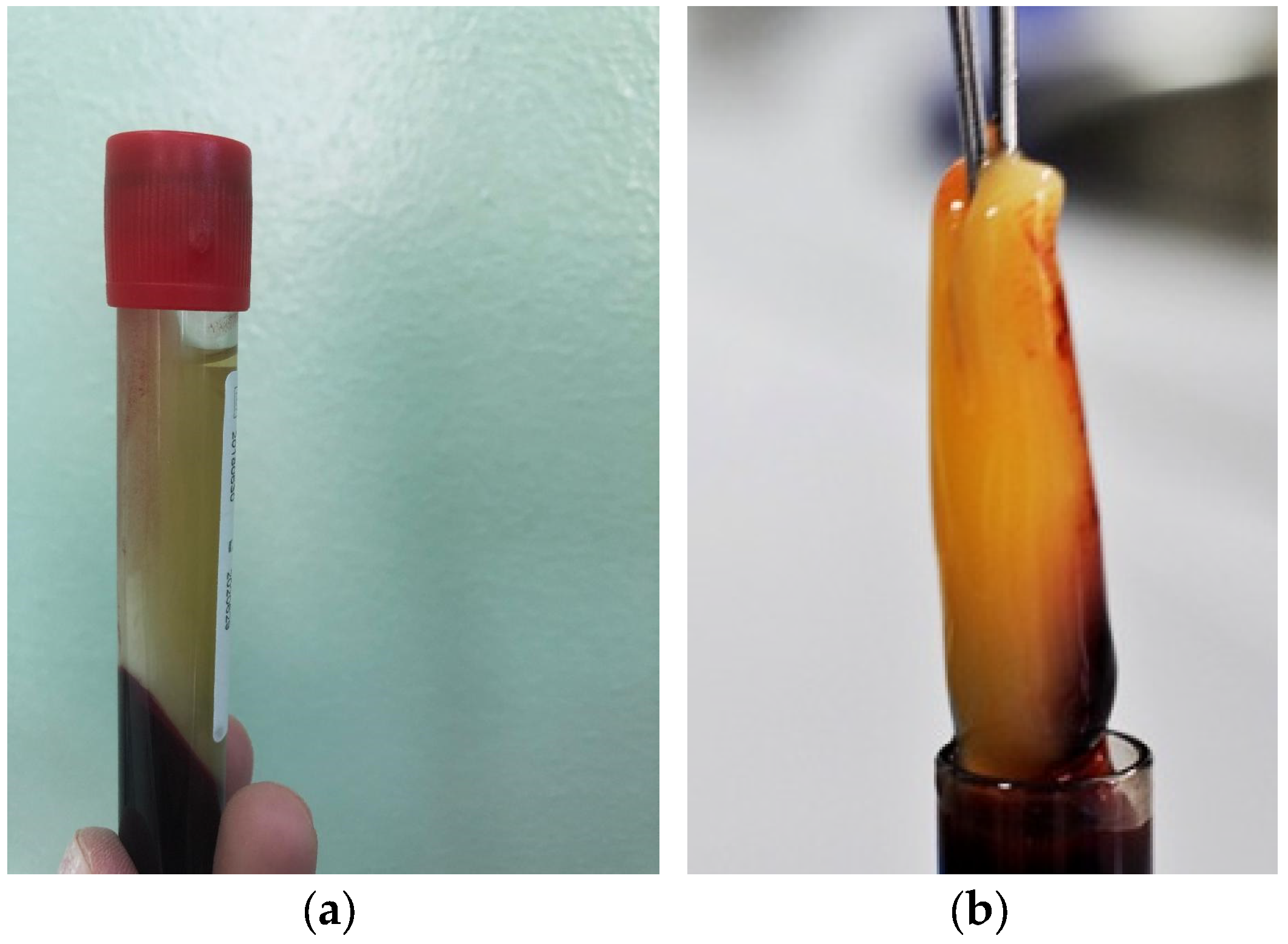
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.D. Application of Platelet Rich Fibrin Matrix to Repair Traumatic Tympanic Membrane Perforations: A Pilot Study. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. S2), 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Klacansky, J. Cartilage myringoplasty. Laryngoscope 2009, 119, 2175–2177. [Google Scholar] [CrossRef] [PubMed]
- Garin, P.; Peerbaccus, Y.; Mullier, F.; Gheldof, D.; Dogne, J.M.; Putz, L.; Van Damme, J.P. Platelet-rich fibrin (PRF): An autologous packing material for middle ear microsurgery. B-ENT 2014, 10, 27–34. [Google Scholar] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng. Part. B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunite en paro-implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Acebes-Huerta, A.; Arias-Fernández, T.; Bernardo, Á.; Muñoz-Turrillas, M.C.; Fernández-Fuertes, J.; Seghatchian, J.; Gutiérrez, L. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfus. Apher. Sci. 2020, 59, 102716. [Google Scholar] [CrossRef]
- Sills, E.S.; Wood, S.H. Autologous activated platelet-rich plasma injection into adult human ovary tissue: Molecular mechanism, analysis, and discussion of reproductive response. Biosci. Rep. 2019, 39, BSR20190805. [Google Scholar] [CrossRef] [Green Version]
- Nazari, L.; Salehpour, S.; Hoseini, S.; Zadehmodarres, S.; Azargashb, E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int. J. Reprod. Biomed. 2019, 17, 443–448. [Google Scholar] [CrossRef]
- Scott, S.; Roberts, M.; Chung, E. Platelet-Rich Plasma and Treatment of Erectile Dysfunction: Critical Review of Literature and Global Trends in Platelet-Rich Plasma Clinics. Sex. Med. Rev. 2019, 7, 306–312. [Google Scholar] [CrossRef]
- Shiomi, Y.; Shiomi, Y. Surgical outcomes of myringoplasty using platelet-rich plasma and evaluation of the outcome-associated factors. Auris Nasus Larynx. 2020, 47, 191–197. [Google Scholar] [CrossRef]
- Nair, N.P.; Alexander, A.; Abhishekh, B.; Hegde, J.S.; Ganesan, S.; Saxena, S.K. Safety and efficacy of autologous platelet-rich fibrin on graft uptake in myringoplasty: A randomized controlled trial. Int. Arch. Otorhinolaryngol. 2019, 23, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Gökçe, K.S.; Özdaş, T. Impact of platelet-rich fibrin therapy in tympanoplasty type 1 surgery on graft survival and frequency-specific hearing outcomes: A retrospective analysis in patients with tympanic membrane perforation due to chronic otitis media. J. Laryngol. Otol. 2019, 133, 1068–1073. [Google Scholar] [CrossRef]
- Shukla, A.; Kaurav, Y.S.; Vatsyayan, R. Novel use of platelet rich fibrin membrane in transcanal myringoplasty: A prospective study. Indian J. Otolaryngol. Head Neck Surg. 2020, 72, 355–362. [Google Scholar] [CrossRef]
- Ankle, N.R.; Rajesh, R.H.S.; Sinha, V. A Prospective Study Assessing the Audiological Outcome of Tympanoplasty with Autologus Platelet Rich Plasma. Biomed. J. Sci. Tech. Res. 2021, 36, 28320–28323. [Google Scholar] [CrossRef]
- Riaz, N.; Muhammad, A.; Khan, M.S. Efficacy of Platelet Rich Fibrin in myringoplasty. Pak. J. Med. Sci. Q. 2021, 37, 212–216. [Google Scholar] [CrossRef]
- Sankaranarayanan, G.; Prithviraj, V.; Kumar, V. A study on efficacy of autologous platelet rich plasma in myringoplasty. J. Otolaryngol. 2013, 3, 36. [Google Scholar]
- El-Anwar, M.W.; El-Ahl, M.A.S.; Zidan, A.A.; Yacoup, M.A.-R.A.-S. Topical use of autologous platelet rich plasma in myringoplasty. Auris Nasus Larynx 2015, 42, 365–368. [Google Scholar] [CrossRef]
- Yadav, S.P.; Malik, J.S.; Malik, P.; Sehgal, P.K.; Gulia, J.S.; Ranga, R.K. Studying the result of underlay myringoplasty using platelet-rich plasma. J. Laryngol. Otol. 2018, 132, 990–994. [Google Scholar] [CrossRef]
- Shanmugam, R.; Gnanavelu, Y.; Shanmugam, V.U.; Swaminathan, B.; Nivas, P.; Bharath, I. Myringoplasty with Autologous Platelet Rich Plasma–A prospective study. J. Med. Sci. Clin. Res. 2018, 6, 1170–1173. [Google Scholar] [CrossRef]
- Gür, Ö.E.; Ensari, N.; Öztürk, M.T.; Boztepe, O.F.; Gün, T.; Selçuk, Ö.T.; Renda, L. Use of a platelet-rich fibrin membrane to repair traumatic tympanic membrane perforations: A comparative study. Acta Otolaryngol. 2016, 136, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Shindy, M.; El-Shimi, O.; Belal, M.; Abdelalim, A. Use of Platelet Rich Fibrin in Acute Traumatic Tympanic Membrane Perforation Compared with the Conservative Treatment. Benha Med. J. 2020, 37, 512–523. [Google Scholar] [CrossRef]
- Huang, J.; Teh, B.M.; Zhou, C.; Shi, Y.; Shen, Y. Tympanic membrane regeneration using platelet-rich fibrin: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2022, 279, 557–565. [Google Scholar] [CrossRef] [PubMed]

| Variable | Control Group | PRF Group | p-Value | |
|---|---|---|---|---|
| Age | 42.4 ± 13.8 | 44.05 ± 9.7 | 0.6 | |
| Sex | M | 12 (44.4%) | 13 (65.0%) | 0.2 |
| F | 15 (55.6%) | 7 (35.0%) | ||
| Perforation * Type | 1 | 5 (18.5%) | - | 0.1 |
| 2 | 9 (33.3%) | 5 (25.0%) | ||
| 3 | 2 (7.4%) | 3 (15.0%) | ||
| 4 | 11 (40.7%) | 12 (60.0%) | ||
| Tympanoplasty | I | 12 (44.4%) | 5 (25.0%) | 0.5 |
| II | 5 (18.5%) | 5 (25.0%) | ||
| III | 4 (14.8%) | 4 (20.0%) | ||
| IV | 6 (22.2%) | 6 (30.0%) | ||
| Variable | Closure Perforation | Control Group | PRF Group | p-Value |
|---|---|---|---|---|
| 1 Month | Yes | 24 (88.9%) | 20 (100%) | 0.2 |
| No | 3 (11.1%) | 0 (0.0%) | ||
| 3 Month | Yes | 21 (77.8%) | 19 (95.0%) | 0.2 |
| No | 6 (22.2%) | 1 (5.0%) | ||
| 6 Month | Yes | 20 (74.1%) | 19 (95.0%) | 0.1 |
| No | 7 (25.9%) | 1 (5.0%) | ||
| 12 Month | Yes | 19 (70.4%) | 19 (95.0%) | 0.05 |
| No | 8 (29.6%) | 1 (5.0%) |
| Variable | Control Group | PRF Group | p | ||
|---|---|---|---|---|---|
| PTA-value | Preoperative | 31.2 (27.5; 40) | 32.5 (28.7; 38.6) | 0.001 | |
| 12 months postoperative | 22.5 (17.5; 31.2) | 15 (11.8; 21.8) | |||
| Air-bone gap | Preoperative | 20 (15; 22.5) | 13.7 (11.2; 18.4) | 0.7 | |
| 12 months postoperative | 5 (2; 10) | 2.5 (1.2; 3.5) | |||
| Preoperative | 500 | 21(16; 23.5) | 16.2 (12.5; 19.6) | 0.8 | |
| 12 months postoperative | 500 | 6 (2.7; 11.2) | 3 (1.7; 4) | ||
| Preoperative | 1000 | 20.5 (15.5; 22.8) | 13.7 (11.2; 19) | 0.7 | |
| 12 months postoperative | 1000 | 5.5 (2.5; 10.5) | 2.7 (1.5; 3.7) | ||
| Preoperative | 2000 | 19.8 (14.8; 21) | 11.8 (10.3; 17.8) | 0.5 | |
| 12 months postoperative | 2000 | 4.5 (2.2; 10) | 2.5 (1.2; 3.5) | ||
| Preoperative | 4000 | 17.5 (13; 19.5) | 10 (10; 15.6) | 0.4 | |
| 12 months postoperative | 4000 | 3.5 (1.5; 9) | 2 (0.7; 3) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiple, C.; Chirila, M.; Vesa, S.C.; Stamate, M.C. Plasma-Rich Fibrin—Regenerative Material in Tympanic Membrane Surgery. Medicina 2023, 59, 1292. https://doi.org/10.3390/medicina59071292
Tiple C, Chirila M, Vesa SC, Stamate MC. Plasma-Rich Fibrin—Regenerative Material in Tympanic Membrane Surgery. Medicina. 2023; 59(7):1292. https://doi.org/10.3390/medicina59071292
Chicago/Turabian StyleTiple, Cristina, Magdalena Chirila, Stefan Cristian Vesa, and Mirela Cristina Stamate. 2023. "Plasma-Rich Fibrin—Regenerative Material in Tympanic Membrane Surgery" Medicina 59, no. 7: 1292. https://doi.org/10.3390/medicina59071292
APA StyleTiple, C., Chirila, M., Vesa, S. C., & Stamate, M. C. (2023). Plasma-Rich Fibrin—Regenerative Material in Tympanic Membrane Surgery. Medicina, 59(7), 1292. https://doi.org/10.3390/medicina59071292







