High-Intensity Interval Training Improves Glycemic Control, Cellular Apoptosis, and Oxidative Stress of Type 2 Diabetic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Exercise Training Program
2.3. Skeletal Muscle Biopsy and Blood Samples
2.4. Quantification of Mitochondrial DNA Content by Real-Time PCR
2.5. Estimation of Glycemic Control Parameters
2.6. Estimation of Serum Cytochrome c (COX) and p53
2.7. Estimation of Oxidative Stress and Antioxidant Capacity
2.8. Sample Calculations
2.9. Statistical Analysis
3. Results
4. Discussion
4.1. Strength
- (a)
- Scientific contribution: By inducing cellular stress through high-intensity exercise bouts, HIIT may activate cellular signaling pathways that improve mitochondrial function, increase antioxidant capacity, and reduce inflammation.
- (b)
- Objective measurement: Measuring biomarkers such as apoptosis (P53, COX), oxidative stress (TAC and 8-OHdG), and mitochondrial DNA can provide an objective measurement of the effects of HIIT on cellular and molecular processes related to type 2 diabetes.
- (c)
- Mechanistic insights: Measuring biomarkers can help to elucidate the mechanisms through which HIIT improves glucose control and insulin sensitivity in individuals with type 2 diabetes.
- (d)
- Personalized medicine: Biomarkers can potentially be used to identify individuals who are most likely to benefit from HIIT and monitor the effects of HIIT individually.
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, P.D.; Crouse, S.F.; Goodpaster, B.; Kelley, D.; Moyna, N.; Pescatello, L. The acute versus the chronic response to exercise. Med. Sci. Sports Exerc. 2001, 33, S438–S445. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [PubMed] [Green Version]
- Wei, M.; Gibbons, L.W.; Kampert, J.B.; Nichaman, M.Z.; Blair, S.N. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann. Intern. Med. 2000, 132, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Cheng, Y.J.; Earnest, C.P.; Barlow, C.E.; Gibbons, L.W.; Priest, E.L.; Blair, S.N. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004, 27, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Galaviz, K.I.; Weber, M.B.; Straus, A.; Haw, J.S.; Narayan, K.M.V.; Ali, M.K. Global Diabetes Prevention Interventions: A Systematic Review and Network Meta-analysis of the Real-World Impact on Incidence, Weight, and Glucose. Diabetes Care 2018, 41, 1526–1534. [Google Scholar] [CrossRef] [Green Version]
- Alberti, G.; Zimmet, P.; Shaw, J.; Bloomgarden, Z.; Kaufman, F.; Silink, M. Type 2 diabetes in the young: The evolving epidemic: The international diabetes federation consensus workshop. Diabetes Care 2004, 27, 1798–1811. [Google Scholar] [CrossRef] [Green Version]
- Booth, F.W.; Chakravarthy, M.V.; Gordon, S.E.; Spangenburg, E.E. Waging war on physical inactivity using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 2002, 93, 3–30. [Google Scholar] [CrossRef] [Green Version]
- Paul, J.; Beisswenger, P.J.; Drummond, K.S.; Nelson, R.G.; Howell, S.K.; Szwergold, B.S.; Mauer, M. Susceptibility to Diabetic Nephropathy Is Related to Dicarbonyl and Oxidative Stress. Diabetes 2005, 54, 3274–3281. [Google Scholar]
- De Muro, P.; Lepedda, A.J.; Nieddu, G.; Idini, M.; Tram Nguyen, H.Q.; Lobina, O.; Fresu, P.; Formato, M. Evaluaion of Early Markers of Nephropathy in Patients with Type 2 Diabetes Mellitus. Biochem. Res. Int. 2016, 2016, 7497614. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.; Boveris, A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell Physiol. 2007, 292, C670–C686. [Google Scholar] [CrossRef]
- Cortopassi, G.A.; Wong, A. Mitochondria in organismal aging and degeneration. Biochim. Biophys. Acta 1999, 1410, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Boveris, A.; Navarro, A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic. Biol. Med. 2008, 44, 224–229. [Google Scholar] [CrossRef]
- Ungvari, Z.; Labinskyy, N.; Gupte, S.; Chander, P.N.; Edwards, J.G.; Csiszar, A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2121–H2128. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 2001, 50, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Asmann, Y.W.; Stump, C.S.; Short, K.R.; Coenen-Schimke, J.M.; Guo, Z.; Bigelow, M.L.; Nair, K.S. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and non-diabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006, 55, 3309–3319. [Google Scholar] [CrossRef] [Green Version]
- Hawley, J.A.; Burke, L.M.; Phillips, S.M.; Spriet, L.L. Nutritional modulation of training-induced skeletal muscle adaptations. J. Appl. Physiol. 2011, 110, 834–845. [Google Scholar] [CrossRef] [Green Version]
- Hood, D.A.; Irrcher, I.; Ljubicic, V.; Joseph, A.M. Coordination of metabolic plasticity in skeletal muscle. J. Exp. Biol. 2006, 209, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef]
- Egan, B.; Carson, B.P.; Garcia-Roves, P.M.; Chibalin, A.V.; Sarsfield, F.M.; Barron, N.; McCaffrey, N.; Moyna, N.M.; Zierath, J.R.; O’Gorman, D.J. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J. Physiol. 2010, 588, 1779–1790. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Rennie, C.D.; Robertshaw, H.A.; Fedak-Tarnopolsky, S.N.; Devries, M.C.; Hamadeh, M.J. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1271–R1278. [Google Scholar] [CrossRef] [Green Version]
- Schrauwen, P.; Hesselink, M. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 2004, 53, 1412–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowell, B.B.; Shulmanz, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokota, T.; Kinugawa, S.; Hirabayashi, K.; Matsushima, S.; Inoue, N.; Ohta, Y.; Hamaguchi, S.; Sobirin, M.A.; Ono, T.; Suga, T.; et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1069–H1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Liu, Q.; Tan, Y.; Liu, L.; Li, X.; Cai, L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin 2009, 33, 370–377. [Google Scholar] [CrossRef]
- Dokken, B.B.; Saengsirisuwan, V.; Kim, J.S.; Teachey, M.K.; Henriksen, E.J. Oxidative stress-induced insulin resistance in rat skeletal muscle: Role of glycogen synthase kinase-3. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E615–E621. [Google Scholar] [CrossRef] [Green Version]
- Virkamäki, A.; Korsheninnikova, E.; Seppälä-Lindroos, A.; Vehkavaara, S.; Goto, T.; Halavaara, J.; Häkkinen, A.M.; Yki-Järvinen, H. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 2001, 50, 2337–2343. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, J.; Zhang, N.; Chen, M.; Wang, H.; Zhu, D. p53 in mitochondrial dynamics and apoptosis. Mitochondrion 2020, 52, 67–73. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Jones, D. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta (BBA)-Bioenerg. 1998, 1366, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Chipuk, J.E.; Bouchier-Hayes, L.; Green, D.R. Mitochondrial outer membrane permeabilization during apoptosis: The innocent bystander scenario. Cell Death Differ. 2006, 13, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Green, D.R.; Galluzzi, L. Getting Mechanistic Insight from Apoptosis Signaling Networks. Cell 2016, 165, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Sung, H.J.; Park, J.Y.; Matoba, S.; Hwang, P.M. A pivotal role for p53: Balancing aerobic respiration and glycolysis. J. Bioenerg. Biomembr. 2007, 39, 243–246. [Google Scholar] [CrossRef]
- Kruse, J.P.; Gu, W. p53 aerobics: The major tumor suppressor fuels your workout. Cell Metab. 2006, 4, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Knuf, M.; Faber, J.; Huth, R.G.; Freisinger, P.; Zepp, F.; Kampmann, C. Identification of a novel compound heterozygote SCO2 mutation in cytochrome c oxidase deficient fatal infantile cardioencephalomyopathy. Acta Paediatr. 2007, 96, 130–132. [Google Scholar] [CrossRef]
- Matoba, S.; Kang, J.G.; Patino, W.D.; Wragg, A.; Boehm, M.; Gavrilova, O.; Hurley, P.J.; Bunz, F.; Hwang, P.M. p53 regulates mitochondrial respiration. Science 2006, 312, 1650–1653. [Google Scholar] [CrossRef]
- Park, J.Y.; Wang, P.Y.; Matsumoto, T.; Sung, H.J.; Ma, W.; Choi, J.W.; Anderson, S.A.; Leary, S.C.; Balaban, R.S.; Kang, J.G.; et al. p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ. Res. 2009, 105, 705–712. [Google Scholar] [CrossRef]
- Phielix, E.; Meex, R.; Moonen-Kornips, E.; Hesselink, M.K.; Schrauwen, P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia 2010, 53, 1714–1721. [Google Scholar] [CrossRef] [Green Version]
- Meex, R.C.; Schrauwen-Hinderling, V.B.; Moonen-Kornips, E.; Schaart, G.; Mensink, M.; Phielix, E.; van de Weijer, T.; Sels, J.P.; Schrauwen, P.; Hesselink, M.K. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010, 59, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Bajpeyi, S.; Pasarica, M.; Moro, C.; Conley, K.; Jubrias, S.; Sereda, O.; Burk, D.H.; Zhang, Z.; Gupta, A.; Kjems, L.; et al. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 1160–1168. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, S.K.; Teede, H.J.; Rachoń, D.; Harrison, C.L.; Strauss, B.J.; Stepto, N.K. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetologia 2012, 55, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Menshikova, E.V.; Distefano, G.; Zheng, D.; Tanner, C.J.; Standley, R.A.; Helbling, N.L.; Dubis, G.S.; Ritov, V.B.; Xie, H.; et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity after Gastric Bypass Surgery. Diabetes 2015, 64, 3737–3750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, H.; Chen, C.; Hood, D. Mitochondria, muscle health, and exercise with advancing age. Physiology 2015, 30, 208–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, D.; Uguccioni, G.; Vainshtein, A.; D’souza, D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: Implications for health and disease. Compr. Physiol. 2011, 1, 1119–1134. [Google Scholar] [PubMed]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; James, N.G.; Scheel, M.M.; Olesen, J.; Holst, J.J.; Pedersen, B.K.; Solomon, T.P. Mechanisms behind the superior effects of interval vs continuous training on glycaemic control in individuals with type 2 diabetes: A randomised controlled trial. Diabetologia 2014, 57, 2081–2093. [Google Scholar] [CrossRef] [Green Version]
- Karstoft, K.; Christensen, C.S.; Pedersen, B.K.; Solomon, T.P. The acute effects of interval- vs continuous- walking exercise on glycemic control in subjects with type 2 diabetes: A crossover, controlled study. J. Clin. Endocrinol. Metab. 2014, 99, 3334–3342. [Google Scholar] [CrossRef] [Green Version]
- Taha, M.M.; Abdeen, H.A.; Abdelsamaia, R.A. High Intensity Interval versus Continuous Moderate Aerobic Training as a Prophylaxsis of Diabetic Nephropathy. Int. J. Diabetes Res. 2016, 5, 14–19. [Google Scholar]
- Cochran, A.J.; Percival, M.E.; Tricarico, S.; Little, J.P.; Cermak, N.; Gillen, J.B.; Tarnopolsky, M.A.; Gibala, M.J. Intermittent and continuous high-intensity exercise training induce similar acute but different chronic muscle adaptations. Exp. Physiol. 2014, 99, 782–791. [Google Scholar] [CrossRef] [Green Version]
- Darmian, M.A.; Hoseini, R.; Amiri, E.; Golshani, S. Downregulated hs-CRP and MAD, upregulated GSH and TAC, and improved metabolic status following combined exercise and turmeric supplementation: A clinical trial in middle-aged women with hyperlipidemic type 2 diabetes. J. Diabetes Metab. Disord. 2022, 21, 275–283. [Google Scholar] [CrossRef]
- Tokmakidis, S.P.; Zois, C.E.; Volaklis, K.A.; Kotsa, K.; Touvra, A.M. The effects of a combined strength and aerobic exercise program on glucose control and insulin action in women with type 2 diabetes. Eur. J. Appl. Physiol. 2004, 92, 437–442. [Google Scholar] [CrossRef]
- Ildarabadi, E.H.; Tabei, M.G.; Khosh, A.M. Effects of Face-To-Face and Online Training on Self-Care of Middle-Aged and Elderly People with Type 2 Diabetes: A Comparative Study. Open Access Maced. J. Med. Sci. 2019, 7, 1214–1219. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, M.; Hakkinen, K.; Ibanez, J.; Garrues, M.; Antón, A.; Zuniga, A.; Larrión, J.L.; Gorostiaga, E.M. Effects of strength training on muscle power and serum hormones in middle-aged and older men. J. Appl. Physiol. 2001, 90, 1497–1507. [Google Scholar] [CrossRef] [Green Version]
- Guezennec, C.Y.; Satabin, P.; Legrand, H.; Bigard, A.X. Physical performance and metabolic changes induced by combined prolonged exercise and different energy intakes in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 525–530. [Google Scholar] [CrossRef]
- Ekblom, B. The muscle biopsy technique. Historical and methodological considerations. Scand. J. Med. Sci. Sports 2017, 27, 458–461. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Kraunsøe, R.; Boushel, R.; Hansen, C.N.; Schjerling, P.; Qvortrup, K.; Støckel, M.; Mikines, K.J.; Dela, F. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J. Physiol. 2010, 588 Pt 12, 2023–2032. [Google Scholar] [CrossRef]
- Rabøl, R.; Svendsen, P.F.; Skovbro, M.; Boushel, R.; Haugaard, S.B.; Schjerling, P.; Schrauwen, P.; Hesselink, M.K.; Nilas, L.; Madsbad, S.; et al. Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism 2009, 58, 1145–1152. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Furuta, M.; Araki-Sasaki, R.; Hori, Y.; Yano, Y.; Adachi, Y. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 2001, 24, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Dincer, Y.; Himmetoglu, S.; Yalin, S.; Damci, T.; Ilkova, H.; Akcay, T. Serum levels of p53 and cytochrome c in subjects with type 2 diabetes and impaired glucose tolerance. Clin. Investig. Med. 2009, 32, E266–E270. [Google Scholar] [CrossRef] [Green Version]
- Alghadir, A.H.; Gabr, S.A.; Al-Eisa, E.S. Effects of Moderate Aerobic Exercise on Cognitive Abilities and Redox State Biomarkers in Older Adults. Oxid. Med. Cell. Longev. 2016, 2016, 2545168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [Green Version]
- Madsen, S.M.; Thorup, A.C.; Overgaard, K.; Jeppesen, P.B. High Intensity Interval Training Improves Glycaemic Control and Pancreatic β Cell Function of Type 2 Diabetes Patients. PLoS ONE 2015, 10, e0133286. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Leonetti, F.; Di Mario, U.; Fallucca, F. Is a long-term aerobic plus resistance training program feasible for and effective on metabolic profiles in type 2 diabetic patients? Diabetes Care 2004, 27, 841–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matoulek, M. Physical activity in patients with microvascular complications of diabetes. Vnitr. Lek. 2015, 61, 340–345. [Google Scholar] [PubMed]
- Tucker, P.S.; Scanlan, A.T.; Dalbo, V.J. High Intensity Interval Training Favourably Affects Angiotensinogen mRNA Expression and Markers of Cardiorenal Health in a Rat Model of Early-Stage ChronicKidney Disease. BioMed Res. Int. 2015, 2015, 156584. [Google Scholar] [CrossRef] [Green Version]
- Giannaki, C.D.; Aphamis, G.; Sakkis, P.; Hadjicharalambous, M. Eight weeks of a combination of high intensity interval training and conventional training reduce visceral adiposity and improve physical fitness: A group-based intervention. J. Sports Med. Phys. Fit. 2016, 56, 483–490. [Google Scholar]
- Marcinko, K.; Sikkema, S.R.; Samaan, M.C.; Kemp, B.E.; Fullerton, M.D.; Steinberg, G.R. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol. Metab. 2015, 4, 903–915. [Google Scholar] [CrossRef]
- Maedler, K.; Donath, M.Y. Beta-cells in type 2 diabetes: A loss of function and mass. Horm. Res. 2004, 62 (Suppl. S3), 67–73. [Google Scholar]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Nishioka, A.; Kobayashi, T.; Kariya, S.; Hamasato, S.; Saibara, T.; Nakayama, K.; Seguchi, H.; Yoshida, S. Mitochondrial cytochrome c release in radiation-induced apoptosis of human peripheral T cells. Int. J. Mol. Med. 2002, 10, 263–268. [Google Scholar] [CrossRef]
- Adeghate, E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: A short review. Mol. Cell. Biochem. 2004, 261, 187–191. [Google Scholar] [CrossRef]
- Zheng, S.J.; Lamhamedi-Cherradi, S.E.; Wang, P.; Xu, L.; Chen, Y.H. Tumor supressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes 2005, 54, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Saleem, A.; Adhihetty, P.J.; Hood, D.A. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol. Genom. 2009, 37, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, J.D.; Hwa Joo, C.; Jeong, T.S.; Louhelainen, J.; Cochran, A.J.; Gibala, M.J.; Gregson, W.; Close, G.L.; Drust, B.; Morton, J.P. Matched work high-intensity interval and continuous running induce similar increases in PGC-1α mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J. Appl. Physiol. 2012, 112, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Saleem, A.; Hood, D.A. Acute exercise induces p53 translocation to the mitochondria and promotes a p53–Tfam–mtDNA complex in skeletal muscle. J. Physiol. 2013, 591, 3625–3636. [Google Scholar] [CrossRef]
- Saleem, A.; Carter, H.N.; Iqbal, S.; Hood, D.A. Role of p53 within the regulatory network controlling muscle mitochondrial biogenesis. Exerc. Sport Sci. Rev. 2011, 39, 199–205. [Google Scholar] [CrossRef]
- Hood, D.A.; Saleem, A. Exercise-induced mitochondrial biogenesis in skeletal muscle. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 332–337. [Google Scholar] [CrossRef]
- Yoshida, Y.; Izumi, H.; Torigoe, T.; Ishiguchi, H.; Itoh, H.; Kang, D.; Kohno, K. P53 physically interacts with mitochondrial transcription factor A differentially regulates binding to damaged DNA. Cancer Res. 2003, 63, 3729–3734. [Google Scholar]
- Achanta, G.; Sasaki, R.; Feng, L.; Carew, J.S.; Lu, W.; Pelicano, H.; Keating, M.J.; Huang, P. Novel role of p53 in maintaining mitochondrial genetic stability through interaction with DNA Pol gamma. EMBO J. 2005, 24, 3482–3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyne, K.; Mannebach, S.; Wuertz, E.; Knaup, K.X.; Mahyar-Roemer, M.; Roemer, K. Identification of a putative p53 binding sequence within the human mitochondrial genome. FEBS Lett. 2004, 578, 198–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.; He, J.; Zhang, Y.; Shao, Y.; Ding, S. Exercise training attenuates oxidative stress and decreases p53 protein content in skeletal muscle of type 2 diabetic Goto-Kakizaki rats. Free Radic. Biol. Med. 2011, 50, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, I.A.; Kravchenko, J.E.; Chumakov, P.M. Homeostatic functions of the p53 tumor suppressor: Regulation of energy metabolism and antioxidant defense. Semin. Cancer Biol. 2009, 19, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Green, D.R.; Chipuk, J.E. p53 and metabolism: Inside the TIGAR. Cell 2006, 126, 30–32. [Google Scholar] [CrossRef] [Green Version]
- Bensaad, K.; Vousden, K.H. p53: New roles in metabolism. Trends Cell Biol. 2007, 17, 286–291. [Google Scholar] [CrossRef]
- DiMauro, S.; Andreu, A.L. Mutations in mitochondrial DNA as a cause of exercise intolerance. Ann. Med. 2001, 33, 472–476. [Google Scholar] [CrossRef]
- Von Kleist-Retzow, J.C.; Schauseil-Zipf, U.; Michalk, D.V.; Kunz, W.S. Mitochondrial diseases—An expanding spectrum of disorders and affected genes. Exp. Physiol. 2003, 88, 155–166. [Google Scholar] [CrossRef]
- Shen, G.X. Mitochondrial dysfunction, oxidative stress and diabetic cardiovascular disorders. Cardiovasc. Hematol. Disord. Drug Targets 2012, 12, 106–112. [Google Scholar] [CrossRef]
- Wu, L.H.; Chang, S.C.; Fu, T.C.; Huang, C.H.; Wang, J.S. High-intensity Interval Training Improves Mitochondrial Function and Suppresses Thrombin Generation in Platelets undergoing Hypoxic Stress. Sci. Rep. 2017, 7, 4191. [Google Scholar] [CrossRef] [Green Version]
- Kessler, H.S.; Sisson, S.B.; Short, K.R. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012, 42, 489–509. [Google Scholar] [CrossRef]
- Little, J.P.; Gillen, J.B.; Percival, M.E.; Safdar, A.; Tarnopolsky, M.A.; Punthakee, Z.; Jung, M.E.; Gibala, M.J. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 2011, 111, 1554–1560. [Google Scholar] [CrossRef] [Green Version]
- Rognmo, Ø.; Hetland, E.; Helgerud, J.; Hoff, J.; Slørdahl, S.A. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 216–222. [Google Scholar] [CrossRef]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-Intensity Interval Training for Patients With Cardiovascular Disease—Is It Safe? A Systematic Review. J. Am. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.X. Oxidative stress and diabetic cardiovascular disorders: Roles of mitochondria and NADPH oxidase. Can. J. Physiol. Pharmacol. 2010, 88, 241–248. [Google Scholar] [CrossRef]
- DiMauro, S.; Schon, E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003, 348, 2656–2668. [Google Scholar] [CrossRef]
- Hoehn, K.L.; Salmon, A.B.; Hohnen-Behrens, C.; Turner, N.; Hoy, A.J.; Maghzal, G.J.; Stocker, R.; Van Remmen, H.; Kraegen, E.W.; Cooney, G.J.; et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 17787–17792. [Google Scholar] [CrossRef]
- Syed, I.; Kyathanahalli, C.N.; Jayaram, B.; Govind, S.; Rhodes, C.J.; Kowluru, R.A.; Kowluru, A. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: Role of Rac1–JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011, 60, 2843–2852. [Google Scholar] [CrossRef] [Green Version]
- Raza, H.; John, A.; Howarth, F.C. Alterations in glutathione redox metabolism, oxidative stress, and mitochondrial function in the left ventricle of elderly Zucker diabetic fatty rat heart. Int. J. Mol. Sci. 2012, 13, 16241–16254. [Google Scholar] [CrossRef] [Green Version]
- Raza, H.; John, A.; Howarth, F.C. Increased metabolic stress in Zucker diabetic fatty rat kidney and pancreas. Cell. Physiol. Biochem. 2013, 32, 1610–1620. [Google Scholar] [CrossRef]
- Raza, H.; Prabu, S.K.; John, A.; Avadhani, N.G. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 3133–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
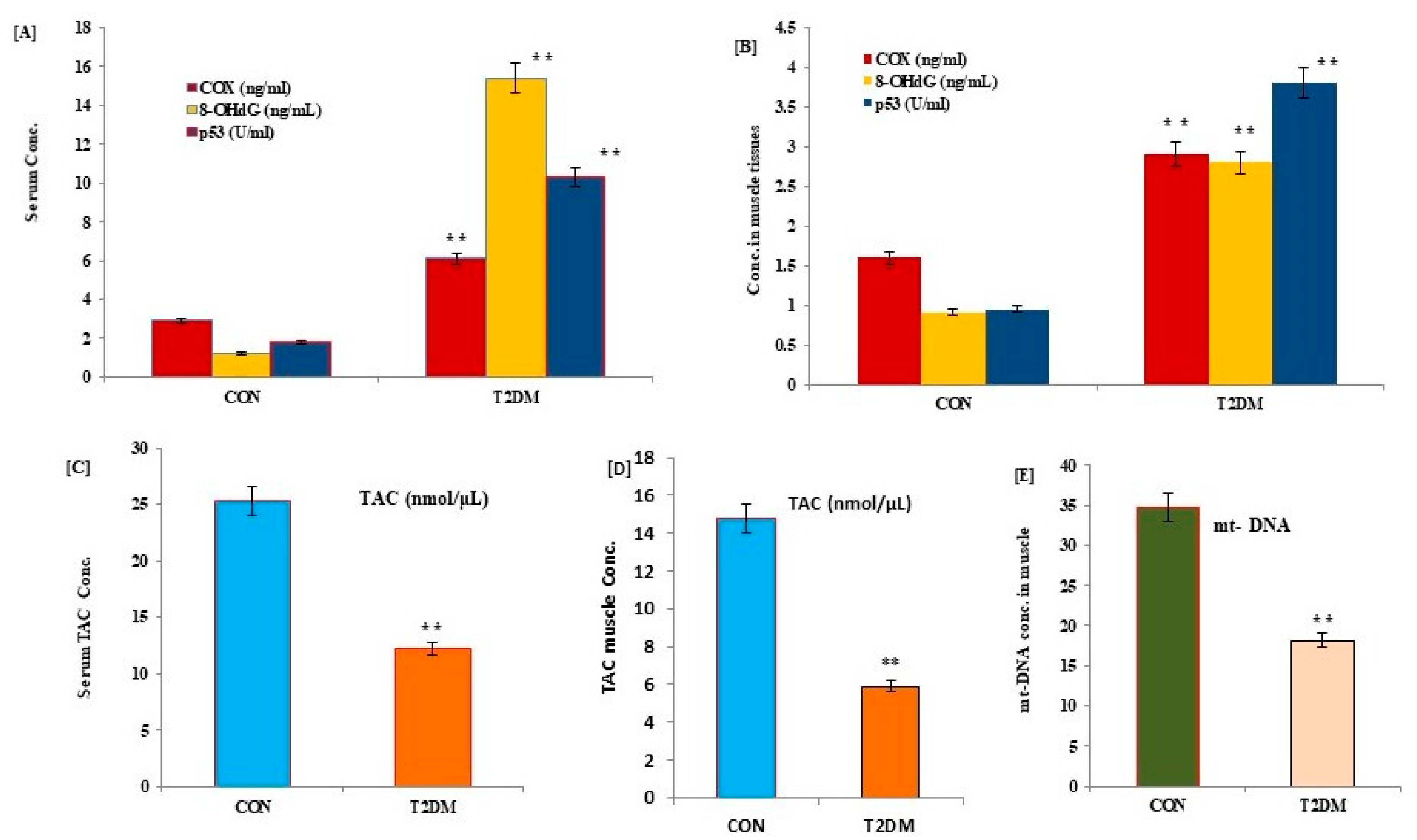


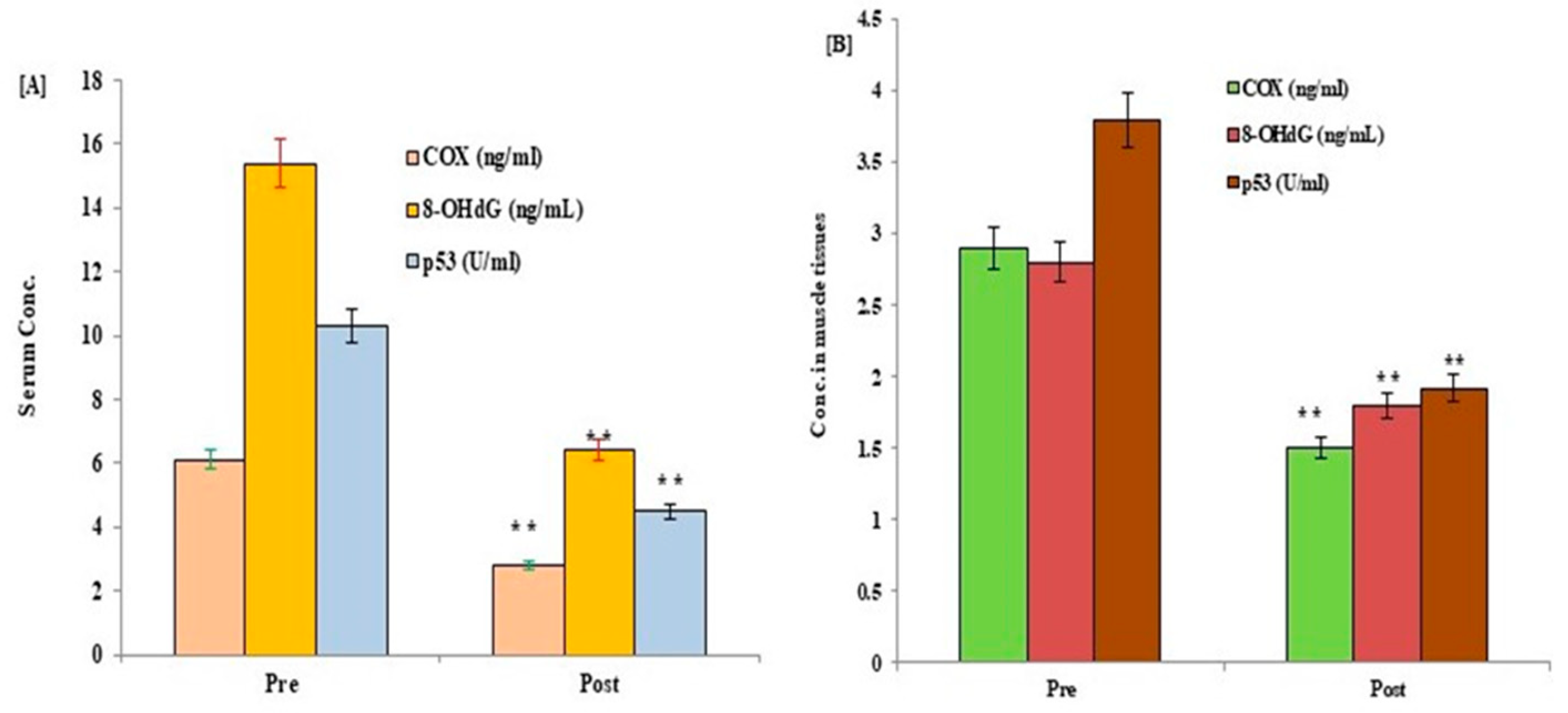

| Parameters | CON (n = 20; Mean Age; 46.3 ± 2.8 Yrs.) | T2DM (n = 30; Mean Age: 46.1 ± 3.1 Yrs.) | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| BMI | 24.5 ± 2.85 | 23.1 ± 1.6 * | 31.8 ± 3.96 | 27.6 ± 2.7 ** |
| Waist (cm) | 98 ± 1.85 | 91.3 ± 1.1 * | 156 ± 3.9 | 148.6 ± 2.4 * |
| Hips (cm) | 115 ± 0.75 | 112 ± 0.81 * | 67 ± 3.7 | 65.5 ± 2.7 * |
| WHR | 0.85 ± 0.95 | 0.46 ± 0.89 * | 2.33 ± 1.2 | 1.6 ± 0.97 ** |
| Fitness score (VO2max; ml/kg × min) | 25.8 ± 2.5 | 34.6 ± 4.6 * | 21.3 ± 1.9 | 32.8 ± 2.8 ** |
| Fasting glucose (mg/dL) | 85.9 ±7.3 | 78.5 ± 2.8 * | 165.2 ± 2.8 | 128.6 ± 3.7 ** |
| Serum C-peptide (ng/mL) | 3.95 ±1.7 | 4. 5 ± 3.9 * | 2.8 ± 1.5 | 5.1 ± 1.5 ** |
| HbA1c (%) | 4.6 ± 0.45 | 3.2 ± 0.65 * | 7.4 ± 1.6 | 5.2 ± 2.5 ** |
| Fasting insulin (FI; μU/mL) | 26.3 ± 7.9 | 32.8 ± 3.6 * | 18.7 ± 5.8 | 35.9 ± 2.6 ** |
| IR (mUmmol/L2) | 5.3 ± 2.6 | 2.8 ± 1.9 * | 12.9 ± 1.9 | 5.9 ± 3.4 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rawaf, H.A.; Gabr, S.A.; Iqbal, A.; Alghadir, A.H. High-Intensity Interval Training Improves Glycemic Control, Cellular Apoptosis, and Oxidative Stress of Type 2 Diabetic Patients. Medicina 2023, 59, 1320. https://doi.org/10.3390/medicina59071320
Al-Rawaf HA, Gabr SA, Iqbal A, Alghadir AH. High-Intensity Interval Training Improves Glycemic Control, Cellular Apoptosis, and Oxidative Stress of Type 2 Diabetic Patients. Medicina. 2023; 59(7):1320. https://doi.org/10.3390/medicina59071320
Chicago/Turabian StyleAl-Rawaf, Hadeel A., Sami A. Gabr, Amir Iqbal, and Ahmad H. Alghadir. 2023. "High-Intensity Interval Training Improves Glycemic Control, Cellular Apoptosis, and Oxidative Stress of Type 2 Diabetic Patients" Medicina 59, no. 7: 1320. https://doi.org/10.3390/medicina59071320
APA StyleAl-Rawaf, H. A., Gabr, S. A., Iqbal, A., & Alghadir, A. H. (2023). High-Intensity Interval Training Improves Glycemic Control, Cellular Apoptosis, and Oxidative Stress of Type 2 Diabetic Patients. Medicina, 59(7), 1320. https://doi.org/10.3390/medicina59071320







