Accuracy of Serological Screening for the Diagnosis of Celiac Disease in Type 1 Diabetes Children
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Detection of Auto-Antibodies
2.3. HLA Typing
2.4. Duodenal Biopsies
2.5. CD Determination
2.6. Ethical Consideration
2.7. Data Analysis
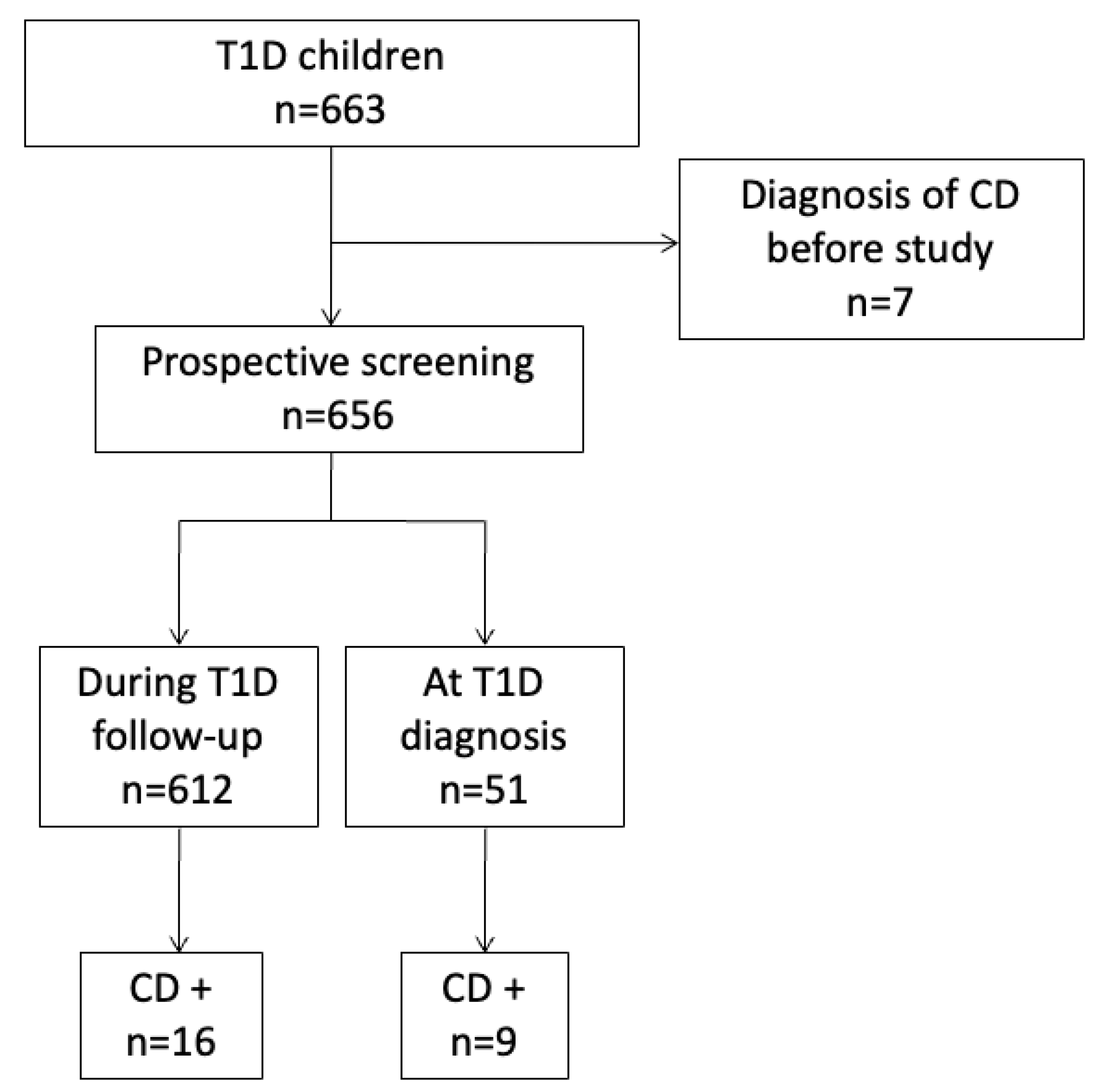
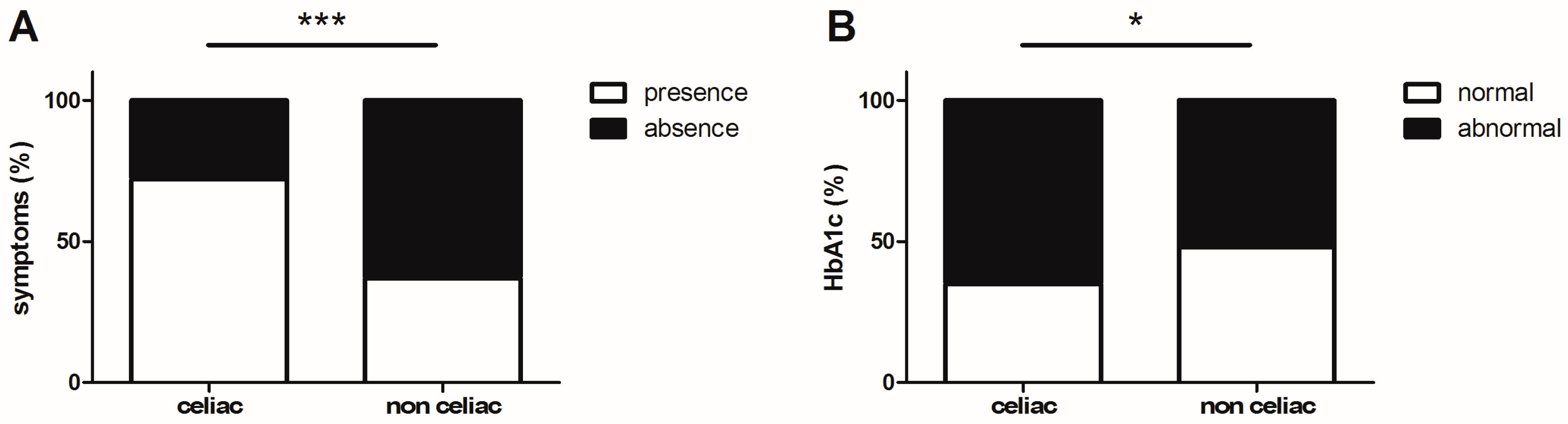
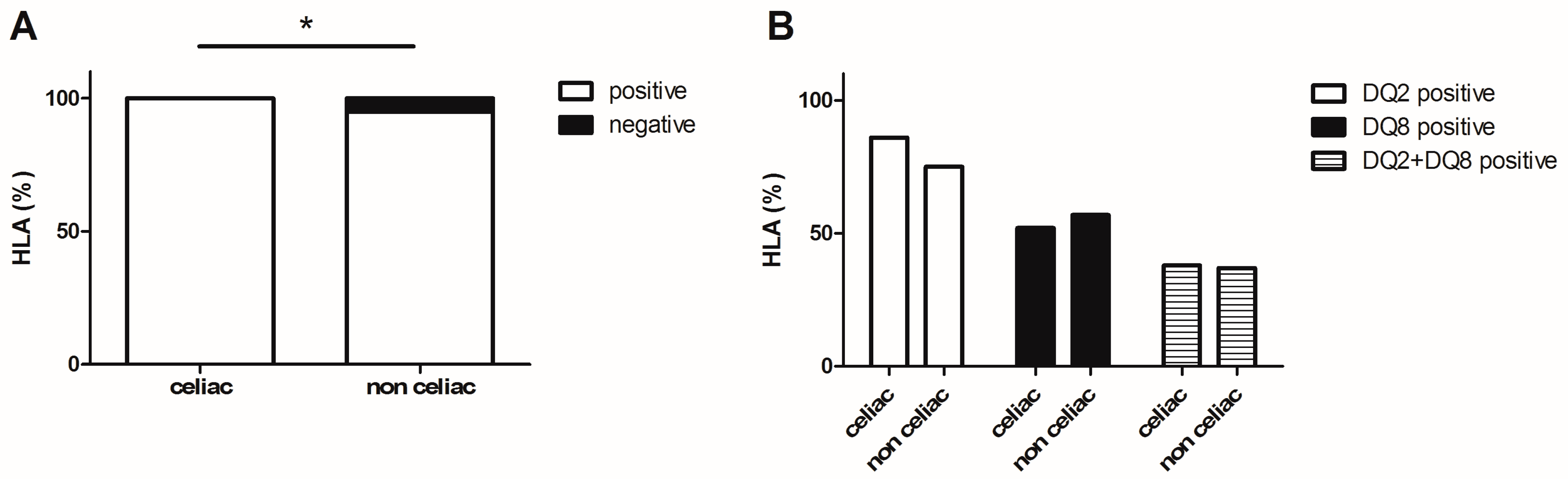
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dube, C.; Rostom, A.; Sy, R.; Cranney, A.; Saloojee, N.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V.; et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: A systematic review. Gastroenterology 2005, 128 (Suppl. S1), S57–S67. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch. Dis. Child. 1990, 65, 909–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieterich, W.; Ehnis, T.; Bauer, M.; Donner, P.; Volta, U.; Riecken, E.O.; Schuppan, D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 1997, 3, 797–801. [Google Scholar] [CrossRef]
- Grodzinsky, E.; Hed, J.; Skogh, T. IgA antiendomysium antibodies have a high positive predictive value for celiac disease in asymptomatic patients. Allergy 1994, 49, 593–597. [Google Scholar] [CrossRef]
- Kaukinen, K.; Partanen, J.; Maki, M.; Collin, P. HLA-DQ typing in the diagnosis of celiac disease. Am. J. Gastroenterol. 2002, 97, 695–699. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werkstetter, K.J.; Korponay-Szabo, I.R.; Popp, A.; Villanacci, V.; Salemme, M.; Heilig, G.; Lillevang, S.T.; Mearin, M.L.; Ribes-Koninckx, C.; Thomas, A.; et al. Accuracy in Diagnosis of Celiac Disease Without Biopsies in Clinical Practice. Gastroenterology 2017, 153, 924–935. [Google Scholar] [CrossRef] [Green Version]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef]
- Patterson, C.C.; Dahlquist, G.G.; Gyurus, E.; Green, A.; Soltesz, G.; Group, E.S. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Triolo, T.M.; Armstrong, T.K.; McFann, K.; Yu, L.; Rewers, M.J.; Klingensmith, G.J.; Eisenbarth, G.S.; Barker, J.M. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care 2011, 34, 1211–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordonouri, O.; Klingensmith, G.; Knip, M.; Holl, R.W.; Aanstoot, H.J.; Menon, P.S.; Craig, M.E.; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Other complications and diabetes-associated conditions in children and adolescents. Pediatr. Diabetes 2014, 15 (Suppl. S20), 270–278. [Google Scholar] [CrossRef] [PubMed]
- Walker-Smith, J.A.; Grigor, W. Coeliac disease in a diabetic child. Lancet 1969, 1, 1021. [Google Scholar] [CrossRef]
- Sud, S.; Marcon, M.; Assor, E.; Palmert, M.R.; Daneman, D.; Mahmud, F.H. Celiac disease and pediatric type 1 diabetes: Diagnostic and treatment dilemmas. Int. J. Pediatr. Endocrinol. 2010, 2010, 161285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Short, A.; Donaghue, K.C.; Ambler, G.; Phelan, H.; Twigg, S.; Craig, M.E. Screening for Celiac Disease in Type 1 Diabetes: A Systematic Review. Pediatrics 2015, 136, e170–e176. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.P.; Sandhu, B.K.; Spray, C.H.; Basude, D.; Ramani, P. Evidence Supporting Serology Based Pathway for Diagnosing Coeliac Disease In Asymptomatic Children From High-Risk Groups. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 641–644. [Google Scholar] [CrossRef]
- Rewers, M.J.; Pillay, K.; de Beaufort, C.; Craig, M.E.; Hanas, R.; Acerini, C.L.; Maahs, D.M.; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr. Diabetes 2014, 15 (Suppl. S20), 102–114. [Google Scholar] [CrossRef]
- Holding, S.; Wilson, F.; Spradbery, D. Clinical evaluation of the BioPlex 2200 Celiac IgA and IgG Kits-a novel multiplex screen incorporating an integral check for IgA deficiency. J. Immunol. Methods 2014, 405, 29–34. [Google Scholar] [CrossRef]
- Marsh, M.N.; Crowe, P.T. Morphology of the mucosal lesion in gluten sensitivity. Baillieres Clin. Gastroenterol. 1995, 9, 273–293. [Google Scholar] [CrossRef]
- Greco, D.; Pisciotta, M.; Gambina, F.; Maggio, F. Celiac disease in subjects with type 1 diabetes mellitus: A prevalence study in western Sicily (Italy). Endocrine 2013, 43, 108–111. [Google Scholar] [CrossRef]
- Atherton, R.; Ross, A.; Jessop, F.; Williams, R.; Heuschkel, R.; Zilbauer, M. Coeliac disease in children with type 1 diabetes: Are current guidelines proving difficult to implement in practice? J. Pediatr. Gastroenterol. Nutr. 2014, 59, 600–603. [Google Scholar] [CrossRef]
- Taler, I.; Phillip, M.; Lebenthal, Y.; de Vries, L.; Shamir, R.; Shalitin, S. Growth and metabolic control in patients with type 1 diabetes and celiac disease: A longitudinal observational case-control study. Pediatr. Diabetes 2012, 13, 597–606. [Google Scholar] [CrossRef]
- Djuric, Z.; Stamenkovic, H.; Stankovic, T.; Milicevic, R.; Brankovic, L.; Ciric, V.; Katic, V. Celiac disease prevalence in children and adolescents with type 1 diabetes from Serbia. Pediatr. Int. 2010, 52, 579–583. [Google Scholar] [CrossRef]
- Sahin, Y.; Cakir, M.D.; Isakoca, M.; Aydin Sahin, D. Prevalence of Celiac Disease in Children with Type 1 Diabetes Mellitus in the South of Turkey. Iran. J. Pediatr. 2020, 30, e97306. [Google Scholar] [CrossRef] [Green Version]
- Taavela, J.; Koskinen, O.; Huhtala, H.; Lahdeaho, M.L.; Popp, A.; Laurila, K.; Collin, P.; Kaukinen, K.; Kurppa, K.; Maki, M. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS ONE 2013, 8, e76163. [Google Scholar] [CrossRef]
- Waisbourd-Zinman, O.; Hojsak, I.; Rosenbach, Y.; Mozer-Glassberg, Y.; Shalitin, S.; Phillip, M.; Shamir, R. Spontaneous normalization of anti-tissue transglutaminase antibody levels is common in children with type 1 diabetes mellitus. Dig. Dis. Sci. 2012, 57, 1314–1320. [Google Scholar] [CrossRef]
- Mitchell, R.T.; Sun, A.; Mayo, A.; Forgan, M.; Comrie, A.; Gillett, P.M. Coeliac screening in a Scottish cohort of children with type 1 diabetes mellitus: Is DQ typing the way forward? Arch. Dis. Child. 2016, 101, 230–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, E.; Loinger, M.; Muhlbacher, A.; Edlinger, M.; Steichen, E.; Meraner, D.; Loacker, L.; Weigel, G.; Muller, T.; Frohlich-Reiterer, E.; et al. Genotyping of coeliac-specific human leucocyte antigen in children with type 1 diabetes: Does this screening method make sense? Arch. Dis. Child. 2017, 102, 603–606. [Google Scholar] [CrossRef]
- Grant, R.W.; Kirkman, M.S. Trends in the evidence level for the American Diabetes Association’s “Standards of Medical Care in Diabetes” from 2005 to 2014. Diabetes Care 2015, 38, 6–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, I.D.; Dirks, M.H.; Liptak, G.S.; Colletti, R.B.; Fasano, A.; Guandalini, S.; Hoffenberg, E.J.; Horvath, K.; Murray, J.A.; Pivor, M.; et al. Guideline for the diagnosis and treatment of celiac disease in children: Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, J.; Hoorweg-Nijman, J.J.; Balemans, W.A. Clinical relevance and cost-effectiveness of HLA genotyping in children with Type 1 diabetes mellitus in screening for coeliac disease in the Netherlands. Diabet. Med. 2015, 32, 834–838. [Google Scholar] [CrossRef]
- Castellaneta, S.; Piccinno, E.; Oliva, M.; Cristofori, F.; Vendemiale, M.; Ortolani, F.; Papadia, F.; Catassi, C.; Cavallo, L.; Francavilla, R. High rate of spontaneous normalization of celiac serology in a cohort of 446 children with type 1 diabetes: A prospective study. Diabetes Care 2015, 38, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Kakleas, K.; Soldatou, A.; Karachaliou, F.; Karavanaki, K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun. Rev. 2015, 14, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Neovius, M.; Hammarstrom, L. Association between IgA deficiency & other autoimmune conditions: A population-based matched cohort study. J. Clin. Immunol. 2014, 34, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kakleas, K.; Karayianni, C.; Critselis, E.; Papathanasiou, A.; Petrou, V.; Fotinou, A.; Karavanaki, K. The prevalence and risk factors for coeliac disease among children and adolescents with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 90, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, A.U.; Agardh, D.; Kivela, L.; Huhtala, H.; Lahdeaho, M.L.; Kaukinen, K.; Kurppa, K. Coeliac patients detected during type 1 diabetes surveillance had similar issues to those diagnosed on a clinical basis. Acta Paediatr. 2017, 106, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Frohlich-Reiterer, E.E.; Kaspers, S.; Hofer, S.; Schober, E.; Kordonouri, O.; Pozza, S.B.; Holl, R.W.; Diabetes Patienten Verlaufsdokumentationssystem-Wiss Study Group. Anthropometry, metabolic control, and follow-up in children and adolescents with type 1 diabetes mellitus and biopsy-proven celiac disease. J. Pediatr. 2011, 158, 589–593.e2. [Google Scholar] [CrossRef]
- Tsouka, A.; Mahmud, F.H.; Marcon, M.A. Celiac Disease Alone and Associated With Type 1 Diabetes Mellitus. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 297–302. [Google Scholar] [CrossRef]
- Pham-Short, A.; Donaghue, K.C.; Ambler, G.; Chan, A.K.; Craig, M.E. Coeliac disease in Type 1 diabetes from 1990 to 2009: Higher incidence in young children after longer diabetes duration. Diabet. Med. 2012, 29, e286–e289. [Google Scholar] [CrossRef]
- Errichiello, S.; Esposito, O.; Di Mase, R.; Camarca, M.E.; Natale, C.; Limongelli, M.G.; Marano, C.; Coruzzo, A.; Lombardo, M.; Strisciuglio, P.; et al. Celiac disease: Predictors of compliance with a gluten-free diet in adolescents and young adults. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 54–60. [Google Scholar] [CrossRef]
- Wagner, G.; Berger, G.; Sinnreich, U.; Grylli, V.; Schober, E.; Huber, W.D.; Karwautz, A. Quality of life in adolescents with treated coeliac disease: Influence of compliance and age at diagnosis. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sud, S.; Marcon, M.; Assor, E.; Daneman, D.; Mahmud, F.H. Quality of life in children with diabetes and celiac disease: Minimal impact of the ‘double diagnosis’. Pediatr. Diabetes 2012, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Pham-Short, A.; Donaghue, K.C.; Ambler, G.; Garnett, S.; Craig, M.E. Quality of Life in Type 1 Diabetes and Celiac Disease: Role of the Gluten-Free Diet. J. Pediatr. 2016, 179, 131–138.e1. [Google Scholar] [CrossRef] [PubMed]
- Mollazadegan, K.; Kugelberg, M.; Montgomery, S.M.; Sanders, D.S.; Ludvigsson, J.; Ludvigsson, J.F. A population-based study of the risk of diabetic retinopathy in patients with type 1 diabetes and celiac disease. Diabetes Care 2013, 36, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Mollazadegan, K.; Sanders, D.S.; Ludvigsson, J.; Ludvigsson, J.F. Long-term coeliac disease influences risk of death in patients with type 1 diabetes. J. Intern. Med. 2013, 274, 273–280. [Google Scholar] [CrossRef]
- Rea, F.; Polito, C.; Marotta, A.; Di Toro, A.; Iovene, A.; Collini, R.; Rea, L.; Sessa, G. Restoration of body composition in celiac children after one year of gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 408–412. [Google Scholar] [CrossRef]
- Assor, E.; Marcon, M.A.; Hamilton, N.; Fry, M.; Cooper, T.; Mahmud, F.H. Design of a dietary intervention to assess the impact of a gluten-free diet in a population with type 1 Diabetes and Celiac Disease. BMC Gastroenterol. 2015, 15, 181. [Google Scholar] [CrossRef] [Green Version]
- Al-Hussaini, A.; Sulaiman, N.; Al-Zahrani, M.; Alenizi, A.; El Haj, I. High prevalence of celiac disease among Saudi children with type 1 diabetes: A prospective cross-sectional study. BMC Gastroenterol. 2012, 12, 180. [Google Scholar] [CrossRef] [Green Version]

| Celiac (n = 32) | Uncertain (n = 12) | Non-Celiac (n = 619) | |
|---|---|---|---|
| Sex ratio | 0.6 | 0.3 | 0.5 |
| Age at diabetes diagnosis (years) | 6.2 ± 0.7 | 5.7 ± 1.1 | 6.6 ± 0.2 |
| Age at study inclusion (years) | 11.3 ± 0.8 ¤¤ | 7.5 ± 0.9 $$ | 11.2 ± 0.2 |
| Weight (SD) | 0.74 ± 0.25 | 0.73 ± 0.45 | 0.98 ± 0.06 |
| Height (SD) | 0.54 ± 0.24 | 0.84 ± 0.38 | 0.74 ± 0.05 |
| HbA1c (%) | 7.9 ± 0.2 *, ¤ | 7.1 ± 0.2 | 7.5 ± 0.4 |
| BMI (z-score) | 0.35 ± 0.23 | 0.10 ± 0.42 | 0.51 ± 0.05 |
| Hb (g/L) | 1.35 ± 0.02 | 1.38 ± 0.03 | 1.35 ± 0.01 |
| Iron (µmol/L) | 14.8 ± 1.4 | 14.3 ± 0.8 | 14.7 ± 0.3 |
| Ferritin (µg/L) | 72.3 ± 16.9 | 58.7 ± 12.1 | 66.9 ± 2.3 |
| ASAT (IU/L) | 25.9 ± 2.0 | 26.8 ± 2.1 | 24.7 ± 0.3 |
| ALAT (IU/L) | 21.3 ± 1.4 | 18.9 ± 2.4 | 18.7 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, C.; De Percin, A.; Morin, C.; Talvard, M.; Fortenfant, F.; Congy-Jolivet, N.; Le Tallec, C.; Olives, J.-P.; Mas, E. Accuracy of Serological Screening for the Diagnosis of Celiac Disease in Type 1 Diabetes Children. Medicina 2023, 59, 1321. https://doi.org/10.3390/medicina59071321
Girard C, De Percin A, Morin C, Talvard M, Fortenfant F, Congy-Jolivet N, Le Tallec C, Olives J-P, Mas E. Accuracy of Serological Screening for the Diagnosis of Celiac Disease in Type 1 Diabetes Children. Medicina. 2023; 59(7):1321. https://doi.org/10.3390/medicina59071321
Chicago/Turabian StyleGirard, Chloé, Aurélie De Percin, Carole Morin, Maeva Talvard, Françoise Fortenfant, Nicolas Congy-Jolivet, Claire Le Tallec, Jean-Pierre Olives, and Emmanuel Mas. 2023. "Accuracy of Serological Screening for the Diagnosis of Celiac Disease in Type 1 Diabetes Children" Medicina 59, no. 7: 1321. https://doi.org/10.3390/medicina59071321
APA StyleGirard, C., De Percin, A., Morin, C., Talvard, M., Fortenfant, F., Congy-Jolivet, N., Le Tallec, C., Olives, J.-P., & Mas, E. (2023). Accuracy of Serological Screening for the Diagnosis of Celiac Disease in Type 1 Diabetes Children. Medicina, 59(7), 1321. https://doi.org/10.3390/medicina59071321






