Association between Bilateral Selective Antegrade Cerebral Perfusion and Postoperative Ischemic Stroke in Patients with Emergency Surgery for Acute Type A Aortic Dissection—Single Centre Experience
Abstract
1. Introduction
2. Methods
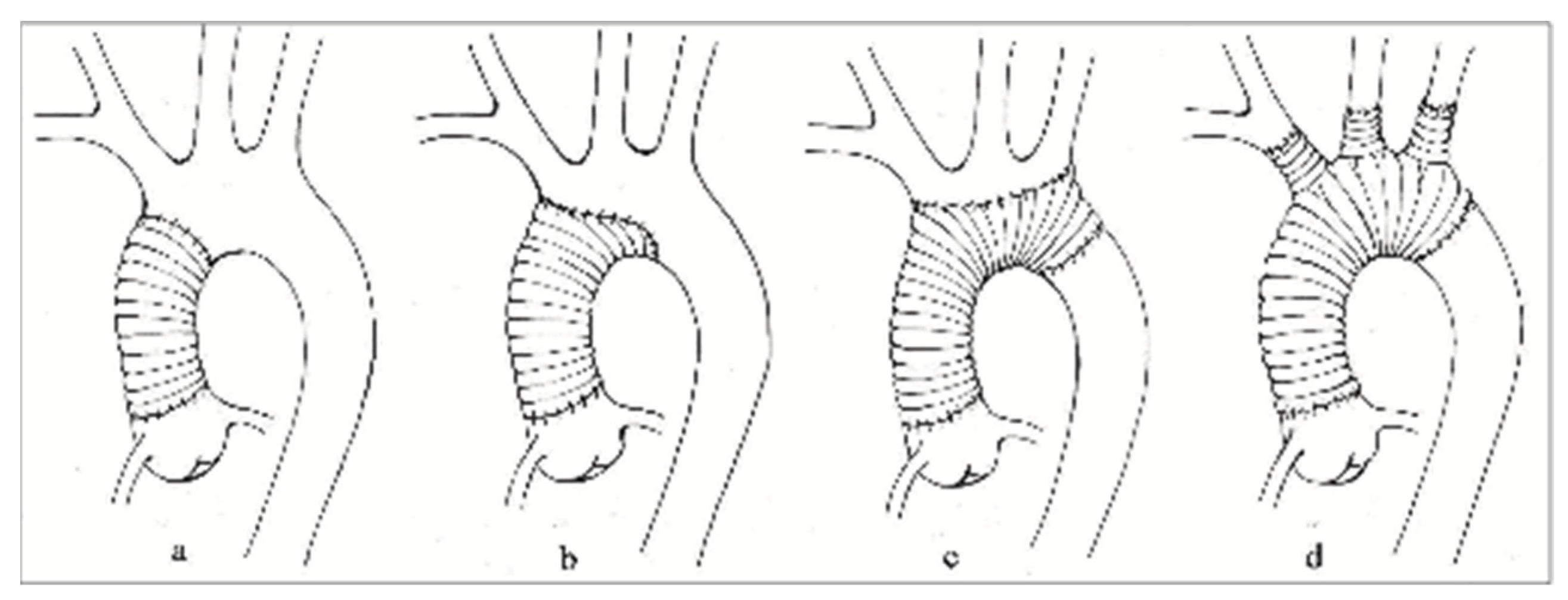
2.1. Surgical Technique
2.2. Statistical Analysis
3. Results
Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, T.T.; Trimarchi, S.; Nienaber, C.A. Acute aortic dissection: Perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur. J. Vasc. Endovasc. Surg. 2009, 37, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Costache, V.S.; Meekel, J.P.; Costache, A.; Melnic, T.; Solomon, C.; Chitic, A.M.; Bucurenciu, C.; Moldovan, H.; Antoniac, I.; Candea, G.; et al. Geometric analysis of type B aortic dissections shows aortic remodeling after intervention using multilayer stents. Materials 2020, 3, 2274. [Google Scholar] [CrossRef] [PubMed]
- Robu, M.; Marian, D.R.; Vasile, R.; Radulescu, B.; Stegaru, A.; Voica, C.; Nica, C.; Gheorghita, D.; Zaharia, O.; Iulian, A.; et al. Delayed Surgical Management of Acute Type A Aortic Dissection in a Patient with Recent COVID-19 Infection and Post-COVID-19 Bronchopneumonia—Case Report and Review of Literature. Medicina 2022, 58, 1357. [Google Scholar] [CrossRef]
- Gemelli, M.; Di Tommaso, E.; Natali, R.; Dixon, L.K.; Mohamed Ahmed, E.; Rajakaruna, C.; Bruno, V.D. Validation of the German Registry for Acute Aortic Dissection Type A Score in predicting 30-day mortality after type A aortic dissection surgery. Eur. J. Cardiothorac. Surg. 2023, 63, ezad141. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.P.; Ergin, M.A.; McCullough, J.N.; Lansman, S.L.; Galla, J.D.; Bodian, C.A.; Apaydin, A.; Griepp, R.B. Results of immediate surgical treatment of all acute type A dissections. Circulation 2000, 102 (Suppl. S3), III248–III252. [Google Scholar] [CrossRef] [PubMed]
- Easo, J.; Weigang, E.; Hölzl, P.P.; Horst, M.; Hoffmann, I.; Blettner, M.; Dapunt, O.E. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection—Analysis of the German Registry for Acute Aortic Dissection type A (GERAADA). Ann. Cardiothorac. Surg. 2013, 2, 175–180. [Google Scholar] [CrossRef]
- Moldovan, H.; Bulescu, C.; Sibisan, A.M.; Tiganasu, R.; Cacoveanu, C.; Nica, C.; Rachieru, A.; Gheorghita, D.; Zaharia, O.; Balanescu, S.; et al. A Large Ascending Aorta Thrombus in a Patient with Acute Myocardial Infarction—Case Report. Medicina 2021, 57, 1176. [Google Scholar] [CrossRef]
- Conzelmann, L.O.; Hoffmann, I.; Blettner, M.; Kallenbach, K.; Karck, M.; Dapunt, O.; Borger, M.A.; Weigang, E.; GERAADA Investigators. Analysis of risk factors for neurological dysfunction in patients with acute aortic dissection type A: Data from the German Registry for Acute Aortic Dissection type A (GERAADA). Eur. J. Cardiothorac. Surg. 2012, 42, 557–565. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Sundt, T.M.; Cameron, D.E.; Holmes, S.D.; Roselli, E.E.; Pasrija, C.; Gammie, J.S.; Patel, H.J.; Bavaria, J.E.; Svensson, L.G.; et al. Factors associated with acute stroke after type A aortic dissection repair: An analysis of the Society of Thoracic Surgeons National Adult Cardiac Surgery Database. J. Thorac. Cardiovasc. Surg. 2020, 159, 2143–2154.e3. [Google Scholar] [CrossRef]
- Dumfarth, J.; Kofler, M.; Stastny, L.; Plaikner, M.; Krapf, C.; Semsroth, S.; Grimm, M. Stroke after emergent surgery for acute type A aortic dissection: Predictors, outcome and neurological recovery. Eur. J. Cardiothorac. Surg. 2018, 53, 1013–1020. [Google Scholar] [CrossRef]
- Svensson, L.G.; Crawford, E.S.; Hess, K.R.; Coselli, J.S.; Raskin, S.; Shenaq, S.A.; Safi, H.J. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J. Thorac. Cardiovasc. Surg. 1993, 106, 19–28; discussion 28–31. [Google Scholar] [CrossRef]
- Dong, S.B.; Xiong, J.X.; Zhang, K.; Zheng, J.; Xu, S.D.; Liu, Y.M.; Sun, L.Z.; Pan, X.D. Different hypothermic and cerebral perfusion strategies in extended arch replacement for acute type a aortic dissection: A retrospective comparative study. J. Cardiothorac. Surg. 2020, 15, 236. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Zhang, X.; Wu, S.; Fang, C.; Pang, X. Effect of different types of cerebral perfusion for acute type A aortic dissection undergoing aortic arch procedure, unilateral versus bilateral. BMC Surg. 2020, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; Scrascia, G.; Vitale, N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact. Cardiovasc. Thorac. Surg. 2008, 7, 891–897. [Google Scholar] [CrossRef]
- Kazui, T.; Inoue, N.; Komatsu, S. Surgical treatment of aneurysms of the transverse aortic arch. J. Cardiovasc. Surg. 1989, 30, 402–406. [Google Scholar]
- Immer, F.F.; Moser, B.; Krähenbühl, E.S.; Englberger, L.; Stalder, M.; Eckstein, F.S.; Carrel, T. Arterial access through the right subclavian artery in surgery of the aortic arch improves neurologic outcome and mid-term quality of life. Ann. Thorac. Surg. 2008, 85, 1614–1618; discussion 1618. [Google Scholar] [CrossRef]
- Zierer, A.; El-Sayed Ahmad, A.; Papadopoulos, N.; Moritz, A.; Diegeler, A.; Urbanski, P.P. Selective antegrade cerebral perfusion and mild (28 °C–30 °C) systemic hypothermic circulatory arrest for aortic arch replacement: Results from 1002 patients. J. Thorac. Cardiovasc. Surg. 2012, 144, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Ergin, M.A.; Galla, J.D.; Lansman, S.L.; Quintana, C.; Bodian, C.; Griepp, R.B. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J. Thorac. Cardiovasc. Surg. 1994, 107, 788–797; discussion 797–799. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Kim, W.K.; Kim, T.H.; Song, S.W. Unilateral versus bilateral antegrade cerebral perfusion during surgical repair for patients with acute type A aortic dissection. JTCVS Open 2022, 11, 37–48. [Google Scholar] [CrossRef]
- Olsson, C.; Thelin, S. Antegrade cerebral perfusion with a simplified technique: Unilateral versus bilateral perfusion. Ann. Thorac. Surg. 2006, 81, 868–874. [Google Scholar] [CrossRef]
- Preventza, O.; Simpson, K.H.; Cooley, D.A.; Cornwell, L.; Bakaeen, F.G.; Omer, S.; Rodriguez, V.; de la Cruz, K.I.; Rosengart, T.; Coselli, J.S. Unilateral versus bilateral cerebral perfusion for acute type A aortic dissection. Ann. Thorac. Surg. 2015, 99, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Halstead, J.C.; Meier, M.; Wurm, M.; Zhang, N.; Spielvogel, D.; Weisz, D.; Bodian, C.; Griepp, R.B. Optimizing selective cerebral perfusion: Deleterious effects of high perfusion pressures. J. Thorac. Cardiovasc. Surg. 2008, 135, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Spielvogel, D.; Tang, G.H. Selective cerebral perfusion for cerebral protection: What we do know. Ann. Cardiothorac. Surg. 2013, 2, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guo, F.; Xu, J.; Zhu, Y.; Wen, D.; Duan, W.; Zheng, M. Preoperative Imaging Risk Findings for Postoperative New Stroke in Patients With Acute Type A Aortic Dissection. Front. Cardiovasc. Med. 2020, 7, 602610. [Google Scholar] [CrossRef] [PubMed]
| Parameter (Unit) | n = 129 (100%) |
|---|---|
| Age (mean, SD) | 59 (11.15) |
| Gender (n, % male) | 83 (64.3%) |
| Euroscore (mean, SD) | 9.03 (2.63) |
| Time from diagnostic CT to surgery (hours) | 4.89 (4.375) |
| Arterial Hypertension (n, %) | 85 (65.9%) |
| Diabetes (n, %) | 8 (6.2%) |
| Dyslipidemia (n, %) | 40 (31%) |
| Chronic Kidney Disease (n, %) | 10 (7.8%) |
| Preoperative Atrial Fibrillation (n, %) | 12 (9.3%) |
| Bicuspid Aortic Valve (n, %) | 11 (8.5%) |
| Cardiac Tamponade at Admission (n, %) | 35 (27.1%) |
| Dissection of Innominate Artery (n, %) | 33 (25.6%) |
| Dissection of Left Common Carotid Artery (n, %) | 21 (16.3%) |
| Dissection of Innominate Artery and Left Common Carotid Artery (n, %) | 9 (6.97%) |
| Severe Aortic Regurgitation (n, %) | 24 (18.6%) |
| Mild left ventricular dysfunction (LVEF 40–50%) (n, %) | 9 (7%) |
| Moderate left ventricle dysfunction (LVEF 30–40%) (n, %) | 2 (1.6%) |
| Severe left ventricle dysfunction (LVEF < 30%) (n, %) | 1 (0.8%) |
| Severe calcifications of ascending aorta or aortic arch (n, %) | 9 (7%) |
| Death Cause | No (%) |
|---|---|
| Cardiogenic shock | 7 (5.4) |
| Septic shock | 12 (9.3) |
| Hemorrhagic stroke | 2 (1.6) |
| Mixed shock (cardiogenic and septic) | 6 (4.7) |
| Parameter (Unit) | n = 129 (100%) |
|---|---|
| Type of operation | |
| Supracoronary ascending aorta and Hemiarch replacement (n, %) | 90 (69.8%) |
| Supracoronary ascending aorta and arch replacement (n, %) | 20 (15.5%) |
| Aortic root, ascending aorta, and Hemiarch replacement (n, %) | 14 (10.9%) |
| Supracoronary ascending aorta replacement (n, %) | 3 (2.3%) |
| Aortic root, ascending aorta, and arch replacement (n, %) | 1 (0.8%) |
| Combined procedures | 13 (10.07%) |
| Mitral valve replacement (n, %) | 4 (3.1%) |
| Coronary artery bypass grafting (n, %) | 6 (4.65%) |
| Peripheral V-A ECMO (n, %) | 1 (0,8%) |
| Femorofemoral Bypass (n, %) | 1 (0.8%) |
| Aortic coarctation repair (n, %) | 1 (0.8%) |
| Cannulation site | |
| Axillary artery (n, %) | 88 (68.2%) |
| Femoral artery (n, %) | 39 (30.2%) |
| Aortic arch (n, %) | 2 (1.6%) |
| Primary entry tear | |
| Ascending aorta/aortic root (n, %) | 56 (43.4%) |
| Aortic arch (n, %) | 42 (32.6%) |
| Ascending aorta/aortic root and aortic arch (n, %) | 12 (9.3%) |
| Not found in the aortic arch or ascending aorta/aortic root (n, %) | 18 (14%) |
| Cardiopulmonary bypass time (min); (mean, SD) | 210 (56,874) |
| Aortic cross-clamp time (min); (mean, SD) | 114,775 (34,602) |
| Cerebral perfusion time (min); (mean, SD) | 37,837 (18,243) |
| Cerebral perfusion below 30 min (n, %) | 57 (44.2) |
| Cerebral perfusion between 30 and 40 min (n, %) | 44 (34.1) |
| Cerebral perfusion over 40 min (n, %) | 41 (31.8) |
| Postoperative atrial fibrillation (n, %) | 34 (26.4) |
| Subsequent intervention for bleeding (n, %) | 34 (26.4) |
| mRS | n/(100%) |
| 0 | 0 (0%) |
| 1 | 3 (9.67%) |
| 2 | 5 (16.12%) |
| 3 | 6 (19.35%) |
| 4 | 4 (12.90%) |
| 5 | 0 (0%) |
| 6 | 13 (41.93%) |
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |
| LCCAD | 2.772 | 1.041–7.381 | 0.041 | 2.941 | 1.034–8.364 | 0.043 |
| Dyslipidemia | 3.048 | 1.077–8.627 | 0.036 | 4.577 | 1.462–14.332 | 0.009 |
| BSACP > 40 | 2.41 | 1.054–5.509 | 0.037 | 3.589 | 1.418–9.085 | 0.007 |
| OR | 95%CI | p | |
|---|---|---|---|
| BSACP 30–40 min | 1.016 | 0.438–2.357 | 0.971 |
| BSACP < 30 min | 0.484 | 0.207–1.128 | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robu, M.; Marian, D.R.; Margarint, I.; Radulescu, B.; Știru, O.; Iosifescu, A.; Voica, C.; Cacoveanu, M.; Ciomag, R.; Gașpar, B.S.; et al. Association between Bilateral Selective Antegrade Cerebral Perfusion and Postoperative Ischemic Stroke in Patients with Emergency Surgery for Acute Type A Aortic Dissection—Single Centre Experience. Medicina 2023, 59, 1365. https://doi.org/10.3390/medicina59081365
Robu M, Marian DR, Margarint I, Radulescu B, Știru O, Iosifescu A, Voica C, Cacoveanu M, Ciomag R, Gașpar BS, et al. Association between Bilateral Selective Antegrade Cerebral Perfusion and Postoperative Ischemic Stroke in Patients with Emergency Surgery for Acute Type A Aortic Dissection—Single Centre Experience. Medicina. 2023; 59(8):1365. https://doi.org/10.3390/medicina59081365
Chicago/Turabian StyleRobu, Mircea, Diana Romina Marian, Irina Margarint, Bogdan Radulescu, Ovidiu Știru, Andrei Iosifescu, Cristian Voica, Mihai Cacoveanu, Raluca Ciomag (Ianula), Bogdan Severus Gașpar, and et al. 2023. "Association between Bilateral Selective Antegrade Cerebral Perfusion and Postoperative Ischemic Stroke in Patients with Emergency Surgery for Acute Type A Aortic Dissection—Single Centre Experience" Medicina 59, no. 8: 1365. https://doi.org/10.3390/medicina59081365
APA StyleRobu, M., Marian, D. R., Margarint, I., Radulescu, B., Știru, O., Iosifescu, A., Voica, C., Cacoveanu, M., Ciomag, R., Gașpar, B. S., Dorobanțu, L., Iliescu, V. A., & Moldovan, H. (2023). Association between Bilateral Selective Antegrade Cerebral Perfusion and Postoperative Ischemic Stroke in Patients with Emergency Surgery for Acute Type A Aortic Dissection—Single Centre Experience. Medicina, 59(8), 1365. https://doi.org/10.3390/medicina59081365








