Blood Biomarkers of Neonatal Sepsis with Special Emphasis on the Monocyte Distribution Width Value as an Early Sepsis Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. Biomarkers
2.3. Inclusion and Exclusion Criteria
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Pathogens and Blood Culture
3.3. Relationship between Peripheral Blood Biomarkers and Neonatal Infections
3.4. Diagnostic Performance of WBC Count, Differential WBC, MDW, and Haematological Parameters in Predicting Sepsis
3.4.1. WBC and Leucocyte Differential Count Biomarkers
3.4.2. RBC Indices
3.4.3. Platelet Biomarkers
3.5. Comparison between the Predictive Values of MDW and Other Biomarkers
3.5.1. ROC Curve Analysis for Sepsis Prediction
3.5.2. Impact of Antibiotic Therapy in Restoring the MDV Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shane, A.L.; Sanchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef]
- Cantey, J.B.; Lee, J.H. Biomarkers for the diagnosis of neonatal sepsis. Clin. Perinatol. 2021, 48, 215–227. [Google Scholar] [CrossRef]
- Chauhan, N.; Tiwari, S.; Jain, U. potential biomarkers for effective screening of neonatal sepsis infection: An overview. Microb. Pathog. 2017, 107, 234–242. [Google Scholar] [CrossRef]
- Brady, M.; Jackson, M.; Kimberlin, D.; Long, S. Red Book, 2018–2021 Report of the Committee on Infectious Diseases; American Academy of Pediatrics: Itaca, IL, USA, 2018. [Google Scholar]
- Al-Matary, A.H.; Heena, A.S.; AlSarheed, W.; Ouda, D.A.; AlShahrani, T.A.; Qaraqei, M.; Abu-Shaheen, A. Characteristics of neonatal Sepsis at a tertiary care hospital in Saudi Arabia. J. Infect. Public Health 2019, 12, 666–672. [Google Scholar] [CrossRef]
- Khan, M.A.; Faiz, A.; Ashshi, A.M. Maternal colonization of group B streptococcus: Prevalence, associated factors and antimicrobial resistance. Ann. Saudi Med. 2015, 35, 423–427. [Google Scholar] [CrossRef]
- Musleh, J.; Al Qahtani, N. Group B streptococcus colonization among Saudi women during labor. Saudi J. Med. Med. Sci. 2018, 6, 18. [Google Scholar]
- Mayer, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Bueno, S.; McCulloh, R.J. Current trend in epidemiology and antimicrobial resistance in neonatal sepsis. In Annual Update in Intensive Care and Emergency Medicine; Vincent, J.L., Ed.; Springer: Cham, Switzerland, 2018; pp. 39–51. [Google Scholar]
- WHO. Available online: https://apps.who.int/iris/bitstream/handle/10665/334216/9789240010789-eng.pdf (accessed on 30 June 2021).
- Seymour, C.W.; Kahn, J.M.; Martin-Gill, C.; Callaway, C.W.; Yealy, D.M.; Scales, D.; Angus, D.C. Delays from First Medical Contact to Antibiotic Administration for Sepsis. Crit. Care Med. 2017, 45, 759–765. [Google Scholar] [CrossRef]
- Molloy, E.J.; Wynn, J.L.; Bliss, J.; Koenig, J.M.; Keij, F.M.; McGovern, M.; Kuester, H.; Turner, M.A.; Giannoni, E.; Mazela, J.; et al. Neonatal sepsis: Need for consensus definition, collaboration and core outcomes. Pediatr. Res. 2020, 88, 2–4. [Google Scholar] [CrossRef]
- Hincu, M.A.; Zonda, G.I.; Stanciu, G.D.; Nemescu, D.; Paduraru, L. Relevance of biomarkers currently in use or research for practical diagnosis approach of neonatal early-onset sepsis. Children 2020, 7, 309. [Google Scholar] [CrossRef]
- Urrechaga, E. Reviewing the value of leukocytes cell population data (CPD) in the management of sepsis. Ann. Transl. Med. 2020, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Parrillo, J.E.; Seymour, C.; Angus, D.C.; Bicking, K.; Tejidor, L.; Magari, R.; Careaga, D.; Williams, J.; Closser, D.R.; et al. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest 2017, 152, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Oh, D.K.; Park, C.J.; Hong, S.B. Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS ONE 2021, 16, e0250101. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Parrillo, J.E.; Martin, G.S.; Huang, D.T.; Hausfater, P.; Grigorov, I.; Careaga, D.; Osborn, T.; Hasan, M.; Tejidor, L. Monocyte distribution width enhances early sepsis detection in the emergency department beyond SIRS and qSOFA. J. Intensive Care 2020, 8, 33. [Google Scholar] [CrossRef]
- Agnello, L.; Iacona, A.; Maestri, S.; Lo Sasso, B.; Giglio, R.V.; Mancuso, S.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Independent Validation of Sepsis Index for Sepsis Screening in the Emergency Department. Diagnostics 2021, 11, 1292. [Google Scholar] [CrossRef]
- Kurul1, Ş.; Simons, S.H.; Ramakers, C.R.; De Rijke, Y.B.; Kornelisse, R.F.; Reiss, I.K.; Taal, H.R. Association of inflammatory biomarkers with subsequent clinical course in suspected late onset sepsis in preterm neonates. Crit. Care 2021, 25, 12. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Waliullah, M.S.; Islam, M.N.; Siddika, M.; Hossain, M.K.; Hossain, M.A. Risk factors, clinical manifestation and bacteriological profile of neonatal sepsis in a tertiary level pediatric hospital. Mymensingh. Med. J. 2009, 18, S66–S72. [Google Scholar] [PubMed]
- Tosson, A.M.; Speer, C.P. Microbial pathogens causative of neonatal sepsis in Arabic countries. J. Matern.-Fetal Neonatal Med. 2011, 24, 990–994. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar]
- Marins, L.R.; Anizelli, L.B.; Romanowski, M.D.; Sarquis, A.L. How does preeclampsia affect neonates? Highlights in the disease’s immunity. J. Matern.-Fetal Neonatal Med. 2019, 32, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Howman, R.A.; Charles, A.K.; Jacques, A.; Doherty, D.A.; Simmer, K.; Strunk, T.; Richmond, P.C.; Cole, C.H.; Burgner, D.P. Inflammatory and haematological markers in the maternal, umbilical cord and infant circulation in histological chorioamnionitis. PLoS ONE 2012, 7, e51836. [Google Scholar] [CrossRef] [PubMed]
- Polilli, E.; Sozio, F.; Frattari, A.; Persichitti, L.; Sensi, M.; Posata, R.; Di Gregorio, M.; Sciacca, A.; Flacco, M.E.; Manzoli, L.; et al. Comparison of Monocyte Distribution Width (MDW) and Procalcitonin for early recognition of sepsis. PLoS ONE 2020, 15, e0227300. [Google Scholar] [CrossRef]
- Fan, S.L.; Miller, N.S.; Lee, J.; Remick, D.G. Diagnosis sepsis—The role of laboratory medicine. Clin. Chim. Acta. 2016, 460, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Plebani, M. can biomarkers help us to better diagnose and manage sepsis? Diagnosis 2015, 2, 81–87. [Google Scholar] [CrossRef]
- Hornik, C.P.; Benjamin, D.K.; Becker, K.C.; Benjamin, D.K., Jr.; Li, J.; Clark, R.H.; Cohen-Wolkowiez, M.; Smith, P.B. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr. Infect. Dis. J. 2012, 31, 799–802. [Google Scholar] [CrossRef]
- Arunachalam, A.R.; Pammi, M. Biomarkers in early-Onset Neonatal Sepsis: An Update. Ann. Clin. Med. Microbio. 2015, 1, 1007. [Google Scholar]
- Gilfillan, M.; Bhandari, V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: Clinical practice guidelines. Early Hum. Dev. 2017, 105, 25–33. [Google Scholar] [CrossRef]
- Ng, P.C.; Ma, T.P.Y.; Lam, H.S. The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotising enterocolitis. Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F448–F452. [Google Scholar] [CrossRef]
- Piva, E.; Zuin, J.; Pelloso, M.; Tosato, F.; Fogar, P.; Plebani, M. Monocyte distribution width (MDW) parameter as a sepsis indicator in intensive care units. Clin. Chem. Lab. Med. 2021, 59, 1307–1314. [Google Scholar] [CrossRef]
- Karne, T.K.; Joshi, D.D.; Zile, U.; Patil, S. Study of platelet count and platelet indices in neonatal sepsis in tertiary care institute. MVP J. Med. Sci. 2017, 4, 55–60. [Google Scholar] [CrossRef]
- Abd-Elrahman, A.A. Early Neonate Sepsis: Hematological Changes and Risk Factors. Ph.D. Thesis, Sudan University of Science and Technology, Khartoum, Sudan, 2016. [Google Scholar]


| Characteristic | Description | Control = 70 | Study Group n = 77 | Study Cases n = 77 | |
|---|---|---|---|---|---|
| Other Health Complications n = 49 | Sepsis n = 28 | ||||
| Blood Culture | Negative | 70 (100.0%) | 49 (100.0%) | 4 (14.29%) | 53 (68.83%) |
| Positive | 0 (0.0) | 0 (0.0) | 24 (85.71%) | 24 (31.17%) | |
| Gender | Female | 34 (48.57%) | 24 (49%) | 14 (50%) | 38 (49.35%) |
| Male | 36 (51.43%) | 25 (51%) | 14 (50%) | 39 (50.65%) | |
| Preterm infant | no | 41 (58.57%) | 17 (34.69%) | 5 (17.86%) | 22 (28.57%) |
| yes | 29 (41.43%) | 32 (65.31%) | 23 (82.14%) | 55 (71.43%) | |
| Gestation age (week) | Mean ± SD | 37.17 ± 3.34 | 35.18 ± 4.22 | 32.86 ± 4.55 | 34.34 ± 4.55 |
| min–max | 30–41 | 24–40 | 24–41 | 24–41 | |
| Age (day) | Mean ± SD | 2.04 ± 2.67 | 2.70 ± 5.50 | 8.51 ± 7.21 | 4.17 ± 6.15 |
| min–max | 1–12 | 1–26 | 1–24 | 1–26 | |
| Baby is well | N/A | 70 (100%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Nationality of neonates | Saudi | Non-Saudi |
| Specimen | Probable specimen | Clotted specimen |
| Anticoagulant tube | EDTA tube for haematological | Other tubes |
| Age | <28 days | ≥28 days |
| Order | CBC and diff | CBC only |
| Antibiotics | Not started antibiotics | Started antibiotics |
| Characteristics | Description | Control n = 70 | Case n = 77 |
|---|---|---|---|
| Sepsis | No | 70 (100.0%) | 49 (63.63%) |
| Yes | 0 (0.0) | 28 (36.37%) | |
| Sepsis type | Early onset ≤ 72 h | 0 (0.0) | 15 (53.6%) |
| Late onset > 72 h | 0 (0.0) | 13 (46.4%) | |
| Organisms | Gram-negative | 0 (0.0) | 9 (37.5%) |
| Gram-positive | 0 (0.0) | 15 (62.5%) | |
| Isolation of the pathogens | Escherichia coli | 0 (0.0) | 5 (6.50%) |
| Enterobacter cloacae | 0 (0.0) | 1 (1.30%) | |
| Klebsiella pneumoniae | 0 (0.0) | 2 (2.6%) | |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 0 (0.0) | 1 (1.30%) | |
| Staphylococcus aureus | 0 (0.0) | 4 (5.19%) | |
| Staphylococcus epidermidis | 0 (0.0) | 6 (7.79%) | |
| Streptococcus agalactiae | 0 (0.0) | 4 (5.19%) | |
| Acinetobacter baumannii | 0 (0.0) | 1 (1.30%) |
| Characteristic | Description | Control (n = 70) | Study (n = 77) | p Value |
|---|---|---|---|---|
| White blood cells (×103/µL) | Mean ± SEM | 15.98 ± 0.70 | 12.16 ± 0.91 | |
| min–max | 9.0–30.0 | 1.5–31.90 | <0.001 | |
| Red blood cells (×106/µL) | Mean ± SEM | 4.88 ± 0.08 | 4.23 ± 0.10 | |
| min–max | 3.80–7.40 | 2.50–6.0 | <0.001 | |
| Haemoglobin (g/L) | Mean ± SEM | 176.00 ± 2.38 | 150.80 ± 3.71 | |
| min–max | 135.0–234.0 | 73.0–207.0 | <0.001 | |
| Haematocrit (%) | Mean ± SEM | 52.10 ± 0.71 | 44.93 ± 1.10 | |
| min–max | 40.10–67.80 | 21.30–60.10 | <0.001 | |
| Red cell distribution width (%) | Mean ± SEM | 16.97 ± 0.13 | 17.76 ± 0.32 | |
| min–max | 14.60–20.4 | 14.0–33.40 | 0.03 | |
| Neutrophil % | Mean ± SEM | 59.38 ± 1.79 | 49.18 ± 2.51 | 0.001 |
| min–max | 13.0–86.10 | 3.70–90.0 | ||
| Lymphocytes % | Mean ± SEM | 27.84 ± 1.72 | 35.58 ± 2.42 | 0.01 |
| min–max | 9.80–84.0 | 3.10–84.90 | ||
| Monocyte Distribution Width | Mean ± SEM | 18.73 ± 0.22 | 24.37 ± 0.82 | <0.001 |
| min–max | 14.90–24 | 15.0–42.0 |
| Characteristic | Other Health Complication (n = 49) | Sepsis (n = 28) | p Value | ||
|---|---|---|---|---|---|
| Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | ||
| Red blood cells (×106/µL) | 2.6–6.0 | 4.39 ± 0.11 | 2.5–6.0 | 3.96 ± 0.17 | 0.03 |
| Haemoglobin (g/L) | 103–207 | 160.14 ±3.89 | 73–200 | 134.6 ± 6.66 | <0.001 |
| Haematocrit (%) | 30.10–60.1 | 47.62 ± 1.15 | 21.3–59.5 | 40.22 ± 2.0 | <0.001 |
| Mean cell volume (fL) | 95–135.9 | 109.02 ±1.19 | 83.0–127.0 | 101.47 ± 1.78 | <0.001 |
| Mean corpuscular haemoglobin (pg) | 31.6–48.10 | 36.93 ± 0.43 | 27.0–40.3 | 33.88 ± 0.60 | <0.001 |
| Red cell distribution width (%) | 14.0–22.6 | 17.42 ± 0.25 | 14.4–33.4 | 18.36 ± 0.76 | 0.16 |
| Mean platelet volume (fL) | 5.9–9.6 | 7.78 ± 0.09 | 5.40–11.20 | 8.85 ± 0.25 | <0.001 |
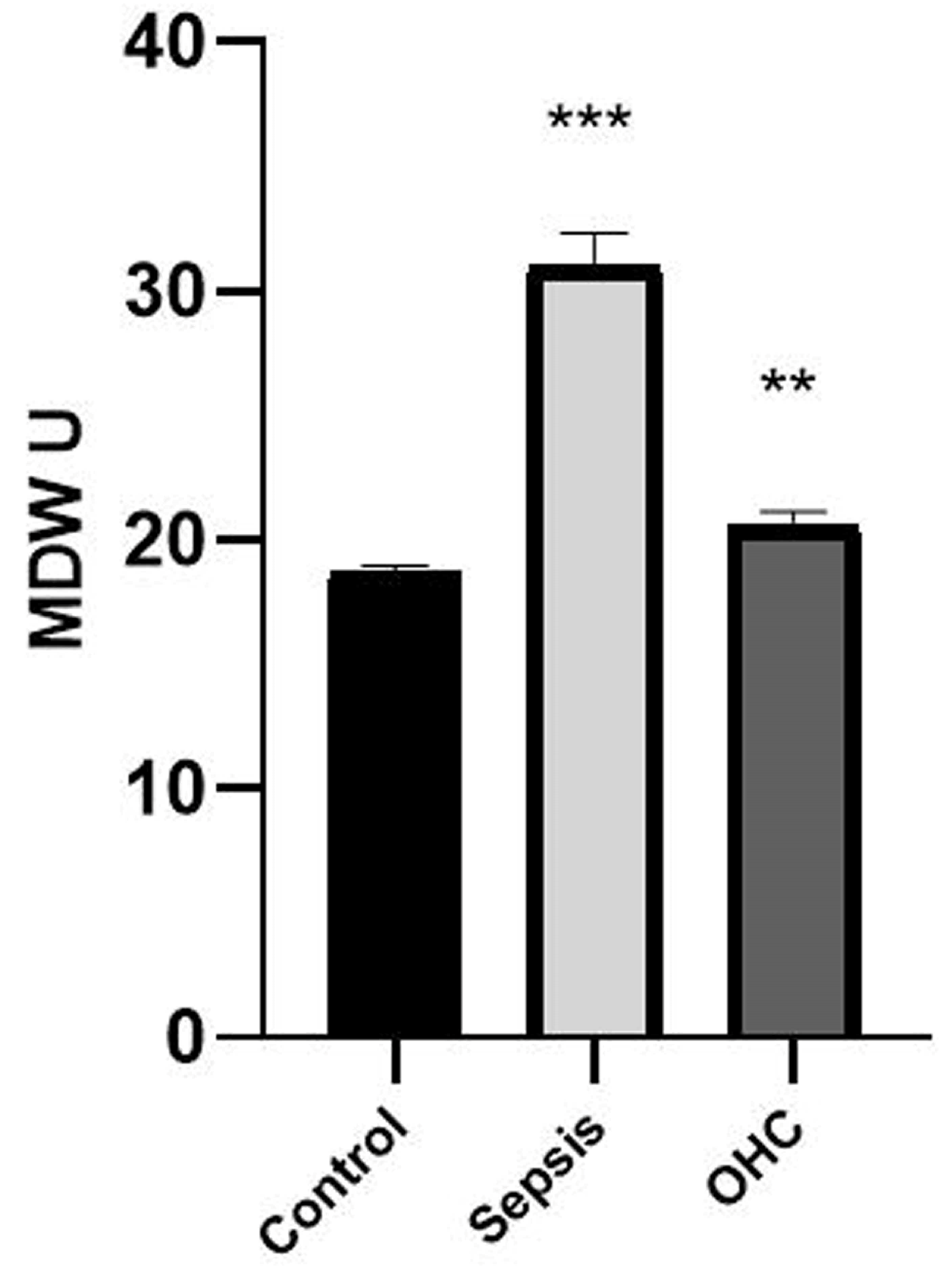
| Monocyte distribution width | 15.0–30.0 | 20.56 ± 0.56 | 17.0–42 | 31.04 ± 1.30 | <0.001 |
| WBC Indices | Description | Normal Control (n = 70) | Other Health Complications (n = 49) | Sepsis (n = 28) | Pearson Chi Square Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | df | p Value | ||||||||
| WBC count | Normal | 68 | 97.1% | 30 | 61.2% | 12 | 42.9% | 42.44 | 4 | <0.001 |
| Leukopenia | - | - | 18 | 36.7% | 13 | 46.4% | ||||
| Leucocytosis | 2 | 2.9% | 1 | 2.0% | 3 | 10.7% | ||||
| MDW | <23 | 69 | 98.6% | 36 | 73.5% | 3 | 10.7% | 79.2 | 2 | <0.001 |
| ≥23 | 1 | 1.4% | 13 | 26.5% | 25 | 89.3% | ||||
| NE% | Normal | 61 | 87.1% | 29 | 59.2% | 15 | 53.6% | 25 | 4 | <0.001 |
| Neutrophilia | 3 | 4.3% | 1 | 2.0% | 5 | 17.9% | ||||
| Neutropenia | 6 | 8.6% | 19 | 38.8% | 8 | 28.6% | ||||
| LY% | Normal | 12 | 17.1% | 6 | 12.2% | 5 | 17.9% | 7.53 | 4 | 0.11 |
| Lymphocytosis | 20 | 28.6% | 26 | 53.1% | 10 | 35.7% | ||||
| Lymphocytopenia | 38 | 54.3% | 17 | 34.7% | 13 | 46.4% | ||||
| MO% | Normal | 13 | 18.6% | 13 | 26.5% | 7 | 25.0% | 1.7 | 4 | 0.792 |
| Monocytosis | 52 | 74.3% | 32 | 65.3% | 18 | 64.3% | ||||
| Monocytopenia | 5 | 7.1% | 4 | 8.2% | 3 | 10.7% | ||||
| EO% | Normal | 66 | 94.3% | 47 | 95.9% | 26 | 92.9% | 0.34 | 2 | 0.842 |
| Eosinophilia | 4 | 5.7% | 2 | 4.1% | 2 | 7.1% | ||||
| BA% | Normal | 51 | 72.9% | 36 | 73.5% | 20 | 71.4% | 0.038 | 2 | 0.981 |
| Basophilia | 19 | 27.1% | 13 | 26.5% | 8 | 28.6% | ||||
| RBC Indices | Description | Normal Control (n = 70) | Other Health Complication (n = 49) | Sepsis (n = 28) | Pearson Chi Square Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | df | p Value | ||||||||
| RBC | Normal | 67 | 95.7% | 38 | 77.6% | 14 | 50.0% | 39.25 | 4 | <0.001 |
| High RBC count | 3 | 4.3% | - | - | - | - | ||||
| Low RBC count | - | - | 11 | 22.4% | 14 | 50.0% | ||||
| Hgb | Normal | 66 | 94.3% | 40 | 81.6% | 12 | 42.9% | 45.02 | 4 | <0.001 |
| High Hgb conc | 3 | 4.3% | - | - | - | - | ||||
| Low Hgb conc | 1 | 1.4% | 9 | 18.4% | 16 | 57.1% | ||||
| HCT | Normal | 61 | 87.1% | 32 | 65.3% | 9 | 32.1% | 37.76 | 4 | <0.001 |
| High HCT | 3 | 4.3% | - | - | - | - | ||||
| Low HCT | 6 | 8.6% | 17 | 34.7% | 19 | 67.9% | ||||
| MCV | Normal | 56 | 80.0% | 35 | 71.4% | 14 | 50.0% | 19.82 | 4 | 0.001 |
| High MCV | 7 | 10.0% | 9 | 18.4% | 2 | 7.1% | ||||
| Low MCV | 7 | 10.0% | 5 | 10.2% | 12 | 42.9% | ||||
| MCH | Normal | 47 | 67.1% | 29 | 59.2% | 21 | 75.0% | 13.05 | 4 | 0.011 |
| High MCH | 22 | 31.4% | 20 | 40.8% | 4 | 14.3% | ||||
| Low MCH | 1 | 1.4% | - | - | 3 | 10.7% | ||||
| MCHC | Normal | 70 | 100.0% | 48 | 98.0% | 27 | 96.4% | 2.15 | 2 | 0.341 |
| High MCHC | - | - | 1 | 2.0% | 1 | 3.6% | ||||
| RDW | Normal | 40 | 57.1% | 25 | 51.0% | 13 | 46.4% | 2.35 | 4 | 0.671 |
| High RDW | 29 | 41.4% | 24 | 49.0% | 15 | 53.6% | ||||
| Low RDW | 1 | 1.4% | - | - | - | - | ||||
| PLT Indices | Description | Normal Control (n = 70) | Other Health Complications (n = 49) | Sepsis (n = 28) | Pearson Chi Square Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | df | p Value | ||||||||
| PLT count | Normal | 61 | 87.1% | 39 | 79.6% | 13 | 46.4% | 20.74 | 4 | <0.001 |
| thrombocytosis | 2 | 2.9% | - | - | 2 | 7.1% | ||||
| thrombocytopenia | 7 | 10.0% | 10 | 20.4% | 13 | 46.4% | ||||
| MPV | Normal | 58 | 82.9% | 42 | 85.7% | 25 | 89.3% | 5.75 | 4 | 0.219 |
| High MPV | - | - | - | - | 1 | 3.6% | ||||
| Low MPV | 12 | 17.1% | 7 | 14.3% | 2 | 7.1% | ||||
| Test Result Variable | Area under ROC Curve | Asymptotic 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| MDW | 0.89 | 0.86 | 0.99 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubaraki, M.A.; Faqihi, A.; AlQhtani, F.; Hafiz, T.A.; Alalhareth, A.; Thagfan, F.A.; Elshanat, S.; Abdel-Gaber, R.A.; Dkhil, M.A. Blood Biomarkers of Neonatal Sepsis with Special Emphasis on the Monocyte Distribution Width Value as an Early Sepsis Index. Medicina 2023, 59, 1425. https://doi.org/10.3390/medicina59081425
Mubaraki MA, Faqihi A, AlQhtani F, Hafiz TA, Alalhareth A, Thagfan FA, Elshanat S, Abdel-Gaber RA, Dkhil MA. Blood Biomarkers of Neonatal Sepsis with Special Emphasis on the Monocyte Distribution Width Value as an Early Sepsis Index. Medicina. 2023; 59(8):1425. https://doi.org/10.3390/medicina59081425
Chicago/Turabian StyleMubaraki, Murad A., Ayman Faqihi, Fatmah AlQhtani, Taghreed A. Hafiz, Ahmed Alalhareth, Felwa A. Thagfan, Sherif Elshanat, Rewaida A. Abdel-Gaber, and Mohamed A. Dkhil. 2023. "Blood Biomarkers of Neonatal Sepsis with Special Emphasis on the Monocyte Distribution Width Value as an Early Sepsis Index" Medicina 59, no. 8: 1425. https://doi.org/10.3390/medicina59081425
APA StyleMubaraki, M. A., Faqihi, A., AlQhtani, F., Hafiz, T. A., Alalhareth, A., Thagfan, F. A., Elshanat, S., Abdel-Gaber, R. A., & Dkhil, M. A. (2023). Blood Biomarkers of Neonatal Sepsis with Special Emphasis on the Monocyte Distribution Width Value as an Early Sepsis Index. Medicina, 59(8), 1425. https://doi.org/10.3390/medicina59081425







