Role of Circular RNAs in Atherosclerosis through Regulation of Inflammation, Cell Proliferation, Migration, and Apoptosis: Focus on Atherosclerotic Cerebrovascular Disease
Abstract
:1. Introduction
2. The Role of CircRNAs in the Pathogenesis of AS
2.1. Endothelial Dysfunction
2.2. Foam Cells Formation and Death
2.3. Fibrous Cap Formation and Atheroma Formation
3. Special CircRNAs
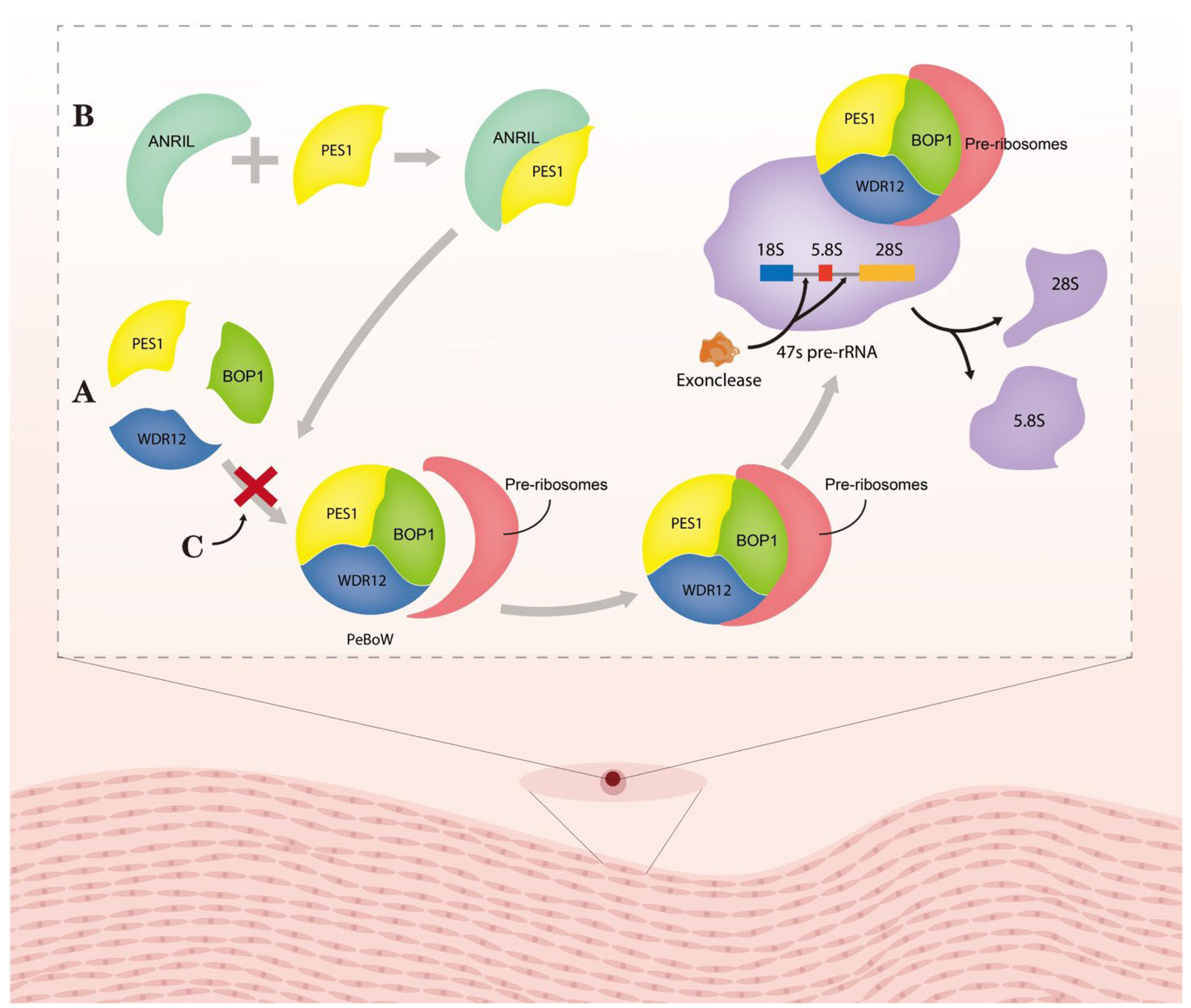
3.1. Circ_ANRIL
3.2. Circ_CHFR
3.3. Circ_USP
4. Clinical Applications of CircRNA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANRIL | antisense non-coding RNA in the INK4 locus |
| Apo- | apolipoprotein |
| AS | atherosclerosis |
| ASPH | aspartyl (asparaginyl) β-hydroxylase |
| CAD | coronary artery disease |
| CHD | coronary heart disease |
| Circ_CHFR | the checkpoint with forkhead-associated and ring-finger domains |
| CircRNAs | circular RNAs |
| Circ_SCAP | circular RNA sterol regulatory element binding transcription factor chaperone |
| Circ_TM7SF3 | circular RNA transmembrane 7 superfamily member 3 |
| Circ_USP36 | circular RNA ubiquitin-specific protease 36 |
| CRP | C-reactive protein |
| DHMEQ | dehydroxymethylepoxyquinomicin |
| EC | endothelial cells |
| EGFR | epidermal growth factor receptor |
| FGF2 | fibroblast growth factor 2 |
| HAEC | human aortic endothelial cell |
| HA-VSMC | human aortic vascular smooth muscle cell |
| HCASMC | human coronary artery smooth muscle cell |
| hMDM | human monocyte-derived macrophage |
| HUVEC | human umbilical vein endothelial cell |
| HUVSMC | human umbilical vein smooth muscle cell |
| HVSMC | human vascular smooth muscle cell |
| IGF2 | insulin-like growth factor 2 |
| KLF5 | kruppel-like factor 5 |
| NF-κB | nuclear factor kappa B |
| NLR | nucleotide binding oligomerization domain-like receptor |
| Nod2 | nucleotide-binding oligomerization domain |
| NOX | NADPH oxidases |
| ox-LDL | oxidized low-density lipoprotein |
| PDE3B | phosphodiesterase 3B |
| PeBoW | PES1–BOP1–WDR12 |
| PES1 | pescadillo homologue 1 |
| SA | stable plaque atherosclerosis |
| SMC | smooth muscle cell |
| UA | unstable/vulnerable plaque atherosclerosis |
| VCAM1 | vascular cell adhesion molecule 1 |
| VECs | vascular endothelial cells |
References
- Zhou, X.; Yu, L.; Zhao, Y.; Ge, J. Panvascular medicine: An emerging discipline focusing on atherosclerotic diseases. Eur. Heart J. 2022, 43, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Gaziano, J.M. Antioxidants and heart disease: Epidemiology and clinical evidence. Clin. Cardiol. 1993, 16, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A. Lipoprotein(a), type 2 diabetes and nephropathy; the mystery continues. J. Nephropathol. 2012, 1, 126–129. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yang, Y.; Wang, Z.; Jiang, S.; Meng, Y.; Song, X.; Zhao, L.; Zou, L.; Li, M.; Yu, T. Targeting non-coding RNAs in unstable atherosclerotic plaques: Mechanism, regulation, possibilities, and limitations. Int. J. Biol. Sci. 2021, 17, 3413–3427. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, C.J.; Gotto, A.M., Jr.; Basson, C.T. The evolving role of statins in the management of atherosclerosis. J. Am. Coll. Cardiol. 2000, 35, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Bakker-Arkema, R.G.; Nawrocki, J.W. An overview of the clinical safety profile of atorvastatin (lipitor), a new HMG-CoA reductase inhibitor. Arch. Intern. Med. 1998, 158, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Stein, E. Cerivastatin in primary hyperlipidemia—A multicenter analysis of efficacy and safety. Atherosclerosis 1998, 139, S15–S22. [Google Scholar] [CrossRef]
- Sabatine, M.S. PCSK9 inhibitors: Clinical evidence and implementation. Nat. Rev. Cardiol. 2019, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, M.A.; Kazi, D.S. PCSK9 Inhibitors: Economics and Policy. J. Am. Coll. Cardiol. 2017, 70, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Z. Efficient backsplicing produces translatable circular mRNAs. RNA 2015, 21, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Burd, C.E.; Jeck, W.R.; Liu, Y.; Sanoff, H.K.; Wang, Z.; Sharpless, N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010, 6, e1001233. [Google Scholar] [CrossRef] [Green Version]
- Lasda, E.; Parker, R. Circular RNAs: Diversity of form and function. RNA 2014, 20, 1829–1842. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Bak, R.O.; Mikkelsen, J.G. miRNA sponges: Soaking up miRNAs for regulation of gene expression. Wiley Interdiscip. Rev. RNA 2014, 5, 317–333. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Ameres, S.L.; Horwich, M.D.; Hung, J.H.; Xu, J.; Ghildiyal, M.; Weng, Z.; Zamore, P.D. Target RNA-directed trimming and tailing of small silencing RNAs. Science 2010, 328, 1534–1539. [Google Scholar] [CrossRef] [Green Version]
- Fuchs Wightman, F.; Giono, L.E.; Fededa, J.P.; de la Mata, M. Target RNAs Strike Back on MicroRNAs. Front. Genet. 2018, 9, 435. [Google Scholar] [CrossRef]
- Kleaveland, B.; Shi, C.Y.; Stefano, J.; Bartel, D.P. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 2018, 174, 350–362.e317. [Google Scholar] [CrossRef] [Green Version]
- De la Mata, M.; Gaidatzis, D.; Vitanescu, M.; Stadler, M.B.; Wentzel, C.; Scheiffele, P.; Filipowicz, W.; Grosshans, H. Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO Rep. 2015, 16, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Qu, S.; Zhong, Y.; Shang, R.; Zhang, X.; Song, W.; Kjems, J.; Li, H. The emerging landscape of circular RNA in life processes. RNA Biol. 2017, 14, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Liu, Z.; Liu, S.; Zhou, X.; Wang, H.; Xu, J.; Wang, D.; Yuan, G. Profile and clinical implication of circular RNAs in human papillary thyroid carcinoma. PeerJ 2018, 6, e5363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, J.; Zhang, Q.; Luo, Q.; Liu, B. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol. Res. 2021, 54, 11. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, W.; Yin, W.; Wang, X.; Wang, J.; Zhu, X.; Xu, S. Circular RNA circ_0090231 promotes atherosclerosis in vitro by enhancing NLR family pyrin domain containing 3-mediated pyroptosis of endothelial cells. Bioengineered 2021, 12, 10837–10848. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 1973, 180, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sasaki, N.; Kasahara, K.; Hirata, K. Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J. Cardiol. 2015, 66, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Berezin, A.; Zulli, A.; Kerrigan, S.; Petrovic, D.; Kruzliak, P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin. Biochem. 2015, 48, 562–568. [Google Scholar] [CrossRef]
- Tavafi, M. Complexity of diabetic nephropathy pathogenesis and design of investigations. J. Renal. Inj. Prev. 2013, 2, 59–62. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Wolf, M.P.; Hunziker, P. Atherosclerosis: Insights into Vascular Pathobiology and Outlook to Novel Treatments. J. Cardiovasc. Transl. Res. 2020, 13, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Harari, F.; Barregard, L.; Ostling, G.; Sallsten, G.; Hedblad, B.; Forsgard, N.; Borne, Y.; Fagerberg, B.; Engstrom, G. Blood Lead Levels and Risk of Atherosclerosis in the Carotid Artery: Results from a Swedish Cohort. Environ. Health Perspect. 2019, 127, 127002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Chun, Y.; Lian, Z.Q.; Yong, Z.W.; Lan, Y.M.; Huan, L.; Xi, C.Y.; Juan, L.S.; Qing, Z.W.; Jia, C.; et al. circRNA-0006896-miR1264-DNMT1 axis plays an important role in carotid plaque destabilization by regulating the behavior of endothelial cells in atherosclerosis. Mol. Med. Rep. 2021, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, H.; Maeda, M.; Ichikawa, N.; Miura, Y.; Umeda, Y.; Hatazaki, S.; Toma, N.; Asakura, F.; Suzuki, H.; Sakaida, H.; et al. High-risk plaque for carotid artery stenting evaluated with 3-dimensional T1-weighted gradient echo sequence. Stroke 2013, 44, 105–110. [Google Scholar] [CrossRef]
- Yamada, K.; Yoshimura, S.; Kawasaki, M.; Enomoto, Y.; Asano, T.; Hara, A.; Minatoguchi, S.; Iwama, T. Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis 2011, 215, 399–404. [Google Scholar] [CrossRef]
- Wang, Z.; Ni, L.; Wang, J.; Lu, C.; Ren, M.; Han, W.; Liu, C. The protective effect of melatonin on smoke-induced vascular injury in rats and humans: A randomized controlled trial. J. Pineal Res. 2016, 60, 217–227. [Google Scholar] [CrossRef]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Bian, W.; Jing, X.; Yang, Z.; Shi, Z.; Chen, R.; Xu, A.; Wang, N.; Jiang, J.; Yang, C.; Zhang, D.; et al. Downregulation of LncRNA NORAD promotes Ox-LDL-induced vascular endothelial cell injury and atherosclerosis. Aging 2020, 12, 6385–6400. [Google Scholar] [CrossRef]
- Di Pietro, N.; Formoso, G.; Pandolfi, A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul. Pharmacol. 2016, 84, 1–7. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Wang, Z.; Gong, W.; Zhang, C. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics 2021, 11, 5404–5417. [Google Scholar] [CrossRef]
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 1998, 9, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Williams, K.J.; Boren, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, X.; Yang, L. Circular RNA circ_0001445 alleviates the ox-LDL-induced endothelial injury in human primary aortic endothelial cells through regulating ABCG1 via acting as a sponge of miR-208b-5p. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 779–792. [Google Scholar] [CrossRef]
- Lei, X.; Yang, Y. Oxidized low-density lipoprotein contributes to injury of endothelial cells via the circ_0090231/miR-9-5p/TXNIP axis. Cent. Eur. J. Immunol. 2022, 47, 41–57. [Google Scholar] [CrossRef]
- Li, X.; Kang, X.; Di, Y.; Sun, S.; Yang, L.; Wang, B.; Ji, Z. CircCHMP5 Contributes to Ox-LDL-induced Endothelial Cell Injury Through the Regulation of MiR-532-5p/ROCK2 axis. Cardiovasc. Drugs Ther. 2022. [Google Scholar] [CrossRef]
- Sawada, N.; Liao, J.K. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid. Redox Signal. 2014, 20, 1251–1267. [Google Scholar] [CrossRef] [Green Version]
- Shimokawa, H.; Rashid, M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol. Sci. 2007, 28, 296–302. [Google Scholar] [CrossRef]
- Shang, L.; Quan, A.; Sun, H.; Xu, Y.; Sun, G.; Cao, P. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene 2019, 711, 143948. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.J.; Wang, L.; Li, H.; Liu, Z.P. FoxO4 inhibits atherosclerosis through its function in bone marrow derived cells. Atherosclerosis 2011, 219, 492–498. [Google Scholar] [CrossRef] [Green Version]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrecher, U.P.; Parthasarathy, S.; Leake, D.S.; Witztum, J.L.; Steinberg, D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc. Natl. Acad. Sci. USA 1984, 81, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dubland, J.A.; Allahverdian, S.; Asonye, E.; Sahin, B.; Jaw, J.E.; Sin, D.D.; Seidman, M.A.; Leeper, N.J.; Francis, G.A. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 876–887. [Google Scholar] [CrossRef]
- Corsini, A.; Bernini, F.; Quarato, P.; Donetti, E.; Bellosta, S.; Fumagalli, R.; Paoletti, R.; Soma, V.M. Non-lipid-related effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Cardiology 1996, 87, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Wang, Y.; Zou, T.; Zhu, H.; Lu, X.; Li, L.; Yang, B.; Chen, J.; Chen, S.; et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis 2019, 286, 88–96. [Google Scholar] [CrossRef]
- Wang, X.; Bai, M. CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc. Disord. 2021, 21, 51. [Google Scholar] [CrossRef]
- He, Q.; Shao, D.; Hao, S.; Yuan, Y.; Liu, H.; Liu, F.; Mu, Q. CircSCAP Aggravates Oxidized Low-density Lipoprotein-induced Macrophage Injury by Upregulating PDE3B by miR-221-5p in Atherosclerosis. J. Cardiovasc. Pharmacol. 2021, 78, e749–e760. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Napolitano, G. The Role of the Transcription Factor Nuclear Factor-kappa B in Thyroid Autoimmunity and Cancer. Front. Endocrinol. 2018, 9, 471. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinet, W.; Coornaert, I.; Puylaert, P.; De Meyer, G.R.Y. Macrophage Death as a Pharmacological Target in Atherosclerosis. Front. Pharmacol. 2019, 10, 306. [Google Scholar] [CrossRef]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell. Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Chen, H.; Zhang, J.; Zhou, K.; Zhuge, Y.; Niu, C.; Qiu, J.; Rong, X.; Shi, Z.; Xiao, J.; et al. Role of pyroptosis in cardiovascular diseases. Int. Immunopharmacol. 2019, 67, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rajamaki, K.; Lappalainen, J.; Oorni, K.; Valimaki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, A.S.; Guarda, G.; D’Ombrain, M.C.; Drexler, S.K. Inflammatory caspases in innate immunity and inflammation. J. Innate Immun. 2010, 2, 228–237. [Google Scholar] [CrossRef]
- Evavold, C.L.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e6. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Li, Y.; Peng, X.; Huang, D.; Gui, L.; Huang, B. Resistance of mitochondrial DNA-depleted cells against oxidized low-density lipoprotein-induced macrophage pyroptosis. Mol. Med. Rep. 2016, 13, 4393–4399. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Yan, R.; Ji, Q.; Yao, H.; Sun, M.; Duan, L.; Xue, Z.; Jia, Y. IFN regulatory Factor-1 induced macrophage pyroptosis by modulating m6A modification of circ_0029589 in patients with acute coronary syndrome. Int. Immunopharmacol. 2020, 86, 106800. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, L.; Zhao, Y.; Fei, J.; Zhang, W. Circular RNA circ_0029589 regulates proliferation, migration, invasion, and apoptosis in ox-LDL-stimulated VSMCs by regulating miR-424-5p/IGF2 axis. Vascul. Pharmacol. 2020, 135, 106782. [Google Scholar] [CrossRef] [PubMed]
- Gerthoffer, W.T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007, 100, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.I.; Pierre-Paul, D.; Sumpio, B.E.; Gahtan, V. Vascular smooth muscle cell migration: Current research and clinical implications. Vasc. Endovascular. Surg. 2004, 38, 11–23. [Google Scholar] [CrossRef]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Campbell, G.R.; Campbell, J.H. The phenotypes of smooth muscle expressed in human atheroma. Ann. N. Y. Acad. Sci. 1990, 598, 143–158. [Google Scholar] [CrossRef]
- Thyberg, J.; Blomgren, K.; Hedin, U.; Dryjski, M. Phenotypic modulation of smooth muscle cells during the formation of neointimal thickenings in the rat carotid artery after balloon injury: An electron-microscopic and stereological study. Cell Tissue Res. 1995, 281, 421–433. [Google Scholar] [CrossRef]
- Pan, J.; Cai, Y.; Liu, M.; Li, Z. Role of vascular smooth muscle cell phenotypic switching in plaque progression: A hybrid modeling study. J. Theor. Biol. 2021, 526, 110794. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.L.; Gabbiani, G. Modulation of smooth muscle cell proliferation and migration: Role of smooth muscle cell heterogeneity. In Atherosclerosis: Diet and Drugs; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Dobnikar, L.; Taylor, A.L.; Chappell, J.; Oldach, P.; Harman, J.L.; Oerton, E.; Dzierzak, E.; Bennett, M.R.; Spivakov, M.; Jorgensen, H.F. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun. 2018, 9, 4567. [Google Scholar] [CrossRef] [Green Version]
- Feil, S.; Fehrenbacher, B.; Lukowski, R.; Essmann, F.; Schulze-Osthoff, K.; Schaller, M.; Feil, R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 2014, 115, 662–667. [Google Scholar] [CrossRef]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Cui, L.; Yuan, J.; Zhang, Y.; Sang, H. Circular RNA WDR77 target FGF-2 to regulate vascular smooth muscle cells proliferation and migration by sponging miR-124. Biochem. Biophys. Res. Commun. 2017, 494, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, T.; Pi, S.; Huang, L.; Liu, Y. Suppression of circular RNA circDHCR24 alleviates aortic smooth muscle cell proliferation and migration by targeting miR-149-5p/MMP9 axis. Biochem. Biophys. Res. Commun. 2020, 529, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tang, T.; Lu, J.; Wang, J. Circ_UBR4 Knockdown Alleviates Oxidized Low-Density Lipoprotein-Provoked Growth and Migration of Human Vascular Smooth Muscle Cells by Acting on the miR-637/FOXO4 Pathway. J. Cardiovasc. Pharmacol. 2021, 78, 534–543. [Google Scholar] [CrossRef]
- Sun, C.; Li, J.; Li, Y.; Li, L.; Huang, G. Circular RNA circUBR4 regulates ox-LDL-induced proliferation and migration of vascular smooth muscle cells through miR-185-5p/FRS2 axis. Mol. Cell. Biochem. 2021, 476, 3899–3910. [Google Scholar] [CrossRef]
- Peng, H.; Liu, S.; Li, Y.; Wang, C.; Zhong, Y. A Novel circUBR4/miR-491-5p/NRP2 ceRNA Network Regulates Oxidized Low-density Lipoprotein-induced Proliferation and Migration in Vascular Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2022, 79, 512–522. [Google Scholar] [CrossRef]
- Tetreault, M.P.; Yang, Y.; Katz, J.P. Kruppel-like factors in cancer. Nat. Rev. Cancer 2013, 13, 701–713. [Google Scholar] [CrossRef]
- Alaiti, M.A.; Orasanu, G.; Tugal, D.; Lu, Y.; Jain, M.K. Kruppel-like factors and vascular inflammation: Implications for atherosclerosis. Curr. Atheroscler. Rep. 2012, 14, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Arkenbout, E.K.; Dekker, R.J.; de Vries, C.J.; Horrevoets, A.J.; Pannekoek, H. Focusing on transcription factor families in atherogenesis: The function of LKLF and TR3. Thromb. Haemost. 2003, 89, 522–529. [Google Scholar] [CrossRef]
- Fang, Y.; Davies, P.F. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, Y.; Kurabayashi, M.; Kanda, T.; Hasegawa, A.; Sakamoto, H.; Okamoto, E.; Kowase, K.; Watanabe, N.; Manabe, I.; Suzuki, T.; et al. Regulated expression of the BTEB2 transcription factor in vascular smooth muscle cells: Analysis of developmental and pathological expression profiles shows implications as a predictive factor for restenosis. Circulation 2000, 102, 2528–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, T.; Kurabayashi, M.; Hoshino, Y.; Ishikawa, S.; Takeyoshi, I.; Morishita, Y.; Nagai, R. Inducible expression of BTEB2, a member of the zinc-finger family of transcription factors, in cardiac allograft arteriosclerosis. Transplant. Proc. 2000, 32, 2032–2033. [Google Scholar] [CrossRef]
- Courboulin, A.; Tremblay, V.L.; Barrier, M.; Meloche, J.; Jacob, M.H.; Chapolard, M.; Bisserier, M.; Paulin, R.; Lambert, C.; Provencher, S.; et al. Kruppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir. Res. 2011, 12, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, R.; Suzuki, T.; Aizawa, K.; Miyamoto, S.; Amaki, T.; Kawai-Kowase, K.; Sekiguchi, K.I.; Kurabayashi, M. Phenotypic modulation of vascular smooth muscle cells: Dissection of transcriptional regulatory mechanisms. Ann. N. Y. Acad. Sci. 2001, 947, 56–66, discussion 66–67. [Google Scholar] [CrossRef]
- Aizawa, K.; Suzuki, T.; Kada, N.; Ishihara, A.; Kawai-Kowase, K.; Matsumura, T.; Sasaki, K.; Munemasa, Y.; Manabe, I.; Kurabayashi, M.; et al. Regulation of platelet-derived growth factor-A chain by Kruppel-like factor 5: New pathway of cooperative activation with nuclear factor-kappaB. J. Biol. Chem. 2004, 279, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bateman, N.W.; Tan, D.; Pestell, R.G.; Black, J.D.; Black, A.R. Intestinal tumor progression is associated with altered function of KLF5. J. Biol. Chem. 2004, 279, 12093–12101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandan, M.O.; Chanchevalap, S.; Dalton, W.B.; Yang, V.W. Kruppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 2005, 579, 4757–4762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Lu, Y.H.; Wang, X.; Zhang, X.J. Circ_USP36/miR-182-5p/KLF5 axis regulates the ox-LDL-induced injury in human umbilical vein smooth muscle cells. Am. J. Transl. Res. 2020, 12, 7855–7869. [Google Scholar]
- Sun, J.; Zhang, Z.; Yang, S. Circ_RUSC2 upregulates the expression of miR-661 target gene SYK and regulates the function of vascular smooth muscle cells. Biochem. Cell Biol. 2019, 97, 709–714. [Google Scholar] [CrossRef]
- Zhang, L.L. CircRNA-PTPRA promoted the progression of atherosclerosis through sponging with miR-636 and upregulating the transcription factor SP1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12437–12449. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, H.; Yang, A.; Ma, P.; Sun, L.; Deng, M.; Mao, C.; Xiong, J.; Sun, J.; Wang, N.; et al. Homocysteine accelerates atherosclerosis by inhibiting scavenger receptor class B member1 via DNMT3b/SP1 pathway. J. Mol. Cell. Cardiol. 2020, 138, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.J.; Zhao, G.J.; Chen, W.J.; Zhang, M.; Zeng, G.F.; Zheng, X.L.; Tang, C.K. Hsp27 promotes ABCA1 expression and cholesterol efflux through the PI3K/PKCzeta/Sp1 pathway in THP-1 macrophages. Eur. J. Pharmacol. 2017, 810, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Deniaud, E.; Baguet, J.; Mathieu, A.L.; Pages, G.; Marvel, J.; Leverrier, Y. Overexpression of Sp1 transcription factor induces apoptosis. Oncogene 2006, 25, 7096–7105. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Hu, Y.; Lou, J.; Yin, S.; Wang, W.; Wang, Y.; Xia, Y.; Wu, W. CircRNA-0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR-107. Mol. Med. Rep. 2019, 19, 3923–3932. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012, 36, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.Y.; Lv, R.R.; Teng, Z. Circular RNA circHIPK3 as a novel circRNA regulator of autophagy and endothelial cell dysfunction in atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12849–12858. [Google Scholar] [CrossRef]
- Kong, Y.; Hsieh, C.H.; Alonso, L.C. ANRIL: A lncRNA at the CDKN2A/B Locus with Roles in Cancer and Metabolic Disease. Front. Endocrinol. 2018, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Skroblin, P.; Mayr, M. “Going long”: Long non-coding RNAs as biomarkers. Circ. Res. 2014, 115, 607–609. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Zhao, H.Y.; Zhang, X.B.; Gao, X.L.; Peng, W.P.; Zhou, Y.; Zhao, W.H.; Yang, H.F. LncRNA ANRIL regulates cell proliferation and migration via sponging miR-339-5p and regulating FRS2 expression in atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1956–1969. [Google Scholar] [CrossRef]
- Song, C.L.; Wang, J.P.; Xue, X.; Liu, N.; Zhang, X.H.; Zhao, Z.; Liu, J.G.; Zhang, C.P.; Piao, Z.H.; Liu, Y.; et al. Effect of Circular ANRIL on the Inflammatory Response of Vascular Endothelial Cells in a Rat Model of Coronary Atherosclerosis. Cell. Physiol. Biochem. 2017, 42, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.J.; Liu, C.A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanderLaan, P.A.; Reardon, C.A.; Getz, G.S. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Han, X.; Wittfeldt, A.; Sun, J.; Liu, C.; Wang, X.; Gan, L.M.; Cao, H.; Liang, Z. Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-κB pathway. RNA Biol. 2016, 13, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Guo, Y.; Liu, J.; Chen, S.; Wang, X.; Zhao, H.; Zuo, T.; Hu, Q.; Dong, Z. Long noncoding RNA ANRIL knockdown attenuates neuroinflammation following ischemic stroke via suppressing the expression of NF-κB in vitro and in vivo. Neurol. Res. 2021, 43, 767–777. [Google Scholar] [CrossRef]
- Gareus, R.; Kotsaki, E.; Xanthoulea, S.; van der Made, I.; Gijbels, M.J.; Kardakaris, R.; Polykratis, A.; Kollias, G.; de Winther, M.P.; Pasparakis, M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008, 8, 372–383. [Google Scholar] [CrossRef] [Green Version]
- Chiba, T.; Kondo, Y.; Shinozaki, S.; Kaneko, E.; Ishigami, A.; Maruyama, N.; Umezawa, K.; Shimokado, K. A selective NFkappaB inhibitor, DHMEQ, reduced atherosclerosis in ApoE-deficient mice. J. Atheroscler. Thromb. 2006, 13, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Bouchier-Hayes, L.; Conroy, H.; Egan, H.; Adrain, C.; Creagh, E.M.; MacFarlane, M.; Martin, S.J. CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF-kappa B activation pathways. J. Biol. Chem. 2001, 276, 44069–44077. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Nie, S.; Jiang, G.; Zhou, Y.; Zhou, M.; Zhao, Y.; Li, S.; Wang, F.; Lv, Q.; Huang, Y.; et al. Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p.C10X) with ischemic stroke. Stroke 2014, 45, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Paramel, G.V.; Karadimou, G.; Eremo, A.G.; Ljungberg, L.U.; Hedin, U.; Olofsson, P.S.; Folkersen, L.; Paulsson-Berne, G.; Sirsjo, A.; Fransen, K. Expression of CARD8 in human atherosclerosis and its regulation of inflammatory proteins in human endothelial cells. Sci. Rep. 2020, 10, 19108. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ge, S.; Gong, W.; Xu, J.; Guo, Z.; Liu, Z.; Gao, X.; Wei, X.; Ge, S. LncRNA ANRIL acts as a modular scaffold of WDR5 and HDAC3 complexes and promotes alteration of the vascular smooth muscle cell phenotype. Cell Death Dis. 2020, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, A.D.; Tansey, W.P. Moonlighting with WDR5: A Cellular Multitasker. J. Clin. Med. 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Chamseddine, A.H.; Carrell, S.; Miller, F.J., Jr. Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014, 2, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Grimm, T.; Holzel, M.; Rohrmoser, M.; Harasim, T.; Malamoussi, A.; Gruber-Eber, A.; Kremmer, E.; Eick, D. Dominant-negative Pes1 mutants inhibit ribosomal RNA processing and cell proliferation via incorporation into the PeBoW-complex. Nucleic Acids Res. 2006, 34, 3030–3043. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.C.; Stillman, B. Yph1p, an ORC-interacting protein: Potential links between cell proliferation control, DNA replication, and ribosome biogenesis. Cell 2002, 109, 835–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Yang, F.; Zhao, H.; Wang, M.; Zhang, Y. Circular RNA circCHFR Facilitates the Proliferation and Migration of Vascular Smooth Muscle via miR-370/FOXO1/Cyclin D1 Pathway. Mol. Ther. Nucleic Acids 2019, 16, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yang, S.; Qu, H. circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells. Open Life Sci. 2021, 16, 1053–1063. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, T.; Hu, X.; Ning, M.; Gao, W.; Lang, Y.; Zheng, W.; Wei, J. Circ_CHFR expedites cell growth, migration and inflammation in ox-LDL-treated human vascular smooth muscle cells via the miR-214-3p/Wnt3/beta-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3282–3292. [Google Scholar]
- Li, Y.; Wang, B. Circular RNA circCHFR downregulation protects against oxidized low-density lipoprotein-induced endothelial injury via regulation of microRNA-15b-5p/growth arrest and DNA damage inducible gamma. Bioengineered 2022, 13, 4481–4492. [Google Scholar] [CrossRef]
- Lu, Q.; Li, Y.; Lou, J.; Li, P.; Gu, Y.; Wang, X. Circ-CHFR modulates the proliferation, migration, and invasion of ox-LDL-induced human aorta vascular smooth muscle cells through the miR-214-3p/PAPPA axis. Clin. Hemorheol. Microcirc. 2021, 80, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Tang, X.; Wang, J.J.; Liu, J.; Chen, P.; Sun, Y. A circular RNA, circUSP36, accelerates endothelial cell dysfunction in atherosclerosis by adsorbing miR-637 to enhance WNT4 expression. Bioengineered 2021, 12, 6759–6770. [Google Scholar] [CrossRef]
- Zhang, S.; Song, G.; Yuan, J.; Qiao, S.; Xu, S.; Si, Z.; Yang, Y.; Xu, X.; Wang, A. Circular RNA circ_0003204 inhibits proliferation, migration and tube formation of endothelial cell in atherosclerosis via miR-370-3p/TGFβR2/phosph-SMAD3 axis. J. Biomed. Sci. 2020, 27, 11. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Jiang, P.; Du, Y.; Zeng, D.; Zhao, J.; Li, M.; Xia, C.; Xie, Z.; Wu, J. Oxidized low-density lipoprotein accelerates the injury of endothelial cells via circ-USP36/miR-98-5p/VCAM1 axis. IUBMB Life 2021, 73, 177–187. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Mao, Z.; Shen, M.; Zhu, J.; Chen, F. Circular RNA has_circ_0003204 inhibits oxLDL-induced vascular endothelial cell proliferation and angiogenesis. Cell. Signal. 2020, 70, 109595. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.F.; Climent, M.; Quintavalle, M.; Farina, F.M.; Schorn, T.; Zani, S.; Carullo, P.; Kunderfranco, P.; Civilini, E.; Condorelli, G.; et al. Circ_Lrp6, a Circular RNA Enriched in Vascular Smooth Muscle Cells, Acts as a Sponge Regulating miRNA-145 Function. Circ. Res. 2019, 124, 498–510. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, G.; Yu, K.; Zhang, X.; Jiang, A. Circ_0003204 knockdown protects endothelial cells against oxidized low-density lipoprotein-induced injuries by targeting the miR-491-5p-ICAM1 pathway. J. Thromb. Thrombolysis 2022, 53, 302–312. [Google Scholar] [CrossRef]
- Rao, R.M.; Yang, L.; Garcia-Cardena, G.; Luscinskas, F.W. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res. 2007, 101, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Morabito, G.; Urban, L.; Ioannone, F.; Serafini, M. Oxidative stress in atherosclerosis development: The central role of LDL and oxidative burst. Endocr. Metab. Immune Disord.-Drug Targets 2012, 12, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Wiese, K.M.; Coates, B.M.; Ridge, K.M. The Role of Nucleotide-Binding Oligomerization Domain-Like Receptors in Pulmonary Infection. Am. J. Respir. Cell Mol. Biol. 2017, 57, 151–161. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, R.; Li, Y.; Yan, W. Knockdown of circular RNA hsa_circ_0003204 inhibits oxidative stress and apoptosis through the miR-330-5p/Nod2 axis to ameliorate endothelial cell injury induced by low-density lipoprotein. Cent. Eur. J. Immunol. 2021, 46, 140–151. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, J.; Wu, L.J.; Li, Y.Y.; Li, M.Q.; Liao, H.Q. CircRNA circUSP36 impairs the stability of NEDD4L mRNA through recruiting PTBP1 to enhance ULK1-mediated autophagic granulosa cell death. J. Reprod. Immunol. 2022, 153, 103681. [Google Scholar] [CrossRef]
- Tang, X.; Yin, R.; Shi, H.; Wang, X.; Shen, D.; Wang, X.; Pan, C. LncRNA ZFAS1 confers inflammatory responses and reduces cholesterol efflux in atherosclerosis through regulating miR-654-3p-ADAM10/RAB22A axis. Int. J. Cardiol. 2020, 315, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Li, J.; Ke, S. CircRNA CORO1C Regulates miR-654-3p/USP7 Axis to Mediate Laryngeal Squamous Cell Carcinoma Progression. Biochem. Genet. 2022, 60, 1615–1629. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Pandya, R.S.; Mao, L.; Zhou, H.; Zhou, S.; Zeng, J.; Popp, A.J.; Wang, X. Central nervous system agents for ischemic stroke: Neuroprotection mechanisms. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 81–97. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.; Zhang, Y.; Bai, Y.; Chen, X.; Huang, R.; Wu, F.; Leng, S.; Chao, J.; Zhang, J.H.; et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: Implications for cerebral ischemic stroke. Autophagy 2018, 14, 1164–1184. [Google Scholar] [CrossRef] [Green Version]
- Si, X.; Zheng, H.; Wei, G.; Li, M.; Li, W.; Wang, H.; Guo, H.; Sun, J.; Li, C.; Zhong, S.; et al. circRNA Hipk3 Induces Cardiac Regeneration after Myocardial Infarction in Mice by Binding to Notch1 and miR-133a. Mol. Ther. Nucleic Acids 2020, 21, 636–655. [Google Scholar] [CrossRef]
- Su, G.; Sun, G.; Lv, J.; Zhang, W.; Liu, H.; Tang, Y.; Su, H. Hsa_circ_0004831 downregulation is partially responsible for atorvastatinalleviated human umbilical vein endothelial cell injuries induced by ox-LDL through targeting the miR-182-5p/CXCL12 axis. BMC Cardiovasc. Disord. 2021, 21, 221. [Google Scholar] [CrossRef]
- Zu, J.; Zuo, L.; Zhang, L.; Wang, Z.; Shi, Y.; Gu, L.; Zhang, Z. Circular RNA FUNDC1 for Prediction of Acute Phase Outcome and Long-Term Survival of Acute Ischemic Stroke. Front. Neurol. 2022, 13, 846198. [Google Scholar] [CrossRef]
- Li, S.; Hu, W.; Deng, F.; Chen, S.; Zhu, P.; Wang, M.; Chen, X.; Wang, Y.; Hu, X.; Zhao, B.; et al. Identification of Circular RNA hsa_circ_0001599 as a Novel Biomarker for Large-Artery Atherosclerotic Stroke. DNA Cell Biol. 2021, 40, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, W.; Wang, L.; Lu, L.; Pang, Y. Circular RNA circLMF1 regulates PDGF-BB-induced proliferation and migration of human aortic smooth muscle cells by regulating the miR-125a-3p/VEGFA or FGF1 axis. Clin. Hemorheol. Microcirc. 2022, 80, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Paganini-Hill, A.; Martin, A.; Cosgrove, M.; Toole, J.F.; Barnett, H.J.; Norris, J. Carotid plaque pathology: Thrombosis, ulceration, and stroke pathogenesis. Stroke 2005, 36, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazan, H.A.; Hatfield, S.A.; Brug, A.; Brooks, A.J.; Lightell, D.J., Jr.; Woods, T.C. Carotid Plaque Rupture Is Accompanied by an Increase in the Ratio of Serum circR-284 to miR-221 Levels. Circ. Cardiovasc. Genet. 2017, 10, e001720. [Google Scholar] [CrossRef] [Green Version]

| CircRNA | Model | Expression Level in Model | Molecular Pathways | Biological Process | References | |
|---|---|---|---|---|---|---|
| Cell/Animal | Chemical | |||||
| Circ_0030042 | HUVEC | Ox-LDL (100 ug/mL) | HUVEC ↓ | Circ_0030042 inhibit eIF4A3 stimulate FOXO1 and beclin 1 | Autophagy | [50] |
| Circ_HIPK3 | HUVEC ApoE−/− mice | Ox-LDL (100 ug/mL, 12 h) High-fat diet (78.85% basic feed, 0.15% cholesterol and 21% fat,16 weeks) | HUVEC ↓ ApoE−/− mice ↓ | Circ_HIPK3 inhibit miR-190b inhibit ATG7 | Autophagy | [118] |
| Circ_RSF1 | HUVEC | Ox-LDL (100 ug/mL, 48 h) | HUVEC ↓ | Circ_RSF1 inhibit miR-135b-5p inhibit HDAC1 | Cell proliferation Cell apoptosis | [33] |
| Circ_0001445 | HAEC | Ox-LDL (100 ug/mL, 0–72 h) | HAEC ↓ | Circ_0001445 inhibit miR-208b-5p inhibit ABCG1 | Cell proliferation Cell migration inflammation | [54] |
| Circ_0090231 | HUVEC | Ox-LDL (75 ug/mL, 48 h) | HUVEC↑ | Circ_0090231 inhibit miR-9-5p inhibit TXNIP | Angiogenesis Oxidative stress Inflammation Apoptosis | [55] |
| Circ_0003575 | HUVEC | Ox-LDL (100 ug/mL, 24 h) | HUVEC↑ | Circ_0003575 inhibit miR-532-5p inhibit ROCK2 | Cell proliferation Angiogenesis Cell apoptosis Inflammation | [56] |
| Circ_DHCR 24 | HA-VSMC | PDGF-BB (2.5 mg/L, 24 h) | HA-VSMC↑ | Circ_DHCR 24 inhibit miR-149-5p inhibit MMP9 | Cell proliferation Cell migration Phenotypic switch | [95] |
| Circ_UBR4 | HVSMC | Ox-LDL (100 ug/mL, 48 h) | HVSMC↑ | Circ_UBR4 inhibit miR-637 inhibit FOXO4 | Cell proliferation Cell migration | [96] |
| Circ _UBR4 | VSMC | Ox-LDL (100 ug/mL, 48 h) | VSMC ↑ | Circ_UBR4 inhibit miR-185-5p inhibit FRS2 | Cell proliferation Cell migration | [97] |
| Circ_UBR4 | Human VSMC | Ox-LDL (0, 25, 50, 100 ug/mL, 48 h) | Human VSMC ↑ | Circ_UBR4 inhibit miR-491-5p inhibit NRP2 | Cell proliferation Cell migration | [98] |
| Circ_PTPRA | VSMC | Ox-LDL (unknow) | VSMC ↑ | Circ_PTPRA inhibit miR-636 inhibit SP1 | Cell proliferation Apoptosis | [112] |
| Circ_0090231 | HAEC | Ox-LDL (25 ug/mL, 24 h) | HAEC ↑ | Circ_0090231 inhibit miR-635 inhibit NLP3 | Cell pyrotosis | [34] |
| Circ_TM7SF3 | THP-1-derived macrophage hMDMs | Ox-LDL (50 ug/mL, 24 h) | Macrophage ↑ | Circ_TM7SF3 inhibit miR-206 inhibit ASPH | Cell apoptosis Inflammation Oxidative stress | [67] |
| Circ_SCAP | THP-1-derived macrophage | Ox-LDL (50 ug/mL, 24 h) | Macrophage ↑ | Circ_SCAP inhibit miR-221-5p inhibit PDE3B | Cell proliferation Cell apoptosis Inflammation Oxidative stress | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, L.; Shi, H.; Chen, B.; Li, X.; Zhang, Y.; Liu, F.; Wei, W.; Zhou, Y.; Liu, K.; et al. Role of Circular RNAs in Atherosclerosis through Regulation of Inflammation, Cell Proliferation, Migration, and Apoptosis: Focus on Atherosclerotic Cerebrovascular Disease. Medicina 2023, 59, 1461. https://doi.org/10.3390/medicina59081461
Zhang Z, Li L, Shi H, Chen B, Li X, Zhang Y, Liu F, Wei W, Zhou Y, Liu K, et al. Role of Circular RNAs in Atherosclerosis through Regulation of Inflammation, Cell Proliferation, Migration, and Apoptosis: Focus on Atherosclerotic Cerebrovascular Disease. Medicina. 2023; 59(8):1461. https://doi.org/10.3390/medicina59081461
Chicago/Turabian StyleZhang, Zheng, Lingfei Li, Huanqing Shi, Biao Chen, Xiaoqin Li, Yuyao Zhang, Fei Liu, Wan Wei, Yongji Zhou, Keqin Liu, and et al. 2023. "Role of Circular RNAs in Atherosclerosis through Regulation of Inflammation, Cell Proliferation, Migration, and Apoptosis: Focus on Atherosclerotic Cerebrovascular Disease" Medicina 59, no. 8: 1461. https://doi.org/10.3390/medicina59081461
APA StyleZhang, Z., Li, L., Shi, H., Chen, B., Li, X., Zhang, Y., Liu, F., Wei, W., Zhou, Y., Liu, K., Xia, W., Gu, X., Huang, J., Tu, S., Yin, C., Shao, A., & Jiang, L. (2023). Role of Circular RNAs in Atherosclerosis through Regulation of Inflammation, Cell Proliferation, Migration, and Apoptosis: Focus on Atherosclerotic Cerebrovascular Disease. Medicina, 59(8), 1461. https://doi.org/10.3390/medicina59081461







