Learning Curve Analysis of Robotic-Assisted Mitral Valve Repair with COVID-19 Exogenous Factor: A Single Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Technique
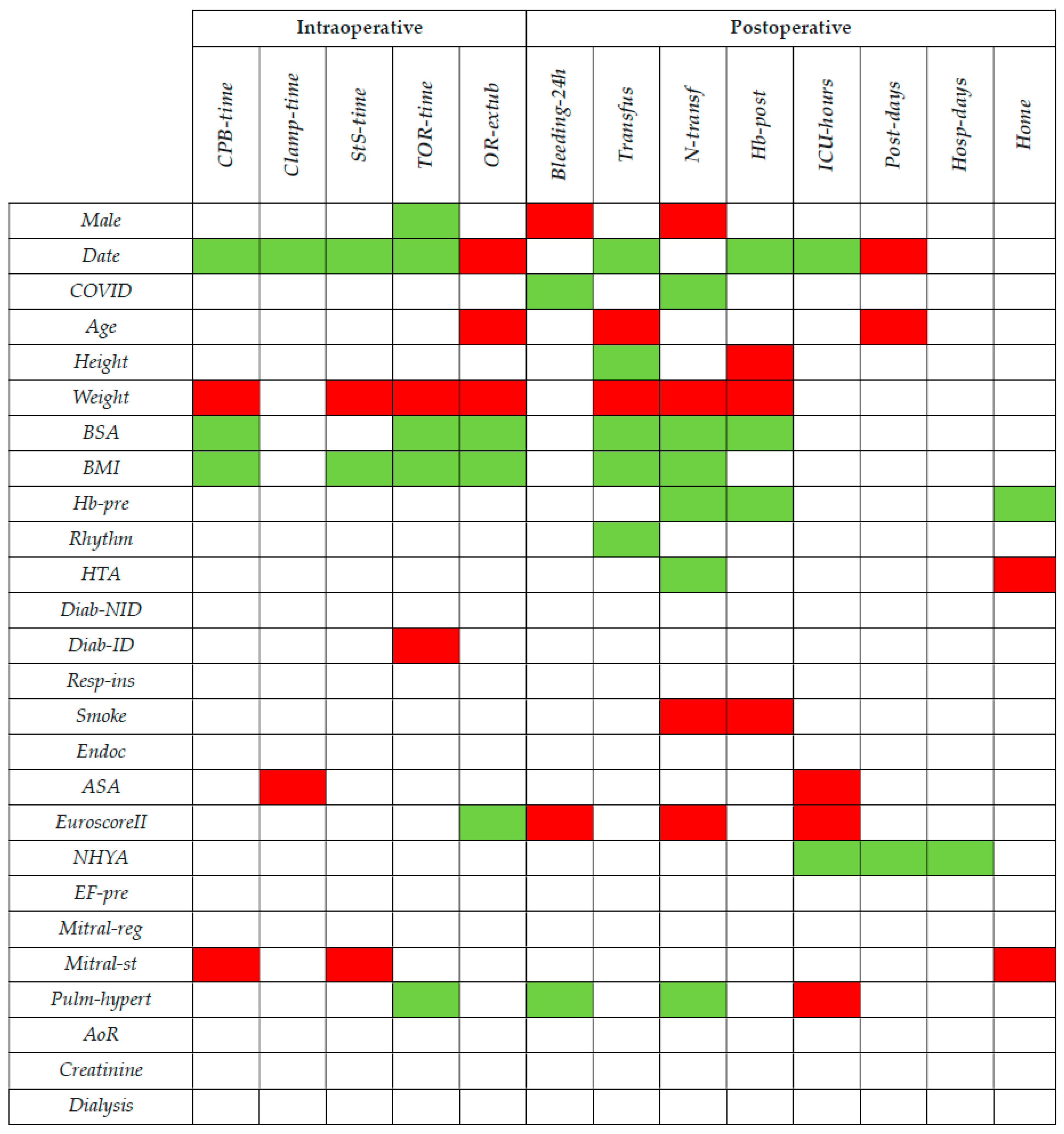
2.3. Collected Data
2.3.1. Preoperative Data
- Male: binary variable equal to 1 if male, and 0 if female;
- Date of the intervention, expressed as number of days since the first RAMVS surgery (performed on 9 May 2019 and with Date = 0);
- COVID: binary variable indicating the altered clinical activities due to the COVID-19 pandemic, which is equal to 1 from Date = 285 (19 February 2020) to Date = 957 (21 December 2021). It is worth noting that the first intervention with COVID = 1 was performed on Date = 397, showing an outage of activities of more than 100 days.
- Age in years;
- Height in centimeters;
- Weight in kilograms;
- BSA (body surface area), equal to 0.007814·Height0.725·Weight0.425;
- BMI (body mass index) equal to Weight/Height2;
- Hb-pre (preoperative hemoglobin) expressed in mg/dL;
- Rhythm: binary variable equal to 1 if the patient’s cardiac rhythm is not sinusal, and 0 if sinusal;
- HTA: binary variable equal to 1 if the patient has arterial hypertension, and 0 otherwise;
- Diab-NID: binary variable equal to 1 if the patient is a non-insulin dependent diabetic patient, and 0 otherwise;
- Diab-ID: binary variable equal to 1 if the patient is an insulin-dependent diabetic patient, and 0 otherwise;
- Resp-ins: binary variable equal to 1 if the patient has respiratory failure, and 0 otherwise;
- Smoke: binary variable equal to 1 if the patient is a smoker or an ex-smoker, and 0 otherwise;
- Endoc: binary variable equal to 1 if the patient has active endocarditis, and 0 otherwise;
- ASA (American Society of Anesthesiology score): categorical variable with 4 levels, from I to IV, which determines if the patient is healthy enough to tolerate surgery and anesthesia;
- EuroscoreII (European System for Cardiac Operative Risk Evaluation): numerical score based on 17 parameters indicating the risk of death from heart surgery [12];
- NHYA (New York Heart Association classification): categorical variable with 4 levels, from I to IV, which represents the heart failure intensity based on the activities the patient is able to perform;
- EF-pre (preoperative ejection fraction): ratio between stroke volume and end-diastolic volume for the left ventricle
- Mitral-reg (severity of mitral regurgitation): categorical variable with 3 levels, from I to III;
- Mitral-st: binary variable equal to 1 if the patient has mitral stenosis, and 0 otherwise;
- Pulm-hypert: binary variable equal to 1 if the patient has pulmonary hypertension, and 0 otherwise;
- AoR: binary variable equal to 1 if the patient has aortic regurgitation, and 0 otherwise;
- Creatinine, expressed in mg/dL;
- Dialysis: binary variable equal to 1 if the patient is currently on dialysis, and 0 otherwise.
2.3.2. Intraoperative Data
- CPB-time (duration of CPB), expressed in minutes;
- Clamp-time (clamping time of the aorta), expressed in minutes;
- StS-time (skin-to-skin time elapsed from incision to suture), expressed in minutes;
- TOR-time (total operating time elapsed from patient entry into the operating room to exit), expressed in minutes;
- OR-extub: binary variable equal to 1 if the patient is extubated directly on the operating table without the need for intubation in the ICU, and 0 otherwise.
2.3.3. Postoperative Data
- Bleeding-24h: volume of fluid collected from the drains in the 24 post-operative hours, expressed in cc (not exclusively blood);
- Transfus: binary variable equal to 1 if the patient has been transfused, including during CPB, and 0 otherwise;
- N-transf: overall number of blood units received by the patient, including during CPB;
- Hb-post: postoperative hemoglobin the day after surgery, expressed in mg/dL;
- ICU-hours: time in hours spent by the patient in the ICU;
- Post-days: number of post-operative hospitalization days including the ICU;
- Hosp-days: total hospitalization days including preoperative stay;
- Home: binary variable equal to 1 if the patient directly returns home upon discharge, and 0 if he/she needs to stay at a rehabilitation center.
2.4. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pettinari, M.; Navarra, E.; Noirhomme, P.; Gutermann, H. The state of robotic cardiac surgery in Europe. Ann. Cardiothorac. Surg. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Cerny, S.; Oosterlinck, W.; Onan, B.; Singh, S.; Segers, P.; Bolcal, C.; Bonatti, J. Robotic Cardiac Surgery in Europe: Status 2020. Front. Cardiovasc. Med. 2022, 8, 827515. [Google Scholar] [CrossRef] [PubMed]
- Agnino, A.; Graniero, A.; Villari, N.; Roscitano, C.; Gerometta, P.; Albano, G.; Anselmi, A. Evaluation of robotic-assisted mitral surgery in a contemporary experience. J. Cardiovasc. Med. 2022, 23, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Husen, T.F.; Kohar, K.; Angelica, R.; Saputro, B.I.L. Robotic vs other surgery techniques for mitral valve repair and/or replacement: A systematic review and meta-analysis. Hell. J. Cardiol. 2023, 71, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Dearani, J.A.; Mihaljevic, T.; Chitwood, W.R.; Murphy, D.A.; Trento, A.; Javadikasgari, H.; Burkhart, H.M.; Nifong, W.L.; Daly, R.C.; et al. Mitral valve repair using robotic technology: Safe, effective, and durable. J. Thorac. Cardiovasc. Surg. 2016, 151, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Gillinov, A.M.; Mihaljevic, T.; Javadikasgari, H.; Suri, R.M.; Mick, S.L.; Navia, J.L.; Desai, M.Y.; Bonatti, J.; Khosravi, M.; Idrees, J.J.; et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J. Thorac. Cardiovasc. Surg. 2018, 155, 82–91.e2. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, T.; Koprivanac, M.; Kelava, M.; Goodman, A.; Jarrett, C.; Williams, S.J.; Gillinov, A.M.; Bajwa, G.; Mick, S.L.; Bonatti, J.; et al. Value of Robotically Assisted Surgery for Mitral Valve Disease. JAMA Surg. 2014, 149, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Thornton, B.A.; Peacock, J.C.; Hollingsworth, K.W.; Smith, C.R.; Oz, M.C.; Argenziano, M. Does Robotic Technology Make Minimally Invasive Cardiac Surgery Too Expensive? A Hospital Cost Analysis of Robotic and Conventional Techniques. J. Card. Surg. 2005, 20, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Coyan, G.; Wei, L.M.; Althouse, A.; Roberts, H.G.; Schauble, D.; Murashita, T.; Badhwar, V. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J. Thorac. Cardiovasc. Surg. 2018, 156, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Wei, S. Comparison of hospital cost between robotic versus thoracoscopic mitral valve repair. J. Card. Surg. 2021, 36, 3459–3460. [Google Scholar] [CrossRef] [PubMed]
- Agnino, A.; Anselmi, A. Robotic Mitral Valve Repair Through Nonresectional Posterior Leaflet Remodeling. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2020, 15, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thoracic Surg. 2012, 41, 734–745, discussion 744–745. [Google Scholar] [CrossRef] [PubMed]
- Charland, P.J.; Robbins, T.; Rodriguez, E.; Nifong, W.L.; Chitwood, R.W., Jr. Learning curve analysis of mitral valve repair using telemanipulative technology. J. Thorac. Cardiovasc. Surg. 2011, 142, 404–410. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakamura, Y.; Hirano, T.; Ito, Y.; Watanabe, T. Cumulative sum analysis for the learning curve of minimally invasive mitral valve repair. Heart Vessel. 2021, 36, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- McFadden, D. Conditional logit analysis of qualitative choice behavior. In Frontiers in Econometrics; Zarembka, P., Ed.; Academic Press: Cambridge, MA, USA, 1974; pp. 105–142. [Google Scholar]
- Güllü, A.; Senay, S.; Kocyigit, M.; Zencirci, E.; Akyol, A.; Degirmencioglu, A.; Karakus, G.; Ersin, E.; Karabiber, A.; Alhan, C. An analysis of the learning curve for robotic-assisted mitral valve repair. J. Card. Surg. 2021, 36, 624–628. [Google Scholar] [CrossRef]
- Palmen, M.; Navarra, E.; Bonatti, J.; Franke, U.; Cerny, S.; Musumeci, F.; Modi, P.; Singh, S.; Sandoval, E.; Pettinari, M.; et al. Current state of the art and recommendations in robotic mitral valve surgery. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac160. [Google Scholar] [CrossRef]
- Agnino, A.; Graniero, A.; Albano, G.; Gerometta, P.S.; Roscitano, C.; Villari, N.; Albertini, A.; Salvi, D.; Persico, I.; Anselmi, A. Towards the age of computer-assisted surgery: How to establish a robotic-assisted mitral surgery program with a last-generation platform. G Ital. Cardiol. 2021, 22, 648–656. [Google Scholar] [CrossRef]
- Seo, Y.J.; Sanaiha, Y.; Bailey, K.L.; Aguayo, E.; Chao, A.; Shemin, R.J.; Benharash, P. Outcomes and Resource Utilization in Robotic Mitral Valve Repair: Beyond the Learning Curve. J. Surg. Res. 2019, 235, 258–263. [Google Scholar] [CrossRef] [PubMed]
| Preoperative Data | Intraoperative Data | Postoperative Data | ||||
|---|---|---|---|---|---|---|
| Variable | Value | Variable | Value | Variable | Value | |
| Male | 70.5% | CPB-time | 164 [147;183] | Bleeding-24h | 370 [250;610] | |
| Date | 956 [649;1203] | Clamp-time | 91 [81;106] | Transfus | 24.6% | |
| COVID | 38.3% | StS-time | 260 [230;290] | N-transf | 0 [0;0] | |
| Age | 59.1 ± 13.3 | TOR-time | 370 [345;410] | Hb-post | 11.3 ± 1.5 | |
| Height | 173.0 ± 9.2 | OR-extub | 65.1% | ICU-hours | 40 [20;45] | |
| Weight | 72.7 ± 13.5 | Post-days | 7 [6;9] | |||
| BSA | 1.9 ± 0.2 | Hosp-days | 10 [9;12] | |||
| BMI | 24.1 ± 3.3 | Home | 89.3% | |||
| Hb-pre | 14.3 [13.6;15.0] | |||||
| Rhythm | 14.8% | |||||
| HTA | 45.6% | |||||
| Diab-NID | 3.4% | |||||
| Diab-ID | 0.7% | |||||
| Resp-ins | 0.0% | |||||
| Smoke | 22.8% | |||||
| Endoc | 0.0% | |||||
| ASA | I | 2.0% | ||||
| II | 28.2% | |||||
| III | 64.4% | |||||
| IV | 5.4% | |||||
| EuroscoreII | 0.9 [0.7;1.2] | |||||
| NHYA | I | 2.0% | ||||
| II | 71.1% | |||||
| III | 26.2% | |||||
| IV | 0.7% | |||||
| EF-pre | 65 [60;68] | |||||
| Mitral-reg | I | 0.7% | ||||
| II | 5.4% | |||||
| III | 94.0% | |||||
| Mitral-st | 2.7% | |||||
| Pulm-hypert | 45.0% | |||||
| AoR | 18.1% | |||||
| Creatinine | 0.93 [0.81;1.07] | |||||
| Dialysis | 0.0% | |||||
| CPB-Time | Clamp-Time | StS-Time | TOR-Time | OR-Extub | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | +8.20 | *** | +5.34 | *** | +7.60 | *** | +6.50 | *** | −2.53 × 101 | ||
| Male | −8.17 × 10−2 | −9.39 × 10−2 | −7.20 × 10−2 | . | −8.70 × 10−2 | *** | — | ||||
| Date | −7.29 × 10−4 | *** | −6.83 × 10−4 | ** | −8.97 × 10−4 | *** | −5.83 × 10−4 | *** | −1.17 × 10−2 | * | |
| Date2 | +3.36 × 10−7 | ** | +3.45 × 10−7 | ** | +4.15 × 10−7 | *** | +2.62 × 10−7 | *** | +4.88 × 10−6 | * | |
| COVID | — | — | — | — | — | ||||||
| Age | — | −1.91 × 10−2 | . | −1.77 × 10−3 | — | −5.97 × 10−2 | * | ||||
| Age2 | — | +1.76 × 10−4 | * | — | — | — | |||||
| Weight | +5.72 × 10−2 | ** | +3.52 × 10−3 | . | +8.86 × 10−2 | ** | +6.57 × 10−2 | ** | −1.99 | ** | |
| BSA | −2.72 | * | — | −1.28 | — | — | |||||
| BSA2 | — | — | −8.21 × 10−1 | . | −8.4 × 10−1 | ** | +2.73 × 101 | ** | |||
| BMI | −7.99 × 10−2 | * | — | −1.15 × 10−1 | * | −8.47 × 10−2 | * | +2.64 | ** | ||
| Rhythm | +8.00 × 10−2 | . | — | +6.36 × 10−2 | . | — | — | ||||
| HTA | — | −7.27 × 10−2 | . | — | — | — | |||||
| Diab-ID | — | — | — | +4.01 × 10−1 | *** | — | |||||
| ASA | = II | +3.23 × 10−2 | +1.47 × 10−1 | +4.41 × 10−2 | — | +1.96 × 101 | |||||
| = III | +1.25 × 10−1 | +2.22 × 10−1 | +9.85 × 10−2 | — | +2.09 × 101 | ||||||
| = IV | +2.42 × 10−1 | . | +3.84 × 10−1 | * | +1.58 × 10−1 | — | +3.79 × 101 | ||||
| EuroscoreII | — | — | +1.30 × 10−2 | . | — | — | |||||
| EuroscoreII2 | — | — | — | — | +5.38 × 10−1 | * | |||||
| Mitral-reg | = II | — | −5.26 × 10−1 | * | — | — | — | ||||
| = III | −3.76 × 10−1 | — | — | — | |||||||
| Mitral-st | +2.29 × 10−1 | * | — | +1.50 × 10−1 | * | +1.03 × 10−1 | . | — | |||
| Pulm-hypert | — | — | — | −4.13 × 10−2 | * | — | |||||
| AoR | — | — | — | — | +1.11 | . | |||||
| Pseudo-R2 | 0.314 | 0.229 | 0.509 | 0.545 | 0.351 | ||||||
| Bleeding-24h | Transfus | N-Transf | Hb-Post | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | +1.41 × 101 | * | +1.39 × 102 | +1.47 × 101 | ** | +2.97 | *** | ||
| Male | +4.67 × 10−1 | ** | — | +1.18 | ** | — | |||
| Date | — | −7.96 × 10−3 | . | — | — | ||||
| Date2 | — | +6.86 × 10−6 | * | — | +5.36 × 10−8 | ** | |||
| COVID | −3.02 × 10−1 | ** | — | −7.14 × 10−1 | * | +3.24 × 10−2 | |||
| Age | +6.46 × 10−2 | * | +1.21 × 10−1 | ** | — | — | |||
| Age2 | −6.47 × 10−4 | * | — | — | — | ||||
| Height | −6.33 × 10−2 | −7.12 × 10−1 | * | — | −3.46 × 10−2 | ** | |||
| Weight | −1.01 × 10−1 | . | +3.99 | ** | +1.18 | * | −4.43 × 10−2 | ** | |
| BSA | — | — | — | +4.39 | ** | ||||
| BSA2 | +2.14 | −4.39 × 101 | * | −1.74 × 101 | * | — | |||
| BMI | — | −6.82 | ** | −1.61 | * | — | |||
| Hb-pre | — | — | −1.84 × 10−1 | * | +3.21 × 10−2 | *** | |||
| Rhythm | — | −2.18 | * | — | — | ||||
| HTA | — | −1.29 | . | −9.07 × 10−1 | * | — | |||
| Diab-NID | +4.32 × 10−1 | — | — | — | |||||
| Smoke | +2.56 × 10−1 | . | +1.29 | . | +1.62 | *** | −5.10 × 10−2 | * | |
| ASA | = II | — | +1.23 × 101 | — | — | ||||
| = III | — | +1.19 × 101 | — | — | |||||
| = IV | — | +1.53 × 101 | — | — | |||||
| EuroscoreII | +5.08 × 10−1 | *** | — | +1.50 | *** | — | |||
| EuroscoreII2 | −2.06 × 10−2 | ** | — | −7.21 × 10−2 | *** | — | |||
| EF-pre | — | — | +3.36 × 10−2 | — | |||||
| Mitral-reg | = II | +3.32 × 10−1 | — | — | — | ||||
| = III | +8.81 × 10−1 | — | — | — | |||||
| Pulm-hypert | −2.69 × 10−1 | * | — | −6.67 × 10−1 | * | — | |||
| Creatinine | — | −1.77 × 101 | ** | −8.29 | ** | — | |||
| Creatinine2 | — | +8.69 | ** | +3.90 | ** | — | |||
| Pseudo-R2 | 0.299 | 0.523 | 0.499 | 0.372 | |||||
| ICU-Hours | Post-Days | Hosp-Days | Home | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | +8.42 | ** | +1.92 | *** | +2.65 | *** | −1.10 × 101 | ||
| Male | −2.14 × 10−1 | — | — | — | |||||
| Date | −2.43 × 10−3 | *** | — | −4.01 × 10−4 | — | ||||
| Date2 | +1.29 × 10−6 | *** | +1.19 × 10−7 | * | +2.53 × 10−7 | — | |||
| COVID | — | — | — | — | |||||
| Age | — | +9.08 × 10−3 | *** | +4.27 × 10−3 | . | +4.13 × 10−1 | . | ||
| Age2 | — | — | — | −3.75 × 10−3 | * | ||||
| BSA | −5.10 | — | — | — | |||||
| BSA2 | 1.22 | — | — | — | |||||
| BMI | +3.40 × 10−2 | . | — | — | — | ||||
| Hb-pre | — | — | — | +8.45 × 10−1 | ** | ||||
| HTA | −1.32 × 10−1 | — | — | −3.05 | * | ||||
| Smoke | +2.01 × 10−1 | . | — | — | −1.53 | . | |||
| ASA | = II | +4.14 × 10−1 | — | — | — | ||||
| = III | +6.20 × 10−1 | . | — | — | — | ||||
| = IV | +1.03 | * | — | — | — | ||||
| EuroscoreII | +2.60 × 10−1 | * | — | +9.12 × 10−2 | . | +1.62 | . | ||
| EuroscoreII2 | −1.12 × 10−2 | * | — | −3.88 × 10−3 | −8.90 × 10−2 | . | |||
| NHYA | = II | −6.20 × 10−1 | . | −4.93 × 10−1 | ** | −5.28 × 10−1 | *** | — | |
| = III | −3.26 × 10−1 | −5.43 × 10−1 | ** | −5.38 × 10−1 | *** | — | |||
| = IV | −1.37 | * | −7.31 × 10−1 | . | −7.17 × 10−1 | * | — | ||
| EF-pre | — | — | — | 8.99 × 10−2 | |||||
| Mitral-reg | = II | +8.11 × 10−1 | — | — | — | ||||
| = III | +2.18 × 10−1 | — | — | — | |||||
| Mitral-st | — | — | −3.79 | * | |||||
| Pulm-hypert | +2.56 × 10−1 | * | — | — | — | ||||
| Creatinine | +8.50 × 10−2 | — | — | −2.10 × 101 | . | ||||
| Creatinine2 | — | — | — | +7.24 | |||||
| Pseudo-R2 | 0.391 | 0.216 | 0.199 | 0.435 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giroletti, L.; Brembilla, V.; Graniero, A.; Albano, G.; Villari, N.; Roscitano, C.; Parrinello, M.; Grazioli, V.; Lanzarone, E.; Agnino, A. Learning Curve Analysis of Robotic-Assisted Mitral Valve Repair with COVID-19 Exogenous Factor: A Single Center Experience. Medicina 2023, 59, 1568. https://doi.org/10.3390/medicina59091568
Giroletti L, Brembilla V, Graniero A, Albano G, Villari N, Roscitano C, Parrinello M, Grazioli V, Lanzarone E, Agnino A. Learning Curve Analysis of Robotic-Assisted Mitral Valve Repair with COVID-19 Exogenous Factor: A Single Center Experience. Medicina. 2023; 59(9):1568. https://doi.org/10.3390/medicina59091568
Chicago/Turabian StyleGiroletti, Laura, Valentina Brembilla, Ascanio Graniero, Giovanni Albano, Nicola Villari, Claudio Roscitano, Matteo Parrinello, Valentina Grazioli, Ettore Lanzarone, and Alfonso Agnino. 2023. "Learning Curve Analysis of Robotic-Assisted Mitral Valve Repair with COVID-19 Exogenous Factor: A Single Center Experience" Medicina 59, no. 9: 1568. https://doi.org/10.3390/medicina59091568
APA StyleGiroletti, L., Brembilla, V., Graniero, A., Albano, G., Villari, N., Roscitano, C., Parrinello, M., Grazioli, V., Lanzarone, E., & Agnino, A. (2023). Learning Curve Analysis of Robotic-Assisted Mitral Valve Repair with COVID-19 Exogenous Factor: A Single Center Experience. Medicina, 59(9), 1568. https://doi.org/10.3390/medicina59091568








