Elucidation of the Mechanism of Occasional Anterior Longitudinal Ligament Rupture with Posterior Correction Procedure for Adult Spinal Deformity Using LLIF–Finite Element Analysis of the Impact of the Lordotic Angle of Intervertebral LLIF Cage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of the 3D FE Model
2.2. Construction of Two Types of FE Models
2.3. Comparison between Two Different Degrees of Lordotic Angle of LLIF Cage in Two Different Types of FEA Model
2.4. Statistical Analysis, etc.
3. Results
3.1. Assessment of the Elongation Degree of ALL in the PS Compression Model
3.2. Assessment of the Rotation Center Location in the PS Compression Model
3.3. Assessment of the Elongation of ALL in Cantilever Technique Model (Spinous Process Displacement Model)
3.4. Assessment of the Rotation Center Location in Cantilever Technique Model (Spinous Process Displacement Model)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Davis, T.T.; Hynes, R.A.; Fung, D.A.; Spann, S.W.; MacMillan, M.; Kwon, B.; Liu, J.; Acosta, F.; Drochner, T.E. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position: An anatomic study. J. Neurosurg. Spine 2014, 21, 785–793. [Google Scholar] [CrossRef]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kepler, C.K.; Girardi, F.P.; Cammisa, F.P.; Huang, R.C.; Sama, A.A. Lateral lumbar interbody fusion: Clinical and radiographic outcomes at 1 year: A preliminary report. J. Spinal Disord. Tech. 2011, 24, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Malham, G.M.; Ellis, N.J.; Parker, R.M.; Seex, K.A. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. Sci. World J. 2012, 2012, 246989. [Google Scholar] [CrossRef]
- Castro, C.; Oliveira, L.; Amaral, R.; Marchi, L.; Pimenta, L. Is the lateral transpsoas approach feasible for the treatment of adult degenerative scoliosis? Clin. Orthop. Relat. Res. 2014, 472, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Rao, P.J.; Scherman, D.B.; Dandie, G.; Mobbs, R.J. Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J. Clin. Neurosci. 2015, 22, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Maharaj, M.; Assem, Y.; Mobbs, R.J. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF). J. Clin. Neurosci. 2016, 31, 23–29. [Google Scholar] [CrossRef]
- Kamal, R.M.W.; James, B.B.; Richard, A.H. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J. 2017, 17, 543–553. [Google Scholar]
- Salzmann, S.N.; Shue, J.; Hughes, A.P. Lateral lumbar interbody fusion outcomes and complications. Curr. Rev. Musculoskelet. Med. 2017, 10, 539–546. [Google Scholar] [CrossRef]
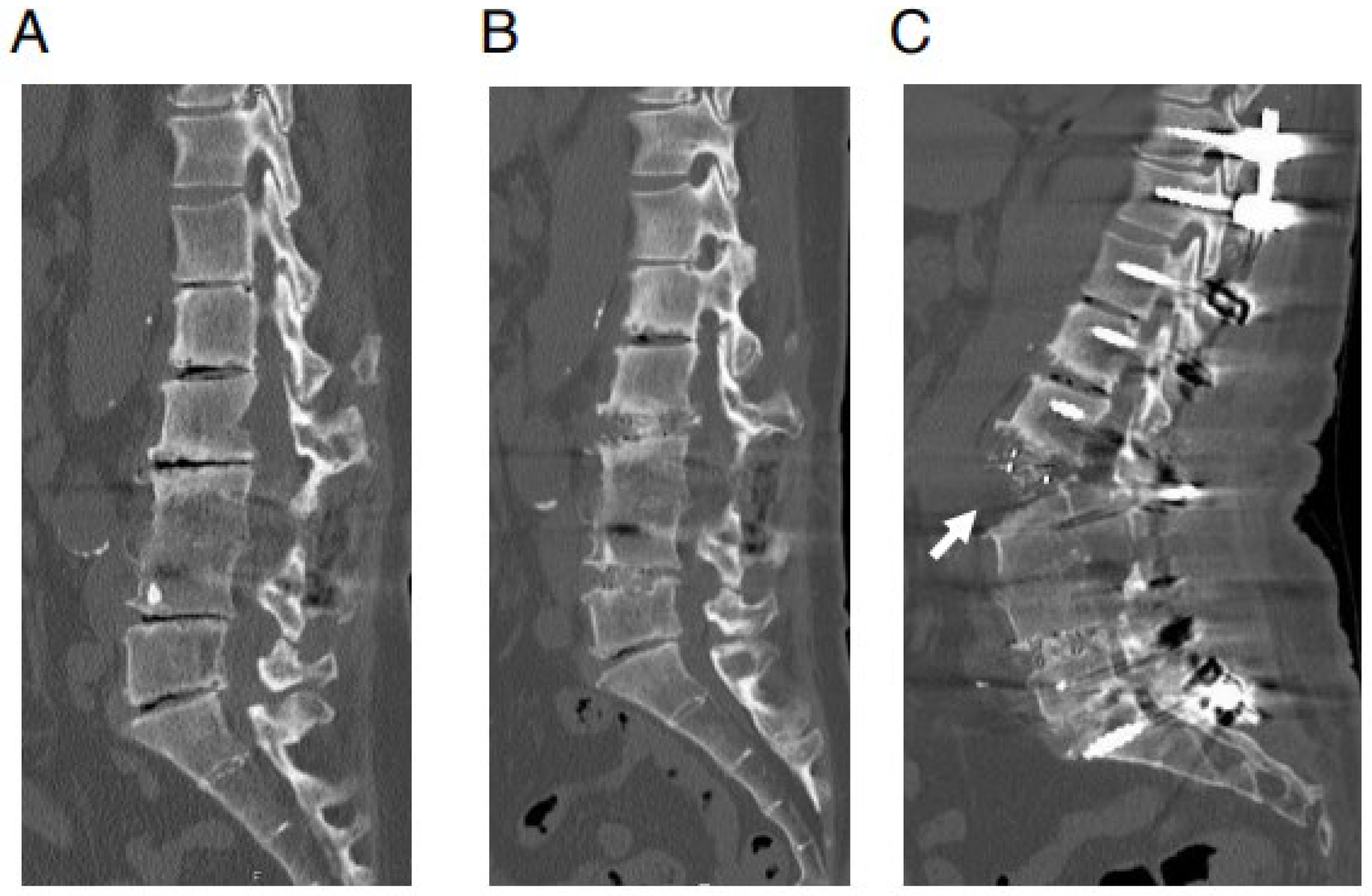
- Nakashima, H.; Kanemura, T.; Satake, K.; Ishikawa, Y.; Ouchida, J.; Segi, N.; Yamaguchi, H.; Imagama, S. Factors affecting postoperative sagittal alignment after lateral lumbar interbody fusion in adult spinal deformity: Posterior osteotomy, anterior longitudinal ligament rupture, and endplate injury. Asian Spine J. 2019, 13, 738–745. [Google Scholar] [CrossRef]
- Schwab, F.; Blondel, B.; Chay, E.; Demakakos, J.; Lenke, L.; Tropiano, P.; Ames, C.; Smith, J.S.; Shaffrey, C.I.; Glassman, S.; et al. The comprehensive anatomical spinal osteotomy classification. Neurosurgery 2014, 74, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Strom, R.G.; Bae, J.; Mizutani, J.; Valone, F., 3rd; Ames, C.P.; Deviren, V. Lateral interbody fusion combined with open posterior surgery for adult spinal deformity. J. Neurosurg. Spine 2016, 25, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.F.; Kaneko, S.; Takeda, H.; Nagai, S.; Kawabata, S.; Ikeda, D.; Fujita, N.; Yato, Y.; Asazuma, T. Circumferential bone fusion in adult spinal deformity via combination of oblique lateral interbody fusion and grade 2 posterior column osteotomy. Glob. Spine J. 2022, 21925682211069936. [Google Scholar] [CrossRef]
- Maruo, K.; Arizumi, F.; Kusuyama, K.; Kishima, K.; Tachibana, T. Incidence and risk factors of anterior longitudinal ligament rupture after posterior corrective surgery using lateral lumbar interbody fusion for adult spinal deformity. Clin. Spine Surg. 2021, 34, E26–E31. [Google Scholar] [CrossRef] [PubMed]
- Shibao, Y.; Koda, M.; Abe, T.; Mataki, K.; Miura, K.; Noguchi, H.; Takahashi, H.; Funayama, T.; Yamazaki, M. Accidental anterior longitudinal ligament rupture during lateral lumbar interbody fusion disclosed after posterior corrective fusion surgery resulting in local hyper-lordosis. J. Rural Med. 2021, 16, 111–114. [Google Scholar] [CrossRef]
- Yamamura, R.; Kudo, Y.; Matsuoka, A.; Maruyama, H.; Ishikawa, K.; Dodo, Y.; Shirahata, T.; Toyone, T.; Inagaki, K. Anterior column reconstruction performed for complete anterior longitudinal ligament rupture caused by surgical correction with lateral interbody fusion for kyphosis. Spine Surg. Relat. Res. 2019, 4, 87–90. [Google Scholar] [CrossRef]
- Taddei, F.; Pancanti, A.; Viceconti, M. An improved method for the automatic mapping of computed tomography numbers onto finite element models. Med. Eng. Phys. 2004, 26, 61–69. [Google Scholar] [CrossRef]
- Grover, P.; Albert, C.; Wang, M.; Harris, G.F. Mechanical characterization of fourth generation composite humerus. Proc. Inst. Mech. Eng. H 2011, 225, 1169–1176. [Google Scholar] [CrossRef]
- Erdemir, A.; Guess, T.M.; Halloran, J.; Tadepalli, S.C.; Morrison, T.M. Considerations for reporting finite element analysis studies in biomechanics. J. Biomech. 2012, 45, 625–633. [Google Scholar] [CrossRef]
- Sabalic, S.; Kodvanj, J.; Pavic, A. Comparative study of three models of extra-articular distal humerus fracture osteosynthesis using the finite element method on an osteoporotic computational model. Injury 2013, 44 (Suppl. S3), S56–S61. [Google Scholar] [CrossRef]
- Nejad, T.M.; Foster, C.; Gongal, D. Finite element modelling of cornea mechanics: A review. Arq. Bras. Oftalmol. 2014, 77, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.M. The use of finite element analysis to enhance research and clinical practice in orthopedics. J. Knee Surg. 2016, 29, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Bess, S.; Harris, J.E.; Turner, A.W.; LaFage, V.; Smith, J.S.; Shaffrey, C.I.; Schwab, F.J.; Haid, R.W., Jr. The effect of posterior polyester tethers on the biomechanics of proximal junctional kyphosis: A finite element analysis. J. Neurosurg. Spine 2017, 26, 125–133. [Google Scholar] [CrossRef]
- Zhao, X.; Du, L.; Xie, Y.; Zhao, J. Effect of Lumbar Lordosis on the Adjacent Segment in Transforaminal Lumbar Interbody Fusion: A Finite Element Analysis. World Neurosurg. 2018, 114, e114–e120. [Google Scholar] [CrossRef]
- Buell, T.J.; Bess, S.; Xu, M.; Schwab, F.J.; Lafage, V.; Ames, C.P.; Shaffrey, C.I.; Smith, J.S. Optimal tether configurations and preload tensioning to prevent proximal junctional kyphosis: A finite element analysis. J. Neurosurg. Spine 2019, 8, 574–584. [Google Scholar] [CrossRef]
- Jiang, S.; Li, W. Biomechanical study of proximal adjacent segment degeneration after posterior lumbar interbody fusion and fixation: A finite element analysis. J. Orthop. Surg. Res. 2019, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Du, C.F.; Cai, X.Y.; Gui, W.; Sun, M.S.; Liu, Z.X.; Liu, C.J.; Zhang, C.Q.; Huang, Y.P. Does oblique lumbar interbody fusion promote adjacent degeneration in degenerative disc disease: A finite element analysis. Comput. Biol. Med. 2021, 128, 104122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; She, L.J.; Zhang, W.; Cheng, X.D.; Fan, J.P. Biomechanics of extreme lateral interbody fusion with different internal fixation methods: A finite element analysis. BMC Musculoskelet. Disord. 2022, 23, 134. [Google Scholar] [CrossRef]
- Kozaki, T.; Lundberg, H.J.; Mell, S.P.; Samartzis, D.; Kawakami, M.; Yamada, H.; Inoue, N.; An, H.S. Effect of Lumbar Fusion and Pelvic Fixation Rigidity on Hip Joint Stress: A Finite Element Analysis. Spine 2023. [Google Scholar] [CrossRef] [PubMed]
- Tachi, H.; Kato, K.; Abe, Y.; Kokabu, T.; Yamada, K.; Iwasaki, N.; Sudo, H. Surgical Outcome Prediction Using a Four-Dimensional Planning Simulation System with Finite Element Analysis Incorporating Pre-bent Rods in Adolescent Idiopathic Scoliosis: Simulation for Spatiotemporal Anatomical Correction Technique. Front. Bioeng. Biotechnol. 2021, 9, 746902. [Google Scholar] [CrossRef]
- Jalilvand, E.; Abollfathi, N.; Khajehzhadeh, M.; Hassani-Gangaraj, M. Optimization of cervical cage and analysis of its base material: A finite element study. Proc. Inst. Mech. Eng. H 2022, 236, 1613–1625. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, H.; Abe, Y.; Imai, T.; Rashid, M.Z.M.; Ikeda, D.; Kawabata, S.; Nagai, S.; Hachiya, K.; Fujita, N.; Kaneko, S. Elucidation of the Mechanism of Occasional Anterior Longitudinal Ligament Rupture with Posterior Correction Procedure for Adult Spinal Deformity Using LLIF–Finite Element Analysis of the Impact of the Lordotic Angle of Intervertebral LLIF Cage. Medicina 2023, 59, 1569. https://doi.org/10.3390/medicina59091569
Takeda H, Abe Y, Imai T, Rashid MZM, Ikeda D, Kawabata S, Nagai S, Hachiya K, Fujita N, Kaneko S. Elucidation of the Mechanism of Occasional Anterior Longitudinal Ligament Rupture with Posterior Correction Procedure for Adult Spinal Deformity Using LLIF–Finite Element Analysis of the Impact of the Lordotic Angle of Intervertebral LLIF Cage. Medicina. 2023; 59(9):1569. https://doi.org/10.3390/medicina59091569
Chicago/Turabian StyleTakeda, Hiroki, Yuichiro Abe, Takaya Imai, Mohd Zaim Mohd Rashid, Daiki Ikeda, Soya Kawabata, Sota Nagai, Kurenai Hachiya, Nobuyuki Fujita, and Shinjiro Kaneko. 2023. "Elucidation of the Mechanism of Occasional Anterior Longitudinal Ligament Rupture with Posterior Correction Procedure for Adult Spinal Deformity Using LLIF–Finite Element Analysis of the Impact of the Lordotic Angle of Intervertebral LLIF Cage" Medicina 59, no. 9: 1569. https://doi.org/10.3390/medicina59091569




