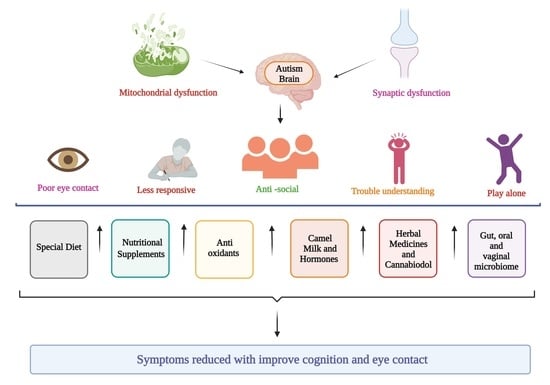
A Spectrum of Solutions: Unveiling Non-Pharmacological Approaches to Manage Autism Spectrum Disorder
Abstract
1. Introduction
2. Diets for ASD
2.1. Elimination Diet for ASD
2.2. Casein and Gluten for ASD
2.3. Specific Carbohydrate Diet for ASD
2.4. Ketogenic Diet for ASD
3. Nutritional Supplements for ASD
3.1. Omega-3 Fatty Acids for ASD
3.2. Zinc for ASD
3.3. Vitamins for ASD
3.4. Iron for ASD
3.5. Magnesium (Mg) for ASD
3.6. Selenium for ASD
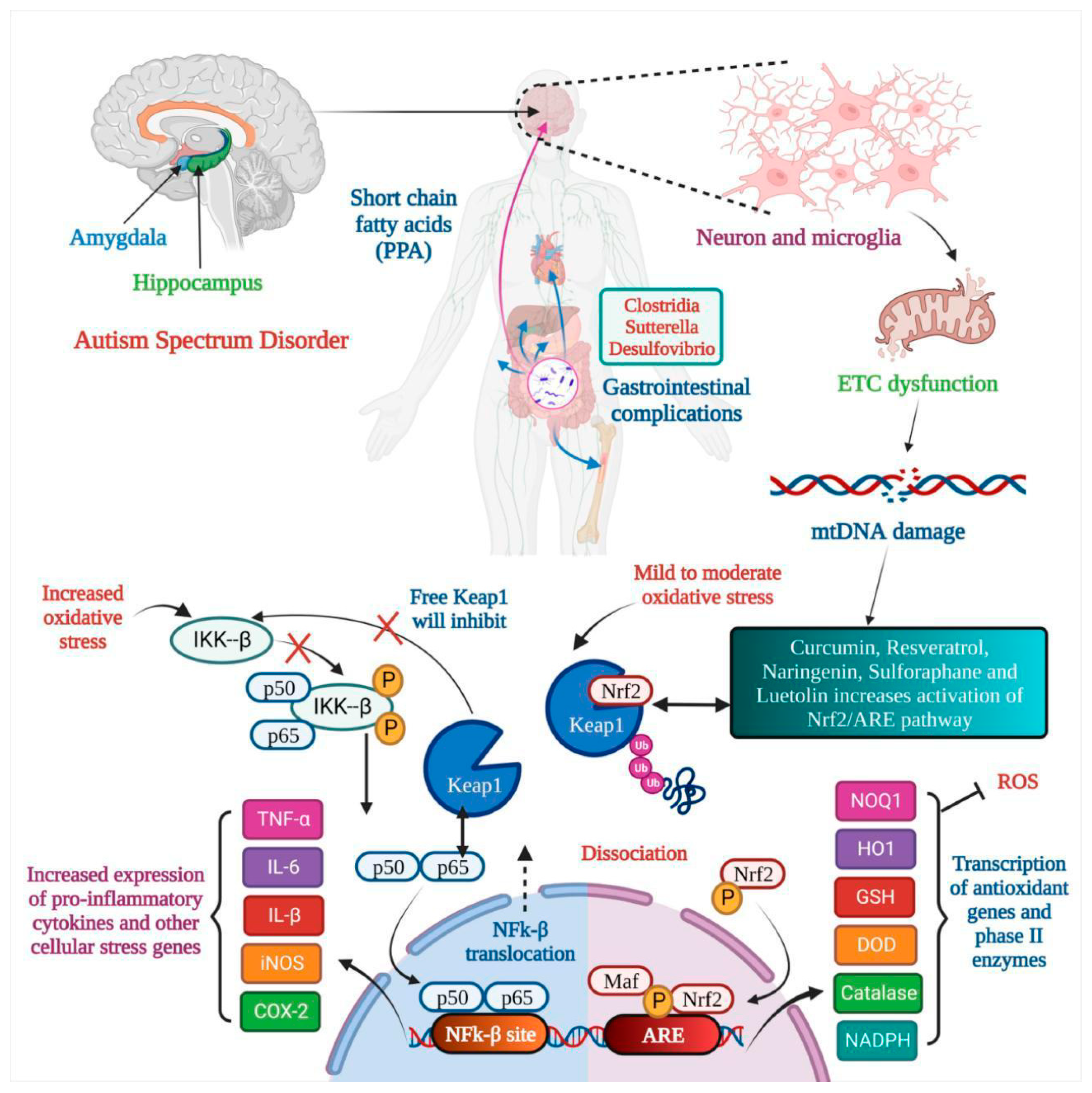
4. Antioxidants for ASD
4.1. Curcumin for ASD
4.2. Resveratrol for ASD
4.3. Naringenin for ASD
4.4. Sulforaphane for ASD
4.5. Luteolin for ASD
5. Camel Milk for ASD
6. Hormone Therapies for ASD
6.1. Melatonin for ASD
6.2. Oxytocin for ASD
6.3. Vasopressin for ASD
7. Herbal Medicine for ASD
8. Cannabidiols for ASD
| Name of Natural Product | Clinical Model of ASD | Method and Duration | Result | References |
|---|---|---|---|---|
| Cannabidiol | 150 participants (5–21 years of age) | Entire plant cannabis extricate that included cannabidiol and \sΔ9-tetrahydrocannabinol at a 20:1 ratio and distilled cannabidiol and Δ9-tetrahydrocannabinol at a 20:1 ratio. | Whole-plant extract showed 49% improvement in behavior with no severe effect, with common adverse effects like decreased appetite and somnolence. | [146] |
| Cannabidiol | 18 autistic patients | Observational study | Cannabidiol and enriched Cannabis sativa extract decreased multiple ASD symptoms, even in epileptic patients | [159] |
| Luteolin | Children (n = 37, 4–14 years old) | Children were given luteolin as well as another supplement for four months | 50% improvement in eye contact and attention, 25% improvement in social skills, 10% improvement in speech, and 75% improvement in GI | [161] |
| Luteolin | 10-year-old male child | Co-ultra peak-LUT | Improved almost all symptoms | [162] |
| Luteolin | Children | Based on serum levels of IL-6 and TNF | Reduction in IL-6 and TNF levels after 26 weeks of treatment improved behavior | [163] |
| Luteolin | 50 children aged 4–10 years old (42 boys, 8 girls) | Open-label trial, one capsule per 10 kg of weight per day with food | Decreased all clinical signs with no significant harmful effects | [164] |
| Cannabidiol | 34 healthy men (half with ASD), 600 mg cannabidiol taken via oral administration | fMRI response to cannabidiol in ASD | Cannabidiol altered the fractional amplitude of low-frequency fluctuations. | [165] |
| Cannabidiol | 34 healthy men (17 neurotypical and 17 ASD) | A single oral dose of 600 mg cannabidiol or placebo | Modulated glutamate GABA system | [166] |
| Cannabidiol | 188 ASD patients | Medical cannabis treatment | 28 patients showed significant improvement, 50 moderate improvement, 6 slight improvement, and 8 no improvement | [167] |
| Ginkgo biloba extract (Ginko T.D., Tolidaru, Iran) | 3 autistic patients | 2 × 100 mg, four weeks | Improvement in behavior | [168] |
| Camel milk | Total of 45 children, three groups of 15 children each | Blood samples for activation-regulated chemokine (TARC) serum level and childhood autism rating scale (CARS) score were taken before and after participants consumed 500 mL of milk per day in their daily diet for two weeks. | Reduced level of TARC and improvement in CAR score | [169] |
| Gluten-free diet | 80 children, two groups (one regular group consisting of 40 children and one gluten-free diet group consisting of 40 children), and 53.9% of children had gastrointestinal abnormalities | The Rome questionnaire was used to examine gastrointestinal symptoms, and the Gilliam Autism Rating Scale 2 (GARS-2) was used to assess psychometric qualities. | Reduction in gastrointestinal symptoms and ASD symptoms | [170] |
| GFCF diet | 37 patients, six months on a regular diet and six months on GFCF | Questionnaires regarding behavior | No change in behavior after consumption of GFCF for 6 months | [171] |
| GFCF diet | 14 children, 3–5 years age | 12-week double-blind, placebo-controlled trial study with continuation of the diet, with a 12-week follow-up and dietary supplement delivered via snacks | No change in behavior or other autism symptoms | [172] |
| Modified ketogenic, gluten-free diet | 15 children, 2–17 years of age | Open-label clinical trial for three months | Improvement in autism symptoms | [173] |
| Vitamin and omega 3 | 111 children | Trial: Vitamin D (2000 IU/day), omega-3 LCPUFA (722 mg/day EPA and DHA, OM), or both for 12 months. | Vitamin D and omega-3 LCPUFA reduced irritability symptoms | [174] |
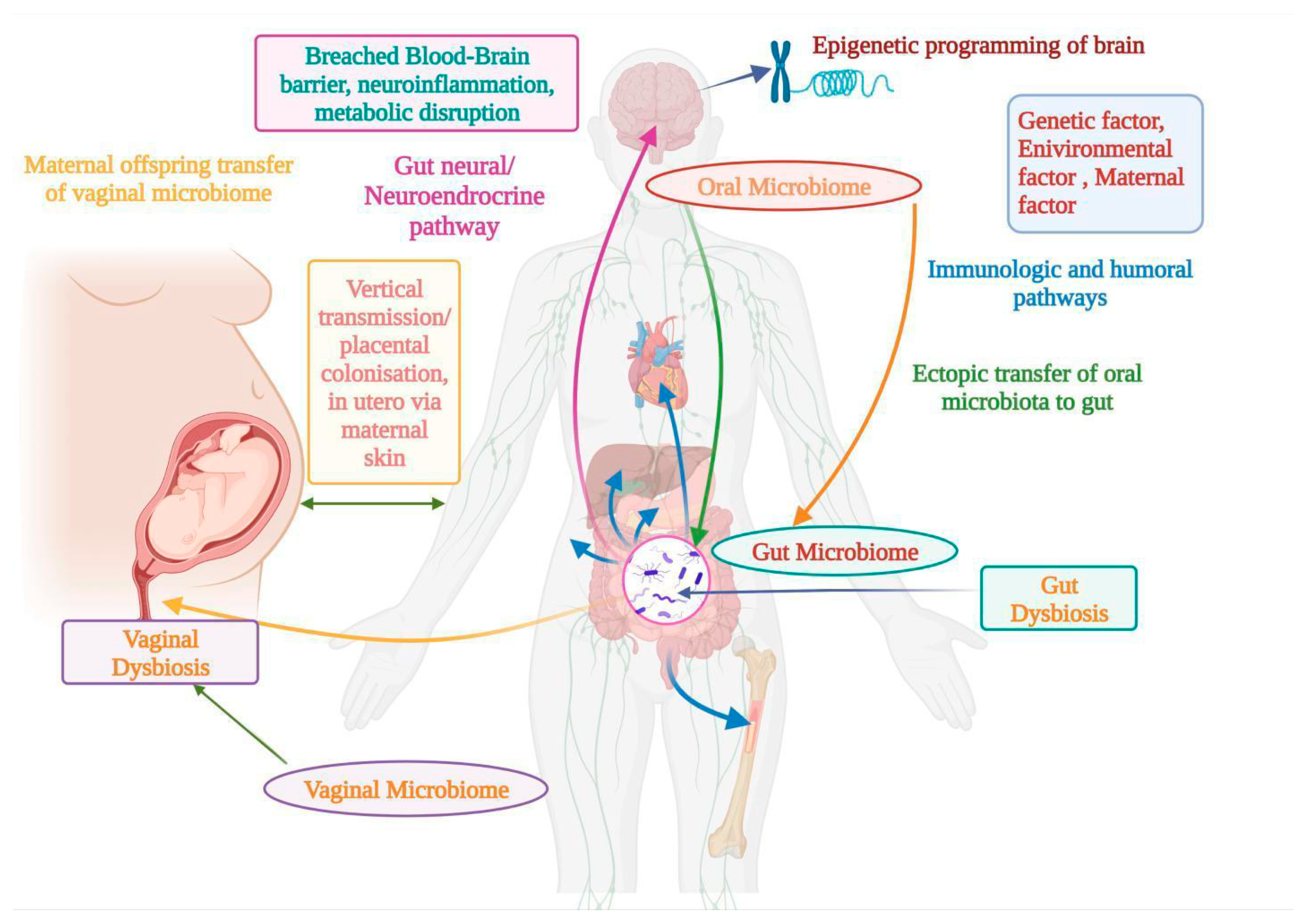
9. Relationship between Microbiome and ASD
9.1. Oral Microbiome for ASD
9.2. Gut Microbiome for ASD
9.3. Vaginal Microbiome for ASD
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder |
| CAM | Complementary and alternative medicine |
| ROS | Reactive oxygen species |
| PDDs | Pervasive developmental disorders |
| AAs | Amino acids |
| AS | Asperger’s syndrome |
| NMDA | N-methyl D-aspartate |
| NO | Nitric oxide |
| BBB | blood–brain barrier |
| MDA | Malondialdehyde |
| DHA | Docosahexaenoic acid |
| ALA | Alpha-linolenic acid |
| EPA | Eicosapentaenoic acid |
| NF-κB | Nuclear factor kappa B |
| PI3K | Phosphatidylinositol 3-Kinase |
| RCTs | Randomized controlled trials |
| BDNF | Brain-derived neurotrophic factor |
| COX | Cyclooxygenase |
| PPARs | Peroxisome proliferator-activated receptors |
| eNOS | Endothelial nitric oxide synthase |
| SIRT1 | Sirtuin (silent mating type information regulation homolog 1) |
| AMPK | AMP-activated protein kinase |
| RSV | Resveratrol |
| VPA | Valproic acid |
| PVC | Parvalbumin |
| PPA | Propionic acid |
| MMP-9 | Matrix metalloproteinase-9 |
| SD | Schizotypal disorder |
| NAR | Naringenin |
| VLDL | Very low-density lipoproteins |
| MCAO | Middle cerebral artery occlusion |
| SOCS3 | Suppressor of cytokine signaling 3 |
| NRF2 | Nuclear factor erythroid 2–related factor 2 |
| CGA | Clinical Global Assessment |
| ABC | Autism Behavior Checklist |
| SRS | Social Responsiveness Scale |
| GSH-Px | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| MPO | Myeloperoxidase |
| ETC | Electron transport chain |
| GI | Gastrointestinal |
| RDBPC | Randomized Double Blind Placebo Control |
| PedPRM | Pediatric-appropriate prolonged-release melatonin |
| RRBs | Restricted and repetitive behaviors |
| IMFAR | International Meeting for Autism Research |
| SRS | Social Responsiveness Scale |
| CBD | Cannabidiol |
| THC | Tetrahydrocannabinol |
| GABAA | Gamma-Aminobutyric Acid-A |
| AEA | N-arachidonoylethanolamine (anandamide) |
| ECS | Endocannabinoid system |
| CNS | Central nervous system |
| DAGL | Diacylglycerol lipase |
| 2-AG | 2-arachidonoylglycerol |
| FXS | Fragile X Syndrome |
| CBDV | Cannabidavarin |
| ADAMS | Anxiety, Depression, and Mood Scale |
| FDA | Food and Drug Administration |
| SCFAs | Short-chain fatty acids |
| Mg | Magnesium |
| PD | Parkinson’s disease |
References
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Asperger, H. Die „Autistischen psychopathen” im kindesalter. Arch. Psychiatr. Nervenkrankh. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179. [Google Scholar]
- Wiśniowiecka-Kowalnik, B.; Nowakowska, B.A. Genetics and epigenetics of autism spectrum disorder—Current evidence in the field. J. Appl. Genet. 2019, 60, 37–47. [Google Scholar] [PubMed]
- Ahmad, F.; Virmani, A.; Irfan, M.; Rankawat, S.; Pathak, U. Critical appraisals on depressions and psychotic symptoms. J. Neurobehav. Sci. 2021, 8, 81–88. [Google Scholar]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T.; et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1. [Google Scholar]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar]
- Williams, J.G.; Higgins, J.P.; Brayne, C.E. Systematic review of prevalence studies of autism spectrum disorders. Arch. Dis. Child. 2006, 91, 8–15. [Google Scholar] [CrossRef]
- Fombonne, E. Epidemiology of autistic disorder and other pervasive developmental disorders. J. Clin. Psychiatry 2005, 66, 3. [Google Scholar]
- Shattuck, P.T. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics 2006, 117, 1028–1037. [Google Scholar]
- Williams, J.; Allison, C.; Scott, F.; Stott, C.; Bolton, P.; Baron-Cohen, S.; Brayne, C. The Childhood Asperger Syndrome Test (CAST): Test-retest reliability. Autism Int. J. Res. Pract. 2006, 10, 415–427. [Google Scholar]
- Wing, L.; Potter, D. The epidemiology of autistic spectrum disorders: Is the prevalence rising? Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 151–161. [Google Scholar] [PubMed]
- Bishop, D.V.; Whitehouse, A.J.; Watt, H.J.; Line, E.A. Autism and diagnostic substitution: Evidence from a study of adults with a history of developmental language disorder. Dev. Med. Child Neurol. 2008, 50, 341–345. [Google Scholar] [PubMed]
- Croen, L.A.; Grether, J.K.; Hoogstrate, J.; Selvin, S. The changing prevalence of autism in California. J. Autism Dev. Disord. 2002, 32, 207–215. [Google Scholar] [PubMed]
- Barbaresi, W.J.; Katusic, S.K.; Colligan, R.C.; Weaver, A.L.; Jacobsen, S.J. The incidence of autism in Olmsted County, Minnesota, 1976–1997: Results from a population-based study. Arch. Pediatr. Adolesc. Med. 2005, 159, 37–44. [Google Scholar]
- Hertz-Picciotto, I.; Delwiche, L. The rise in autism and the role of age at diagnosis. Epidemiology 2009, 20, 84. [Google Scholar]
- Mandell, D.S.; Palmer, R. Differences among states in the identification of autistic spectrum disorders. Arch. Pediatr. Adolesc. Med. 2005, 159, 266–269. [Google Scholar]
- Parner, E.T.; Schendel, D.E.; Thorsen, P. Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Arch. Pediatr. Adolesc. Med. 2008, 162, 1150–1156. [Google Scholar]
- Loomes, R.; Hull, L.; Mandy, W. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar]
- Asherson, P.J.; Curran, S. Approaches to gene mapping in complex disorders and their application in child psychiatry and psychology. Br. J. Psychiatry 2001, 179, 122–128. [Google Scholar]
- Zbiciak, A.; Markiewicz, T. A new extraordinary means of appeal in the Polish criminal procedure: The basic principles of a fair trial and a complaint against a cassatory judgment. Access Justice East. Eur. 2023, 6, 1–18. [Google Scholar] [CrossRef]
- Fombonne, E.; Zakarian, R.; Bennett, A.; Meng, L.; McLean-Heywood, D. Pervasive developmental disorders in Montreal, Quebec, Canada: Prevalence and links with immunizations. Pediatrics 2006, 118, e139–e150. [Google Scholar] [CrossRef]
- Jorde, L.B.; Hasstedt, S.J.; Ritvo, E.R.; Mason-Brothers, A.; Freeman, B.J.; Pingree, C.; McMahon, W.M.; Petersen, B.; Jenson, W.R.; Mo, A. Complex segregation analysis of autism. Am. J. Hum. Genet. 1991, 49, 932–938. [Google Scholar]
- Lauritsen, M.B.; Pedersen, C.B.; Mortensen, P.B. Effects of familial risk factors and place of birth on the risk of autism: A nationwide register-based study. J. Child Psychol. Psychiatry Allied Discip. 2005, 46, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The genetics of autism. Pediatrics 2004, 113, e472–e486. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Dobkins, K.; et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics 2011, 128, e488–e495. [Google Scholar] [CrossRef]
- Piven, J.; Gayle, J.; Chase, G.A.; Fink, B.; Landa, R.; Wzorek, M.M.; Folstein, S.E. A family history study of neuropsychiatric disorders in the adult siblings of autistic individuals. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 177–183. [Google Scholar] [CrossRef]
- Risch, N.; Spiker, D.; Lotspeich, L.; Nouri, N.; Hinds, D.; Hallmayer, J.; Kalaydjieva, L.; McCague, P.; Dimiceli, S.; Pitts, T.; et al. A genomic screen of autism: Evidence for a multilocus etiology. Am. J. Hum. Genet. 1999, 65, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, G.B.; Mendelsohn, N.J.; Professional Practice and Guidelines Committee. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet. Med. Off. J. Am. Coll. Med. Genet. 2013, 15, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.N.; Zhang, Y.; Frazier, T.; Abbacchi, A.M.; Law, P. Sibling recurrence and the genetic epidemiology of autism. Am. J. Psychiatry 2010, 167, 1349–1356. [Google Scholar] [CrossRef]
- Palmer, N.; Beam, A.; Agniel, D.; Eran, A.; Manrai, A.; Spettell, C.; Steinberg, G.; Mandl, K.; Fox, K.; Nelson, S.F.; et al. Association of Sex with Recurrence of Autism Spectrum Disorder Among Siblings. JAMA Pediatr. 2017, 171, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, P.; Mehdi, I.; Kaith, R.; Ahmad, F.; Anwar, M.S. Potential natural products for the management of autism spectrum disorder. Ibrain 2022, 8, 365–376. [Google Scholar] [CrossRef]
- Dalton, K.M.; Nacewicz, B.M.; Alexander, A.L.; Davidson, R.J. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol. Psychiatry 2007, 61, 512–520. [Google Scholar] [CrossRef]
- Gamliel, I.; Yirmiya, N.; Jaffe, D.H.; Manor, O.; Sigman, M. Developmental trajectories in siblings of children with autism: Cognition and language from 4 months to 7 years. J. Autism Dev. Disord. 2009, 39, 1131–1144. [Google Scholar] [CrossRef]
- Gamliel, I.; Yirmiya, N.; Sigman, M. The development of young siblings of children with autism from 4 to 54 months. J. Autism Dev. Disord. 2007, 37, 171–183. [Google Scholar] [CrossRef]
- Piven, J.; Palmer, P.; Jacobi, D.; Childress, D.; Arndt, S. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. Am. J. Psychiatry 1997, 154, 185–190. [Google Scholar]
- Yirmiya, N.; Gamliel, I.; Shaked, M.; Sigman, M. Cognitive and verbal abilities of 24- to 36-month-old siblings of children with autism. J. Autism Dev. Disord. 2007, 37, 218–229. [Google Scholar] [CrossRef]
- Baron-Cohen, S. Two new theories of autism: Hyper-systemising and assortative mating. Arch. Dis. Child. 2006, 91, 2–5. [Google Scholar] [CrossRef]
- Ecker, C.; Bookheimer, S.Y.; Murphy, D.G. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. The Lancet. Neurology 2015, 14, 1121–1134. [Google Scholar] [CrossRef]
- Muhle, R.A.; Reed, H.E.; Stratigos, K.A.; Veenstra-VanderWeele, J. The Emerging Clinical Neuroscience of Autism Spectrum Disorder: A Review. JAMA Psychiatry 2018, 75, 514–523. [Google Scholar] [CrossRef]
- Lopez-Rangel, E.; Lewis, M.E. Loud and clear evidence for gene silencing by epigenetic mechanisms in autism spectrum and related neurodevelopmental disorders. Clin. Genet. 2006, 69, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Samaco, R.C.; Nagarajan, R.P.; Braunschweig, D.; LaSalle, J.M. Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum. Mol. Genet. 2004, 13, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Christison, G.W.; Ivany, K. Elimination diets in autism spectrum disorders: Any wheat amidst the chaff? J. Dev. Behav. Pediatr. JDBP 2006, 27 (Suppl. 2), S162–S171. [Google Scholar] [CrossRef] [PubMed]
- Buie, T. The relationship of autism and gluten. Clin. Ther. 2013, 35, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Sun, S.; Itokazu, N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology 2002, 46, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.M.; Green, P.H.; Taylor, A.K.; Hellberg, D.; Ajamian, M.; Tan, C.Z.; Kosofsky, B.E.; Higgins, J.J.; Rajadhyaksha, A.M.; Alaedini, A. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS ONE 2013, 8, e66155. [Google Scholar] [CrossRef]
- Quan, L.; Xu, X.; Cui, Y.; Han, H.; Hendren, R.L.; Zhao, L.; You, X. A systematic review and meta-analysis of the benefits of a gluten-free diet and/or casein-free diet for children with autism spectrum disorder. Nutr. Rev. 2022, 80, 1237–1246. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Evidence of the gluten-free and casein-free diet in autism spectrum disorders: A systematic review. J. Child Neurol. 2014, 29, 1718–1727. [Google Scholar] [CrossRef]
- Geraghty, M.E.; Bates-Wall, J.; Ratliff-Schaub, K.; Lane, A.E. Nutritional interventions and therapies in autism: A spectrum of what we know: Part 2. ICAN Infant Child Adolesc. Nutr. 2010, 2, 120–133. [Google Scholar] [CrossRef][Green Version]
- Horvath, K.; Perman, J.A. Autism and gastrointestinal symptoms. Curr. Gastroenterol. Rep. 2002, 4, 251–258. [Google Scholar] [CrossRef]
- White, J.F. Intestinal pathophysiology in autism. Exp. Biol. Med. 2003, 228, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, A.; Landini, M.; Facchiano, F.; Raggi, M.E.; Villa, L.; Molteni, M.; De Santis, B.; Brera, C.; Caroli, A.M.; Milanesi, L.; et al. Environment, dysbiosis, immunity and sex-specific susceptibility: A translational hypothesis for regressive autism pathogenesis. Nutr. Neurosci. 2015, 18, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Kawicka, A.; Regulska-Ilow, B. How nutritional status, diet and dietary supplements can affect autism. A review. Rocz. Państwowego Zakładu Hig. 2013, 64, 1–12. [Google Scholar]
- Gottschall, E. Digestion-gut-autism connection: The specific carbohydrate diet. Med. Veritas 2004, 1, 261–271. [Google Scholar] [CrossRef]
- Żarnowska, I.; Chrapko, B.; Gwizda, G.; Nocuń, A.; Mitosek-Szewczyk, K.; Gasior, M. Therapeutic use of carbohydrate-restricted diets in an autistic child; a case report of clinical and 18FDG PET findings. Metab. Brain Dis. 2018, 33, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, D.N.; Svedova, J.; Cote, J.L.; Sandau, U.; Rho, J.M.; Kawamura, M., Jr.; Boison, D.; Masino, S.A. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS ONE 2013, 8, e65021. [Google Scholar] [CrossRef]
- Napoli, E.; Dueñas, N.; Giulivi, C. Potential therapeutic use of the ketogenic diet in autism spectrum disorders. Front. Pediatr. 2014, 2, 69. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, Y.; Tomi, M.; Shin, B.C.; Thamotharan, S.; Mazarati, A.; Sankar, R.; Wang, E.A.; Cepeda, C.; Levine, M.S.; et al. Sex-Specific Life Course Changes in the Neuro-Metabolic Phenotype of Glut3 Null Heterozygous Mice: Ketogenic Diet Ameliorates Electroencephalographic Seizures and Improves Sociability. Endocrinology 2017, 158, 936–949. [Google Scholar] [CrossRef]
- Kasprowska-Liśkiewicz, D.; Liśkiewicz, A.D.; Nowacka-Chmielewska, M.M.; Nowicka, J.; Małecki, A.; Barski, J.J. The ketogenic diet affects the social behavior of young male rats. Physiol. Behav. 2017, 179, 168–177. [Google Scholar] [CrossRef]
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: A case-control study. Metab. Brain Dis. 2017, 32, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Ahmed, A.; Tollefsbol, T.O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenet. 2010, 1, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Rollett, A. Zur Kenntnis der Linolensäure und des Leinöls; De Gruyter: Berlin, Germany, 1909. [Google Scholar]
- Cheng, Y.S.; Tseng, P.T.; Chen, Y.W.; Stubbs, B.; Yang, W.C.; Chen, T.Y.; Wu, C.K.; Lin, P.Y. Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: A meta-analysis of randomized controlled trials. Neuropsychiatr. Dis. Treat. 2017, 13, 2531–2543. [Google Scholar] [CrossRef] [PubMed]
- Hagmeyer, S.; Sauer, A.K.; Grabrucker, A.M. Prospects of Zinc Supplementation in Autism Spectrum Disorders and Shankopathies Such as Phelan McDermid Syndrome. Front. Synaptic Neurosci. 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Saneto, R.; Falk, M.J.; Anselm, I.; Cohen, B.H.; Haas, R.; Medicine Society, T.M. A modern approach to the treatment of mitochondrial disease. Curr. Treat. Options Neurol. 2009, 11, 414–430. [Google Scholar] [CrossRef]
- Bou Khalil, R.; Yazbek, J.C. Potential importance of supplementation with zinc for autism spectrum disorder. L’Encephale 2021, 47, 514–517. [Google Scholar] [CrossRef]
- Gunes, S.; Ekinci, O.; Celik, T. Iron deficiency parameters in autism spectrum disorder: Clinical correlates and associated factors. Ital. J. Pediatr. 2017, 43, 86. [Google Scholar] [CrossRef]
- Prakash, P.; Kumari, R.; Sinha, N.; Kumar, S.; Sinha, P. Evaluation of Iron Status in Children with Autism Spectral Disorder: A Case-control Study. J. Clin. Diagn. Res. 2021, 15, BC01–BC04. [Google Scholar] [CrossRef]
- Mousain-Bosc, M.; Roche, M.; Polge, A.; Pradal-Prat, D.; Rapin, J.; Bali, J.P. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. Magnes. Res. 2006, 19, 46–52. [Google Scholar]
- Galland, L. Magnesium, stress and neuropsychiatric disorders. Magnes. Trace Elem. 1993, 10, 287. [Google Scholar]
- Błażewicz, A.; Szymańska, I.; Dolliver, W.; Suchocki, P.; Turło, J.; Makarewicz, A.; Skórzyńska-Dziduszko, K. Are Obese Patients with Autism Spectrum Disorder More Likely to Be Selenium Deficient? Research Findings on Pre- and Post-Pubertal Children. Nutrients 2020, 12, 3581. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007, 595, 197–212. [Google Scholar] [PubMed]
- Al-Askar, M.; Bhat, R.S.; Selim, M.; Al-Ayadhi, L.; El-Ansary, A. Postnatal treatment using curcumin supplements to amend the damage in VPA-induced rodent models of autism. BMC Complement. Altern. Med. 2017, 17, 259. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Neuropsychopharmacotherapeutic efficacy of curcumin in experimental paradigm of autism spectrum disorders. Life Sci. 2015, 141, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Sahebkar, A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother. Res. PTR 2015, 29, 17–21. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother. Res. PTR 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin enhances neurogenesis and cognition in aged rats: Implications for transcriptional interactions related to growth and synaptic plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef]
- Tizabi, Y.; Hurley, L.L.; Qualls, Z.; Akinfiresoye, L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules 2014, 19, 20864–20879. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflamm. 2011, 8, 125. [Google Scholar] [CrossRef]
- Tegenge, M.A.; Rajbhandari, L.; Shrestha, S.; Mithal, A.; Hosmane, S.; Venkatesan, A. Curcumin protects axons from degeneration in the setting of local neuroinflammation. Exp. Neurol. 2014, 253, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Paliwal, J.K.; Kuhad, A. Dietary phytochemicals as neurotherapeutics for autism spectrum disorder: Plausible mechanism and evidence. In Personalized Food Intervention and Therapy for Autism Spectrum Disorder Management; Springer: Cham, Switzerland, 2020; pp. 615–646. [Google Scholar]
- Bassani, T.B.; Turnes, J.M.; Moura, E.; Bonato, J.M.; Cóppola-Segovia, V.; Zanata, S.M.; Oliveira, R.; Vital, M. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017, 335, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin Improves Amyloid β-Peptide (1-42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS ONE 2015, 10, e0131525. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef]
- Jacob, A.; Wu, R.; Zhou, M.; Wang, P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-gamma Activation. PPAR Res. 2007, 2007, 89369. [Google Scholar] [CrossRef]
- Saja, K.; Babu, M.S.; Karunagaran, D.; Sudhakaran, P.R. Anti-inflammatory effect of curcumin involves downregulation of MMP-9 in blood mononuclear cells. Int. Immunopharmacol. 2007, 7, 1659–1667. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Wendeburg, L.; de Oliveira, A.C.; Bhatia, H.S.; Candelario-Jalil, E.; Fiebich, B.L. Resveratrol inhibits prostaglandin formation in IL-1beta-stimulated SK-N-SH neuronal cells. J. Neuroinflamm. 2009, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Steinberg, G.R. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes 2010, 59, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.T.; Gruber, J.; Jenner, A.M.; Ng, M.P.; Ruan, R.; Tay, F.E. Elevation of oxidative-damage biomarkers during aging in F2 hybrid mice: Protection by chronic oral intake of resveratrol. Free Radic. Biol. Med. 2009, 46, 799–809. [Google Scholar] [CrossRef]
- Fontes-Dutra, M.; Santos-Terra, J.; Deckmann, I.; Brum Schwingel, G.; Della-Flora Nunes, G.; Hirsch, M.M.; Bauer-Negrini, G.; Riesgo, R.S.; Bambini-Júnior, V.; Hedin-Pereira, C.; et al. Resveratrol Prevents Cellular and Behavioral Sensory Alterations in the Animal Model of Autism Induced by Valproic Acid. Front. Synaptic Neurosci. 2018, 10, 9. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, G.Y.; Park, K.Y.; Choi, Y.H. Resveratrol inhibits nitric oxide and prostaglandin E2 production by lipopolysaccharide-activated C6 microglia. J. Med. Food 2007, 10, 218–224. [Google Scholar] [CrossRef]
- Bambini-Junior, V.; Zanatta, G.; Della Flora Nunes, G.; Mueller de Melo, G.; Michels, M.; Fontes-Dutra, M.; Nogueira Freire, V.; Riesgo, R.; Gottfried, C. Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci. Lett. 2014, 583, 176–181. [Google Scholar] [CrossRef]
- Roullet, F.I.; Lai, J.K.; Foster, J.A. In utero exposure to valproic acid and autism--a current review of clinical and animal studies. Neurotoxicol. Teratol. 2013, 36, 47–56. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- McCalley, A.E.; Kaja, S.; Payne, A.J.; Koulen, P. Resveratrol and calcium signaling: Molecular mechanisms and clinical relevance. Molecules 2014, 19, 7327–7340. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, X.; Gao, D.; Jiang, X.; Dong, W. Resveratrol inhibits MMP-9 expression by up-regulating PPAR alpha expression in an oxygen glucose deprivation-exposed neuron model. Neurosci. Lett. 2009, 451, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, X.; Jiang, X.; Peng, Y.; Huang, W.; Cheng, G.; Song, L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006, 78, 2564–2570. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martínez, P.; Martín-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J. Agric. Food Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, U.; Klittich, K.; Staib, A.H. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br. J. Clin. Pharmacol. 1993, 35, 431–436. [Google Scholar] [CrossRef]
- Raza, S.S.; Khan, M.M.; Ahmad, A.; Ashafaq, M.; Islam, F.; Wagner, A.P.; Safhi, M.M.; Islam, F. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience 2013, 230, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Liu, B.B.; Li, J.; Luo, L.; Liu, Q.; Geng, D.; Tang, Y.; Xia, Y.; Wu, D. BDNF signaling is necessary for the antidepressant-like effect of naringenin. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 135–141. [Google Scholar] [CrossRef]
- Galluzzo, P.; Ascenzi, P.; Bulzomi, P.; Marino, M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor alpha-palmitoylation. Endocrinology 2008, 149, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- Nahmias, Y.; Goldwasser, J.; Casali, M.; van Poll, D.; Wakita, T.; Chung, R.T.; Yarmush, M.L. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 2008, 47, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Lin, C.; Lin, H.Y.; Liu, Y.S.; Wu, C.Y.; Tsai, C.F.; Chang, P.C.; Yeh, W.L.; Lu, D.Y. Naringenin Suppresses Neuroinflammatory Responses Through Inducing Suppressor of Cytokine Signaling 3 Expression. Mol. Neurobiol. 2016, 53, 1080–1091. [Google Scholar] [CrossRef]
- Bhandari, R.; Paliwal, J.K.; Kuhad, A. Naringenin and its nanocarriers as potential phytotherapy for autism spectrum disorders. J. Funct. Foods 2018, 47, 361–375. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1148–G1154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tiku, A.B. Biochemical and Molecular Mechanisms of Radioprotective Effects of Naringenin, a Phytochemical from Citrus Fruits. J. Agric. Food Chem. 2016, 64, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Tapia, E.; Pedraza-Chaverri, J. Modulation of mitochondrial functions by the indirect antioxidant sulforaphane: A seemingly contradictory dual role and an integrative hypothesis. Free Radic. Biol. Med. 2013, 65, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kensler, T.W.; Cho, C.G.; Posner, G.H.; Talalay, P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fang, H.; Zhen, Y.; Xu, G.; Tian, J.; Zhang, Y.; Zhang, D.; Zhang, G.; Xu, J.; Zhang, Z.; et al. Sulforaphane Prevents Neuronal Apoptosis and Memory Impairment in Diabetic Rats. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 39, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Soane, L.; Li, D.W.; Fiskum, G.; Bambrick, L.L. Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation. J. Neurosci. Res. 2010, 88, 1355–1363. [Google Scholar] [CrossRef]
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006, 393, 108–112. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef]
- Gan, N.; Wu, Y.C.; Brunet, M.; Garrido, C.; Chung, F.L.; Dai, C.; Mi, L. Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J. Biol. Chem. 2010, 285, 35528–35536. [Google Scholar] [CrossRef]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Bent, S.; Lawton, B.; Warren, T.; Widjaja, F.; Dang, K.; Fahey, J.W.; Cornblatt, B.; Kinchen, J.M.; Delucchi, K.; Hendren, R.L. Identification of urinary metabolites that correlate with clinical improvements in children with autism treated with sulforaphane from broccoli. Mol. Autism 2018, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Parker-Athill, E.; Luo, D.; Bailey, A.; Giunta, B.; Tian, J.; Shytle, R.D.; Murphy, T.; Legradi, G.; Tan, J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J. Neuroimmunol. 2009, 217, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, M.; Mottolese, N.; Galvani, G.; Ferrara, D.; Gennaccaro, L.; Loi, M.; Medici, G.; Candini, G.; Rimondini, R.; Ciani, E.; et al. Luteolin Treatment Ameliorates Brain Development and Behavioral Performance in a Mouse Model of CDKL5 Deficiency Disorder. Int. J. Mol. Sci. 2022, 23, 8719. [Google Scholar] [CrossRef]
- Kappeler, S.; Farah, Z.; Puhan, Z. Sequence analysis of Camelus dromedarius milk caseins. J. Dairy Res. 1998, 65, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Al-Ayadhi, L.Y. Effect of camel milk on thymus and activation-regulated chemokine in autistic children: Double-blind study. Pediatr. Res. 2014, 75, 559–563. [Google Scholar] [CrossRef]
- Shabo, Y.; Barzel, R.; Margoulis, M.; Yagil, R. Camel milk for food allergies in children. Isr. Med. Assoc. J. IMAJ 2005, 7, 796–798. [Google Scholar] [PubMed]
- Zafra, O.; Fraile, S.; Gutiérrez, C.; Haro, A.; Páez-Espino, A.D.; Jiménez, J.I.; de Lorenzo, V. Monitoring biodegradative enzymes with nanobodies raised in Camelus dromedarius with mixtures of catabolic proteins. Environ. Microbiol. 2011, 13, 960–974. [Google Scholar] [CrossRef]
- Tillib, S.V.; Ivanova, T.I.; Vasilev, L.A. Fingerprint-like Analysis of “Nanoantibody” Selection by Phage Display Using Two Helper Phage Variants. Acta Naturae 2010, 2, 85–93. [Google Scholar] [CrossRef]
- Abdel Galil, M.A.G.; Abdulqader, A.A. The unique medicinal properties of camel products: A review of the scientific evidence. J. Taibah Univ. Med. Sci. 2016, 11, 98–103. [Google Scholar] [CrossRef]
- Al-Ayadhi, L.Y.; Halepoto, D.M.; Al-Dress, A.M.; Mitwali, Y.; Zainah, R. Behavioral Benefits of Camel Milk in Subjects with Autism Spectrum Disorder. J. Coll. Physicians Surg.-Pak. JCPSP 2015, 25, 819–823. [Google Scholar] [PubMed]
- Gagnon, K.; Godbout, R. Melatonin and Comorbidities in Children with Autism Spectrum Disorder. Curr. Dev. Disord. Rep. 2018, 5, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, B.M.; Vaz, S.; Lee, E.; Thompson, C.; Rogerson, J.M.; Falkmer, T. Effectiveness of Sleep-Based Interventions for Children with Autism Spectrum Disorder: A Meta-Synthesis. Pharmacotherapy 2017, 37, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Damiani, J.M.; Sweet, B.V.; Sohoni, P. Melatonin: An option for managing sleep disorders in children with autism spectrum disorder. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2014, 71, 95–101. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef]
- Braam, W.; Ehrhart, F.; Maas, A.; Smits, M.G.; Curfs, L. Low maternal melatonin level increases autism spectrum disorder risk in children. Res. Dev. Disabil. 2018, 82, 79–89. [Google Scholar] [CrossRef]
- Yamasue, H.; Domes, G. Oxytocin and Autism Spectrum Disorders. Curr. Top. Behav. Neurosci. 2018, 35, 449–465. [Google Scholar]
- Wilczyński, K.M.; Zasada, I.; Siwiec, A.; Janas-Kozik, M. Differences in oxytocin and vasopressin levels in individuals suffering from the autism spectrum disorders vs. general population—A systematic review. Neuropsychiatr. Dis. Treat. 2019, 15, 2613–2620. [Google Scholar] [CrossRef]
- Voinsky, I.; Bennuri, S.C.; Svigals, J.; Frye, R.E.; Rose, S.; Gurwitz, D. Peripheral Blood Mononuclear Cell Oxytocin and Vasopressin Receptor Expression Positively Correlates with Social and Behavioral Function in Children with Autism. Sci. Rep. 2019, 9, 13443. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Jomha, F.A.; Alhammadi, A.H. Vasopressin in the Amelioration of Social Functioning in Autism Spectrum Disorder. J. Clin. Med. 2019, 8, 1061. [Google Scholar] [CrossRef]
- Bang, M.; Lee, S.H.; Cho, S.H.; Yu, S.A.; Kim, K.; Lu, H.Y.; Chang, G.T.; Min, S.Y. Herbal Medicine Treatment for Children with Autism Spectrum Disorder: A Systematic Review. Evid.-Based Complement. Altern. Med. Ecam 2017, 2017, 8614680. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, S.; Bahmani, M.; Afsordeh, O.; Rafieian, R.; Sheikhian, A. Herbal medicines: A new hope for autism therapy. J. Herbmed Pharmacol. 2016, 5, 89–91. [Google Scholar]
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martínez, A.; Limón, I.D. Cannabidiol as a Therapeutic Target: Evidence of its Neuroprotective and Neuromodulatory Function in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 595635. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Harel, M.; Cassuto, H.; Polyansky, L.; Schnapp, A.; Wattad, N.; Shmueli, D.; Golan, D.; Castellanos, F.X. Cannabinoid treatment for autism: A proof-of-concept randomized trial. Mol. Autism 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Aishworiya, R.; Valica, T.; Hagerman, R.; Restrepo, B. An Update on Psychopharmacological Treatment of Autism Spectrum Disorder. Neurother. J. Am. Soc. Exp. Neurother. 2022, 19, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef]
- Wei, D.; Dinh, D.; Lee, D.; Li, D.; Anguren, A.; Moreno-Sanz, G.; Gall, C.M.; Piomelli, D. Enhancement of Anandamide-Mediated Endocannabinoid Signaling Corrects Autism-Related Social Impairment. Cannabis Cannabinoid Res. 2016, 1, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zeidler, Z.; Moulton, K.; Krych, L.; Xia, Z.; Smith, C.B. Endocannabinoid-mediated improvement on a test of aversive memory in a mouse model of fragile X syndrome. Behav. Brain Res. 2015, 291, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Heussler, H.; Duhig, M.; Hurst, T.; O’Neill, C.; Gutterman, D.; Palumbo, J.M.; Sebree, T. Longer Term Tolerability and Efficacy of ZYN002 Cannabidiol Transdermal Gel in Children and Adolescents with Autism Spectrum Disorder (ASD): An Open-Label Phase 2 Study (BRIGHT [ZYN2-CL-030]). Adolesc. Psychiatry 2020, 12, 24. [Google Scholar]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Cassuto, H.; Lubotzky, A.; Wattad, N.; Hazan, E. Brief Report: Cannabidiol-Rich Cannabis in Children with Autism Spectrum Disorder and Severe Behavioral Problems-A Retrospective Feasibility Study. J. Autism Dev. Disord. 2019, 49, 1284–1288. [Google Scholar] [CrossRef]
- Pretzsch, C.M.; Freyberg, J.; Voinescu, B.; Lythgoe, D.; Horder, J.; Mendez, M.A.; Wichers, R.; Ajram, L.; Ivin, G.; Heasman, M.; et al. Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology 2019, 44, 1398–1405. [Google Scholar] [CrossRef]
- Fleury-Teixeira, P.; Caixeta, F.V.; Ramires da Silva, L.C.; Brasil-Neto, J.P.; Malcher-Lopes, R. Effects of CBD-enriched cannabis sativa extract on autism spectrum disorder symptoms: An observational study of 18 participants undergoing compassionate use. Front. Neurol. 2019, 10, 1145. [Google Scholar] [CrossRef]
- Bar-Lev Schleider, L.; Mechoulam, R.; Saban, N.; Meiri, G.; Novack, V. Real life experience of medical cannabis treatment in autism: Analysis of safety and efficacy. Sci. Rep. 2019, 9, 200. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Asadi, S.; Panagiotidou, S. A case series of a luteolin formulation (NeuroProtek®) in children with autism spectrum disorders. Int. J. Immunopathol. Pharmacol. 2012, 25, 317–323. [Google Scholar] [CrossRef]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial effects of co-ultramicronized palmitoylethanolamide/luteolin in a mouse model of autism and in a case report of autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Das, A.S.; Majumder, M.; Mukhopadhyay, R. Antiatherogenic Roles of Dietary Flavonoids Chrysin, Quercetin, and Luteolin. J. Cardiovasc. Pharmacol. 2016, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Wilson, R.; Appiah-Kusi, E.; O’Neill, A.; Brammer, M.; Perez, J.; Murray, R.; Allen, P.; Bossong, M.G.; McGuire, P. Effect of Cannabidiol on Medial Temporal, Midbrain, and Striatal Dysfunction in People at Clinical High Risk of Psychosis: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Falkenberg, I.; Martin-Santos, R.; Atakan, Z.; Crippa, J.A.; Giampietro, V.; Brammer, M.; McGuire, P. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 1343–1352. [Google Scholar] [CrossRef]
- Davidson, B.L.; Gao, G.; Berry-Kravis, E.; Bradbury, A.; Bönnemann, C.; Buxbaum, J.D.; Corcoran, G.R.; Gray, S.J.; Gray-Edwards, H.; Kleiman, R.J.; et al. Gene-based therapeutics for rare genetic neurodevelopmental psychiatric disorders. Mol. Ther. 2022, 30, 2416–2428. [Google Scholar] [CrossRef]
- Niederhofer, H. First preliminary results of an observation of Ginkgo Biloba treating patients with autistic disorder. Phytother. Res. 2009, 23, 1645–1646. [Google Scholar] [CrossRef]
- Al-Ayadhi, L.Y.; Elamin, N.E. Camel milk as a potential therapy as an antioxidant in autism spectrum disorder (ASD). Evid.-Based Complement. Altern. Med. 2013, 2013, 602834. [Google Scholar] [CrossRef]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J. Pediatr. 2016, 12, 436–442. [Google Scholar] [CrossRef]
- González-Domenech, P.J.; Díaz Atienza, F.; García Pablos, C.; Fernández Soto, M.L.; Martínez-Ortega, J.M.; Gutiérrez-Rojas, L. Influence of a combined gluten-free and casein-free diet on behavior disorders in children and adolescents diagnosed with autism spectrum disorder: A 12-month follow-up clinical trial. J. Autism Dev. Disord. 2020, 50, 935–948. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Foley, J.; Peck, R.; Morris, D.D.; Wang, H.; Smith, T. The gluten-free/casein-free diet: A double-blind challenge trial in children with autism. J. Autism Dev. Disord. 2016, 46, 205–220. [Google Scholar] [CrossRef]
- Lee, R.W.; Corley, M.J.; Pang, A.; Arakaki, G.; Abbott, L.; Nishimoto, M.; Miyamoto, R.; Lee, E.; Yamamoto, S.; Maunakea, A.K.; et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol. Behav. 2018, 188, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H. The Role of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids in Children with Autism Spectrum Disorder. Ph.D. Dissertation, Massey University, Albany, New Zealand, 2018. [Google Scholar]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Proctor, L.M. The national institutes of health human microbiome project. In Seminars in Fetal and Neonatal Medicine; WB Saunders: Philadelphia, PA, USA, 2016; Volume 21, pp. 368–372. [Google Scholar]
- Umbrello, G.; Esposito, S. Microbiota and neurologic diseases: Potential effects of probiotics. J. Transl. Med. 2016, 14, 1–11. [Google Scholar] [CrossRef]
- Johnson, D.; Letchumanan, V.; Thurairajasingam, S.; Lee, L.H. A revolutionizing approach to autism spectrum disorder using the microbiome. Nutrients 2020, 12, 1983. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.O.; Shattuck, P.T.; Wagner, M.; Cooper, B.P. Prevalence and correlates of screen-based media use among youths with autism spectrum disorders. J. Autism Dev. Disord. 2012, 42, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Uhlig, R.; Afshari, P.; Williams, J.; Chroneos, M.; Tierney-Aves, C.; Wagner, K.; Middleton, F.A. Oral microbiome activity in children with autism spectrum disorder. Autism Res. 2018, 11, 1286–1299. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): Implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Olsen, I.; Singhrao, S.K. Can oral infection be a risk factor for Alzheimer’s disease? J. Oral Microbiol. 2015, 7, 29143. [Google Scholar] [CrossRef]
- Curtin, C.; Hubbard, K.; Anderson, S.E.; Mick, E.; Must, A.; Bandini, L.G. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Hicks, S.D. Oral microbiota and autism spectrum disorder (ASD). J. Oral Microbiol. 2020, 12, 1702806. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.E.; Lopez, C.A.; Bäumler, A.J. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013, 14, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Darveau, R.P.; Hajishengallis, G.; Curtis, M.A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef]
- Jaber, M.A. Dental caries experience, oral health status and treatment needs of dental patients with autism. J. Appl. Oral Sci. 2011, 19, 212–217. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Jeffcoat, M.K.; Hauth, J.C.; Geurs, N.C.; Reddy, M.S.; Cliver, S.P.; Hodgkins, P.M.; Goldenberg, R.L. Periodontal disease and preterm birth: Results of a pilot intervention study. J. Periodontol. 2003, 74, 1214–1218. [Google Scholar] [CrossRef]
- Han, Y.W.; Ikegami, A.; Bissada, N.F.; Herbst, M.; Redline, R.W.; Ashmead, G.G. Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J. Clin. Microbiol. 2006, 44, 1475–1483. [Google Scholar] [CrossRef]
- Aagaard, K.; Ganu, R.; Ma, J.; Racusin, D.; Arndt, M.; Riehle, K.; Petrosino, J.; Versalovic, J. 8: Whole metagenomic shotgun sequencing reveals a vibrant placental microbiome harboring metabolic function. Am. J. Obstet. Gynecol. 2013, 208, S5. [Google Scholar] [CrossRef]
- Bearfield, C.; Davenport, E.S.; Sivapathasundaram, V.; Allaker, R.P. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fiscella, K.A.; Gill, S.R. Oral microbiome: Possible harbinger for children’s health. Int. J. Oral Sci. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chen, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Neurological diseases with autism spectrum disorder: Role of ASD risk genes. Front. Neurosci. 2019, 13, 349. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Anwar, H.; Irfan, S.; Hussain, G.; Faisal, M.N.; Muzaffar, H.; Mustafa, I.; Mukhtar, I.; Malik, S.; Ullah, M.I. Gut microbiome: A new organ system in body. Parasitol. Microbiol. Res. 2019, 1, 17–21. [Google Scholar]
- Principi, N.; Esposito, S. Gut microbiota and central nervous system development. J. Infect. 2016, 73, 536–546. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Zhang, J.; He, Y.; Wang, J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci. Rep. 2018, 8, 13918. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, M.; Feng, Y.; Zhou, Z.; Chen, L.; Chen, F. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci. Rep. 2018, 8, 1597. [Google Scholar] [CrossRef] [PubMed]
- Van Ameringen, M.; Turna, J.; Patterson, B.; Pipe, A.; Mao, R.Q.; Anglin, R.; Surette, M.G. The gut microbiome in psychiatry: A primer for clinicians. Depress. Anxiety 2019, 36, 1004–1025. [Google Scholar] [CrossRef] [PubMed]
- Bronson, S.L.; Bale, T.L. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 2014, 155, 2635–2646. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Connolly, N.; Anixt, J.; Manning, P.; Ping-I Lin, D.; Marsolo, K.A.; Bowers, K. Maternal metabolic risk factors for autism spectrum disorder—An analysis of electronic medical records and linked birth data. Autism Res. 2016, 9, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; Forslund, K.; Bork, P.; De Vos, W.M. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 2016, 7, 10410. [Google Scholar] [CrossRef]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism spectrum disorders and the gut microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.; Bocking, A.; Challis, J.; Reid, G. Effect of extracorporeal shock wave lithotripsy on bacterial viability-Relationship to the treatment of struvite stones. Colloids Surf. B Biointerfaces 2007, 55, 138. [Google Scholar] [CrossRef]
- Cribby, S.; Taylor, M.; Reid, G. Vaginal microbiota and the use of probiotics. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 256490. [Google Scholar] [CrossRef]
- Bokobza, C.; Van Steenwinckel, J.; Mani, S.; Mezger, V.; Fleiss, B.; Gressens, P. Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr. Res. 2019, 85, 155–165. [Google Scholar] [CrossRef]
- Careaga, M.; Murai, T.; Bauman, M.D. Maternal immune activation and autism spectrum disorder: From rodents to nonhuman and human primates. Biol. Psychiatry 2017, 81, 391–401. [Google Scholar] [CrossRef]
- Brown, A.S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012, 72, 1272–1276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, A.; Sharma, R.; Abiha, U.; Ahmad, F.; Karan, A.; Jayaraj, R.L.; Sundar, V. A Spectrum of Solutions: Unveiling Non-Pharmacological Approaches to Manage Autism Spectrum Disorder. Medicina 2023, 59, 1584. https://doi.org/10.3390/medicina59091584
Mondal A, Sharma R, Abiha U, Ahmad F, Karan A, Jayaraj RL, Sundar V. A Spectrum of Solutions: Unveiling Non-Pharmacological Approaches to Manage Autism Spectrum Disorder. Medicina. 2023; 59(9):1584. https://doi.org/10.3390/medicina59091584
Chicago/Turabian StyleMondal, Arunima, Rashi Sharma, Umme Abiha, Faizan Ahmad, Anik Karan, Richard L. Jayaraj, and Vaishnavi Sundar. 2023. "A Spectrum of Solutions: Unveiling Non-Pharmacological Approaches to Manage Autism Spectrum Disorder" Medicina 59, no. 9: 1584. https://doi.org/10.3390/medicina59091584
APA StyleMondal, A., Sharma, R., Abiha, U., Ahmad, F., Karan, A., Jayaraj, R. L., & Sundar, V. (2023). A Spectrum of Solutions: Unveiling Non-Pharmacological Approaches to Manage Autism Spectrum Disorder. Medicina, 59(9), 1584. https://doi.org/10.3390/medicina59091584