The Efficiency of Sclerotherapy for the Management of Endometrioma: A Systematic Review and Meta-Analysis of Clinical and Fertility Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Method
2.2. Study Selection
2.3. Statistical Analysis
2.4. Quality Assessment
3. Results
3.1. Studies’ Characteristics
3.2. Outcomes
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Single-Arm Studies | ||||||
| Author, Year of Publication | Country | Study Design | Selection | Comparability | Exposure | Tot. |
| Chang et al., 1997 [17] | China | Prospective cohort | 3 | 1 | 3 | 7 |
| Mesogitis et al., 2000 [18] | Greece | Prospective cohort | 2 | 1 | 3 | 6 |
| Koike et al., 2002 [19] | Japan | Retrospective cohort | 3 | 2 | 3 | 8 |
| Fisch et al., 2004 [20] | USA | Prospective cohort | 3 | 2 | 3 | 8 |
| Agostini et al., 2006 [21] | France | Prospective cohort | 3 | 2 | 3 | 9 |
| Ikuta et al., 2006 [22] | Japan | Prospective cohort | 3 | 2 | 3 | 8 |
| Hsieh et al., 2008 [23] | Taiwan | Prospective cohort | 3 | 2 | 2 | 7 |
| Gatta et al., 2010 [24] | Italy | Prospective cross-sectional | 3 | 2 | 3 | 8 |
| André et al., 2011 [25] | Brazil | Prospective cohort | 3 | 1 | 2 | 6 |
| Shawki et al., 2011 [26] | Egypt | Randomized controlled trial | 3 | 2 | 2 | 7 |
| Wang et al., 2011 [27] | China | Prospective cross-sectional | 3 | 2 | 2 | 7 |
| Aflatoonian et al., 2013 [28] | Iran | Randomized controlled trial | 3 | 1 | 2 | 6 |
| Garcia-Tejedor et al., 2015 [29] | Spain | Prospective cohort | 3 | 1 | 2 | 6 |
| Begum et al., 2015 [30] | Bangladesh | Prospective cohort | 3 | 1 | 3 | 7 |
| Wang et al., 2015 [31] | China | Prospective cohort | 3 | 1 | 2 | 6 |
| Han et al., 2018 [32] | South Korea | Prospective cohort | 3 | 1 | 3 | 7 |
| Aflatoonian et al., 2020 [33] | Iran | Retrospective cross-sectional | 3 | 1 | 2 | 6 |
| Miquel et al., 2020 [34] | France | Retrospective cohort | 3 | 1 | 2 | 6 |
| Huang et al., 2021 [35] | Taiwan | Retrospective cross-sectional | 3 | 1 | 3 | 7 |
| Lee et al., 2022 [36] | South Korea | Retrospective cohort | 3 | 1 | 3 | 7 |
| Meng et al., 2022 [37] | China | Prospective cohort | 3 | 1 | 3 | 7 |
| Comparative studies, included for meta-analysis | ||||||
| Noma et al., 2001 [38] | Japan | Retrospective cross-sectional | 3 | 3 | 2 | 8 |
| Suganuma et al., 2002 [39] | Japan | Prospective cross-sectional | 3 | 1 | 2 | 6 |
| Yazbeck et al., 2009 [40] | France | Prospective cross-sectional | 3 | 3 | 3 | 9 |
| Lee et al., 2014 [41] | South Korea | Retrospective cohort | 3 | 1 | 2 | 6 |
| Garcia-Tejedor et al., 2020 [42] | Spain | Prospective cohort pilot | 3 | 3 | 2 | 8 |
| Alborzi et al., 2021 [43] | Iran | Prospective cross-sectional | 2 | 3 | 2 | 7 |
| Koo et al., 2021 [44] | South Korea | Randomized controlled trial | 3 | 3 | 2 | 8 |
| Martinez-Garcia et al., 2021 [45] | Spain | Prospective cohort pilot | 3 | 2 | 2 | 7 |
Appendix B
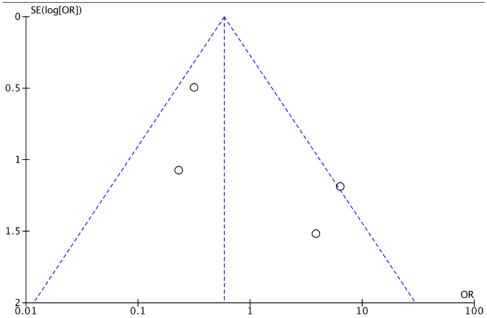
Appendix C
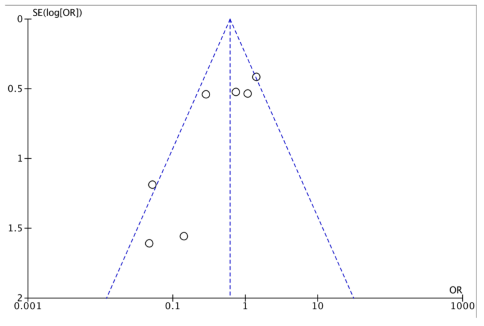
References
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Viganò, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update 2014, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; Hickey, M.; Maouris, P.; Buckett, W.; Garry, R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst. Rev. 2005, CD004992. [Google Scholar] [CrossRef]
- Uncu, G.; Kasapoglu, I.; Ozerkan, K.; Seyhan, A.; Oral Yilmaztepe, A.; Ata, B. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum Reprod. 2013, 28, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Riemma, G.; De Franciscis, P.; La Verde, M.; Ravo, M.; Fumiento, P.; Fasulo, D.D.; Della Corte, L.; Ronsini, C.; Torella, M.; Cobellis, L. Impact of the hemostatic approach after laparoscopic endometrioma excision on ovarian reserve: Systematic review and network meta-analysis of randomized controlled trials [published online ahead of print, 2022 Dec 11]. Int. J. Gynaecol. Obstet. 2022, 162, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Scala, C.; Racca, A.; Calanni, L.; Remorgida, V.; Venturini, P.L.; Maggiore, U.L.R. Second surgery for recurrent unilateral endometriomas and impact on ovarian reserve: A case-control study. Fertil Steril. 2015, 103, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Castellarnau Visus, M.; Ponce Sebastia, J.; Carreras Collado, R.; Cayuela Font, E.; Garcia Tejedor, A. Preliminary results: Ethanol sclerotherapy after ultrasound-guided fine needle aspiration without anesthesia in the management of simple ovarian cysts. J. Minim. Invasive Gynecol. 2015, 22, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Flores, F.J.; Garcia-Velasco, J.A. Is there a benefit for surgery in endometrioma-associated infertility? Curr. Opin. Obstet. Gynecol. 2012, 24, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, R.; Osuga, Y.; Momoeda, M.; Tsutsumi, O.; Taketani, Y. Laparoscopic findings after ultrasound-guided transvaginal ethanol sclerotherapy for ovarian endometrial cyst. Hum. Reprod. 1999, 14, 270. [Google Scholar] [CrossRef]
- Radosa, M.P.; Meyberg-Solomayer, G.; Radosa, J.; Vorwergk, J.; Oettler, K.; Mothes, A.; Baum, S.; Juhasz-Boess, I.; Petri, E.; Solomayer, E.F.; et al. Standardised Registration of Surgical Complications in Laparoscopic-Gynaecological Therapeutic Procedures Using the Clavien-Dindo Classification. Geburtshilfe Frauenheilkd. 2014, 74, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Almog, B.; Tulandi, T. Sclerotherapy in the management of ovarian endometrioma: Systematic review and meta-analysis. Fertil. Steril. 2017, 108, 117–124.e5. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, A. Sclerosants for variceal sclerotherapy: A critical appraisal. Am J Gastroenterol. 1990, 85, 641–649. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef] [PubMed]
- Kansagara, D.; O’Neil, M.; Nugent, S.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’apuaka, M.; Paynter, R.; et al. Benefits and Harms of Cannabis in Chronic Pain or Post-traumatic Stress Disorder: A Systematic Review [Internet]. Washington (DC): Department of Veterans Affairs (US); 2017 Aug. [Table], Quality Assessment Criteria for Observational Studies, Based on the Newcastle-Ottawa Scale. Available online: https://www.ncbi.nlm.nih.gov/books/NBK476448/table/appc.t4/ (accessed on 12 August 2017).
- Chang, C.C.; Lee, H.F.; Tsai, H.D.; Lo, H.Y. Sclerotherapy—An adjuvant therapy to endometriosis. Int. J. Gynaecol. Obstet. 1997, 59, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Mesogitis, S.; Antsaklis, A.; Daskalakis, G.; Papantoniou, N.; Michalas, S. Combined ultrasonographically guided drainage and methotrexate administration for treatment of endometriotic cysts. Lancet. 2000, 355, 1160. [Google Scholar] [CrossRef]
- Koike, T.; Minakami, H.; Motoyama, M.; Ogawa, S.; Fujiwara, H.; Sato, I. Reproductive performance after ultrasound-guided transvaginal ethanol sclerotherapy for ovarian endometriotic cysts. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 105, 39. [Google Scholar] [CrossRef]
- Fisch, J.D.; Sher, G. Sclerotherapy with 5% tetracycline is a simple alternative to potentially complex surgical treatment of ovarian endometriomas before in vitro fertilization. Fertil. Steril. 2004, 82, 437–441. [Google Scholar] [CrossRef]
- Agostini, A.; De Lapparent, T.; Collette, E.; Capelle, M.; Cravello, L.; Blanc, B. In situ methotrexate injection for treatment of recurrent endometriotic cysts. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 130, 129–131. [Google Scholar] [CrossRef]
- Ikuta, A.; Tanaka, Y.; Mizokami, T.; Tsutsumi, A.; Sato, M.; Tanaka, M.; Kajihara, H.; Kanzaki, H. Management of transvaginal ultrasound-guided absolute ethanol sclerotherapy for ovarian endometriotic cysts. J. Med. Ultrason. 2006, 33, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Shiau, C.S.; Lo, L.M.; Hsieh, T.T.; Chang, M.Y. Effectiveness of ultrasound-guided aspiration and sclerotherapy with 95% ethanol for treatment of recurrent ovarian endometriomas. Fertil. Steril. 2009, 91, 2709–2713. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Parlato, V.; Di Grezia, G.; Porto, A.; Cappabianca, S.; Grassi, R.; Rotondo, A. Ultrasound-guided aspiration and ethanol sclerotherapy for treating endometrial cysts. Radiol. Med. 2010, 115, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- André, G.M.; Vilarino, F.L.; Christofolini, D.M.; Bianco, B.; Barbosa, C.P. Aspiration and ethanol sclerotherapy to treat recurrent ovarian endometriomas prior to in vitro fertilization—A pilot study. Einstein 2011, 9, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Shawki, H.E.; Elmorsy, M.; Samir, A.; Eissa Mostafa, K. In situ methotrexate injection after transvaginal ultrasound–guided aspiration of ovarian endometriomas: A randomized controlled trial. Middle East. Fertil. Soc. J. 2011, 16, 224–231. [Google Scholar] [CrossRef]
- Wang, L.L.; Dong, X.Q.; Shao, X.H.; Wang, S.M. Ultrasound-guided interventional therapy for recurrent ovarian chocolate cysts. Ultrasound Med. Biol. 2011, 37, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Aflatoonian, A.; Rahmani, E.; Rahsepar, M. Assessing the efficacy of aspiration and ethanol injection in recurrent endometrioma before IVF cycle: A randomized clinical trial. Iran. J. Reprod. Med. 2013, 11, 179–184. [Google Scholar] [PubMed]
- García-Tejedor, A.; Castellarnau, M.; Ponce, J.; Fernández, M.; Burdio, F. Ethanol sclerotherapy of ovarian endometrioma: A safe and effective minimal invasive procedure. Preliminary results. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 187, 25–29. [Google Scholar] [CrossRef]
- Begum, M.R.; Ehsan, M.; Khan, F.; Ehsan, N.; Santa, M.S.B.; Sharmin, F. Sclerotherapy with Ethanol: An Effective and Safe Alternative to Potentially Complex Surgical Treatment of Recurrent Ovarian Endometrioma. J. South Asian Fed. Obstet. Gynaecol. 2015, 7, 97–101. [Google Scholar] [CrossRef]
- Wang, S.M.; Cai, H.Q.; Dong, X.Q.; Fan, Q.L.; Wang, L.L.; Shao, X.H.; Zhang, L.W. Correlation between ovarian chocolate cyst and serum carbohydrate antigen 125 level and the effect of ultrasound-guided interventional sclerotherapy on serum carbohydrate antigen 125 level. J. Obstet. Gynaecol. Res. 2015, 41, 92–98. [Google Scholar] [CrossRef]
- Han, K.; Seo, S.K.; Kim, M.-D.; Kim, G.M.; Kwon, J.H.; Kim, H.J.; Won, J.Y.; Lee, D.Y. Catheter-directed Sclerotherapy for Ovarian Endometrioma: Short-term Outcomes. Radiology 2018, 289, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Aflatoonian, A.; Tabibnejad, N. Aspiration versus retention ultrasound-guided ethanol sclerotherapy for treating endometrioma: A retrospective cross-sectional study. Int. J. Reprod. Biomed. 2020, 18, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Miquel, L.; Preaubert, L.; Gnisci, A.; Resseguier, N.; Pivano, A.; Perrin, J.; Courbiere, B. Endometrioma ethanol sclerotherapy could increase IVF live birth rate in women with moderate-severe endometriosis. PLoS ONE 2020, 15, e0239846. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chang, M.-Y.; Shiau, C.-S.; Hsieh, T.-T. Changes in anti-müllerian hormone after ultrasound guided aspiration and ethanol sclerotic therapy of ovarian cyst. Taiwan. J. Obstet. Gynecol. 2021, 60, 509–512. [Google Scholar] [CrossRef]
- Lee, J.K.; Ahn, S.H.; Kim, H.I.; Lee, Y.J.; Kim, S.; Han, K.; Kim, M.-D.; Seo, S.K. Therapeutic Efficacy of Catheter-directed Ethanol Sclerotherapy and Its Impact on Ovarian Reserve in Patients with Ovarian Endometrioma at Risk of Decreased Ovarian Reserve: A Preliminary Study. J. Minim. Invasive Gynecol. 2022, 29, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Xu, M. Pelvic Artificial Isolation with Ultrasound-Guided Fluid: A New Technique In Ovarian Endometriotic Cyst Transabdominal Sclerotherapy. Med. Sci. Monit. 2022, 28, e937855. [Google Scholar] [CrossRef] [PubMed]
- Noma, J.; Yoshida, N. Efficacy of ethanol sclerotherapy for ovarian endometriomas. Int. J. Gynaecol. Obstet. 2001, 72, 35–39. [Google Scholar] [CrossRef]
- Suganuma, N.; Wakahara, Y.; Ishida, D.; Asano, M.; Kitagawa, T.; Katsumata, Y.; Moriwaki, T.; Furuhashi, M. Pretreatment for ovarian endometrial cyst before in vitro fertilization. Gynecol. Obstet. Investig. 2002, 54 (Suppl. 1), 36–42. [Google Scholar] [CrossRef]
- Yazbeck, C.; Madelenat, P.; Ayel, J.P.; Jacquesson, L.; Bontoux, L.; Solal, P.; Hazout, A. Ethanol sclerotherapy: A treatment option for ovarian endometriomas before ovarian stimulation. Reprod. Biomed. Online 2009, 19, 121–125. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, C.H.; Lee, Y.J.; Kim, S.H.; Chae, H.D.; Kang, B.M. Surgical resection or aspiration with ethanol sclerotherapy of endometrioma before in vitro fertilization in infertilie women with endometrioma. Obstet. Gynecol. Sci. 2014, 57, 297–303. [Google Scholar] [CrossRef]
- Garcia-Tejedor, A.; Martinez-Garcia, J.M.; Candas, B.; Suarez, E.; Mañalich, L.; Gomez, M.; Merino, E.; Castellarnau, M.; Regueiro, P.; Carreras, M.; et al. Ethanol Sclerotherapy versus Laparoscopic Surgery for Endometrioma Treatment: A Prospective, Multicenter, Cohort Pilot Study. J. Minim. Invasive Gynecol. 2020, 27, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, S.; Askary, E.; Keramati, P.; Alamdarloo, S.M.; Poordast, T.; Ashraf, M.A.; Shomali, Z.; Jahromi, B.N.; Sorouri, Z.Z. Assisted reproductive technique outcomes in patients with endometrioma undergoing sclerotherapy vs. laparoscopic cystectomy: Prospective cross-sectional study. Reprod. Med. Biol. 2021, 20, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Lee, I.; Han, K.; Seo, S.K.; Kim, M.-D.; Lee, J.K.; Kwon, J.H.; Kim, G.M.; Lee, J.; Won, J.Y. Comparison of the therapeutic efficacy and ovarian reserve between catheter-directed sclerotherapy and surgical excision for ovarian endometrioma. Eur. Radiol. 2021, 31, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, J.M.; Candas, B.; Suarez-Salvador, E.; Gomez, M.; Merino, E.; Castellarnau, M.; Carreras, M.; Carrarach, M.; Subirats, N.; Gonzalez, S.; et al. Comparing the effects of alcohol sclerotherapy with those of surgery on anti-Müllerian hormone and ovarian reserve after endometrioma treatment. A prospective multicenter pilot cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.C.; Andres, M.P.; Passman, L.J.; Gonçalves, M.O.; Podgaec, S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int. J. Gynaecol. Obstet. 2016, 134, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Albanese, G.; Kondo, K.L. Pharmacology of sclerotherapy. Semin. Interv. Radiol. 2010, 27, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Psaroudakis, D.; Hirsch, M.; Davis, C. Review of the management of ovarian endometriosis: Paradigm shift towards conservative approaches. Curr. Opin. Obstet. Gynecol. 2014, 26, 266–274. [Google Scholar] [CrossRef]
- Raffi, F.; Metwally, M.; Amer, S. The impact of excision of ovarian endometrioma on ovarian reserve: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 3146–3154. [Google Scholar] [CrossRef]
- Muzii, L.; Achilli, C.; Lecce, F.; Bianchi, A.; Franceschetti, S.; Marchetti, C.; Perniola, G.; Panici, P.B. Second surgery for recurrent endometriomas is more harmful to healthy ovarian tissue and ovarian reserve than first surgery. Fertil. Steril. 2015, 103, 738–743. [Google Scholar] [CrossRef]
- Somigliana, E.; Berlanda, N.; Benaglia, L.; Viganò, P.; Vercellini, P.; Fedele, L. Surgical excision of endometriomas and ovarian reserve: A systematic review on serum antimüllerian hormone level modifications. Fertil. Steril. 2012, 98, 1531–1538. [Google Scholar] [CrossRef]
- Almog, B.; Sheizaf, B.; Shalom-Paz, E.; Shehata, F.; Al-Talib, A.; Tulandi, T. Effects of excision of ovarian endometrioma on the antral follicle count and collected oocytes for in vitro fertilization. Fertil. Steril. 2010, 94, 2340–2342. [Google Scholar] [CrossRef] [PubMed]
- Mercorio, A.; Della Corte, L.; De Angelis, M.C.; Buonfantino, C.; Ronsini, C.; Bifulco, G.; Giampaolino, P. Ovarian Drilling: Back to the Future. Medicina 2022, 58, 1002. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Querleu, D.; Bruhat, M.A.; Madelenat, P.; Fernandez, H.; Pierre, F.; Dubuisson, J.B. Surgical complications of diagnostic and operative gynaecological laparoscopy: A series of 29,966 cases. Hum. Reprod. 1998, 13, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Alson, S.; Jokubkiene, L.; Henic, E.; Sladkevicius, P. Prevalence of endometrioma and deep infiltrating endometriosis at transvaginal ultrasound examination of subfertile women undergoing assisted reproductive treatment. Fertil. Steril. 2022, 118, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Horng, H.C.; Su, M.H.; Wang, P.H. Does any serum marker predict the ovarian endometrioma accompanied with or without deep infiltrative endometriosis? J. Chin. Med. Assoc. 2020, 83, 797–798. [Google Scholar] [CrossRef]
- Perelló, M.; Martínez-Zamora, M.A.; Torres, X.; Munrós, J.; Llecha, S.; De Lazzari, E.; Balasch, J.; Carmona, F. Markers of deep infiltrating endometriosis in patients with ovarian endometrioma: A predictive model. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Antonaci, D.; Schiavi, M.C.; Carletti, V.; Yacoub, V.; Morgani, C.; Grilli, D.; Galanti, F.; Ligato, A.; Valensise, H.C.; Palazzetti, P.; et al. Laparoscopic stripping versus endometrioma ethanol sclerotherapy in women with endometrioma awaiting IVF: A long-term analysis of ovarian reserve and pregnancy outcome. Minerva Obstet. Gynecol. 2022, 74, 410–418. [Google Scholar] [CrossRef]
- Alborzi, S.; Sorouri, Z.Z.; Askari, E.; Poordast, T.; Chamanara, K. The success of various endometrioma treatments in infertility: A systematic review and meta-analysis of prospective studies. Reprod. Med. Biol. 2019, 18, 312–322. [Google Scholar] [CrossRef]
- Ronsini, C.; Fumiento, P.; Iavarone, I.; Greco, P.F.; Cobellis, L.; De Franciscis, P. Liquid Biopsy in Endometriosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6116. [Google Scholar] [CrossRef]
- Iavarone, I.; Greco, P.F.; La Verde, M.; Morlando, M.; Torella, M.; de Franciscis, P.; Ronsini, C. Correlations between Gut Microbial Composition, Pathophysiological and Surgical Aspects in Endometriosis: A Review of the Literature. Medicina 2023, 59, 347. [Google Scholar] [CrossRef]
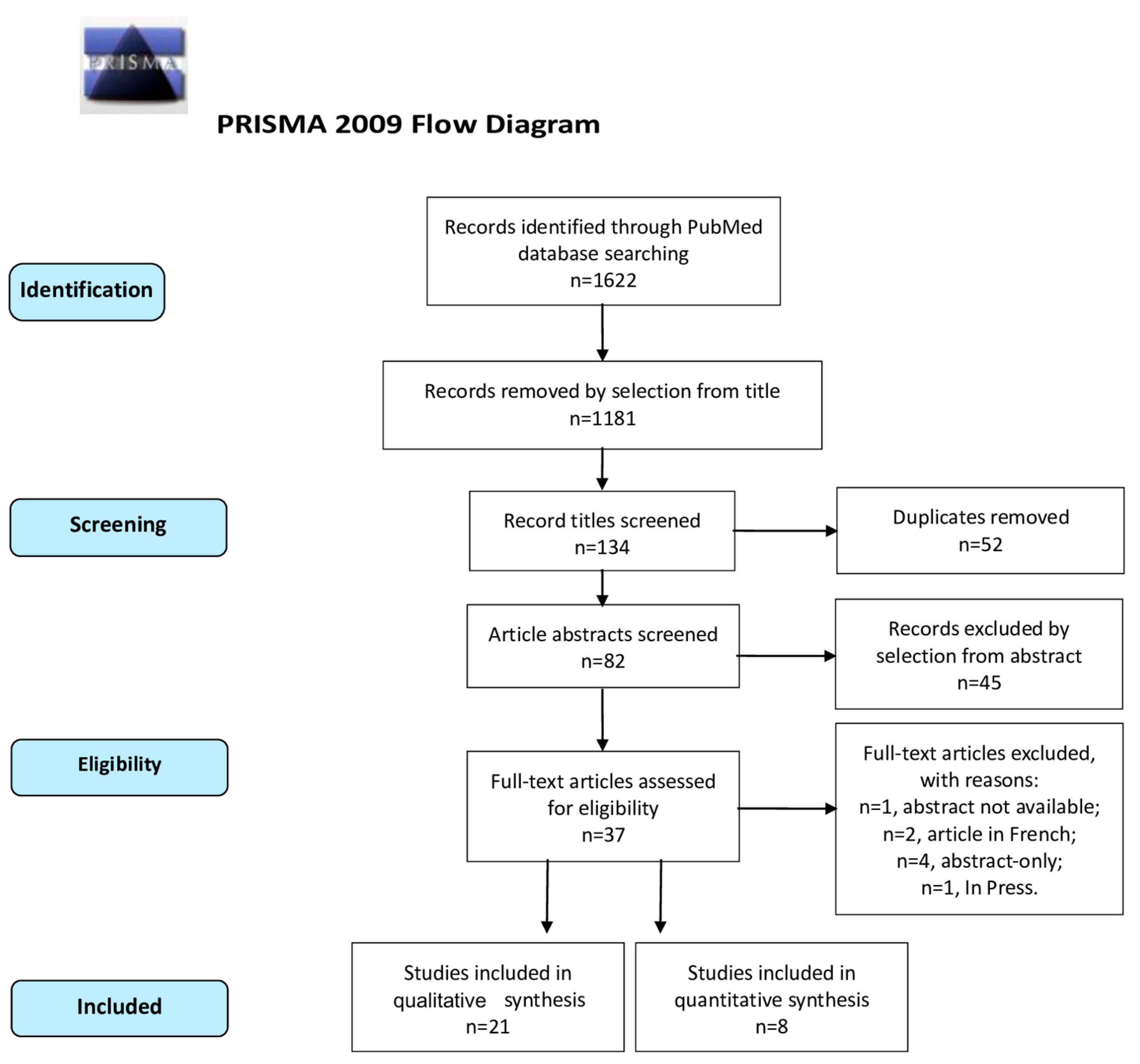
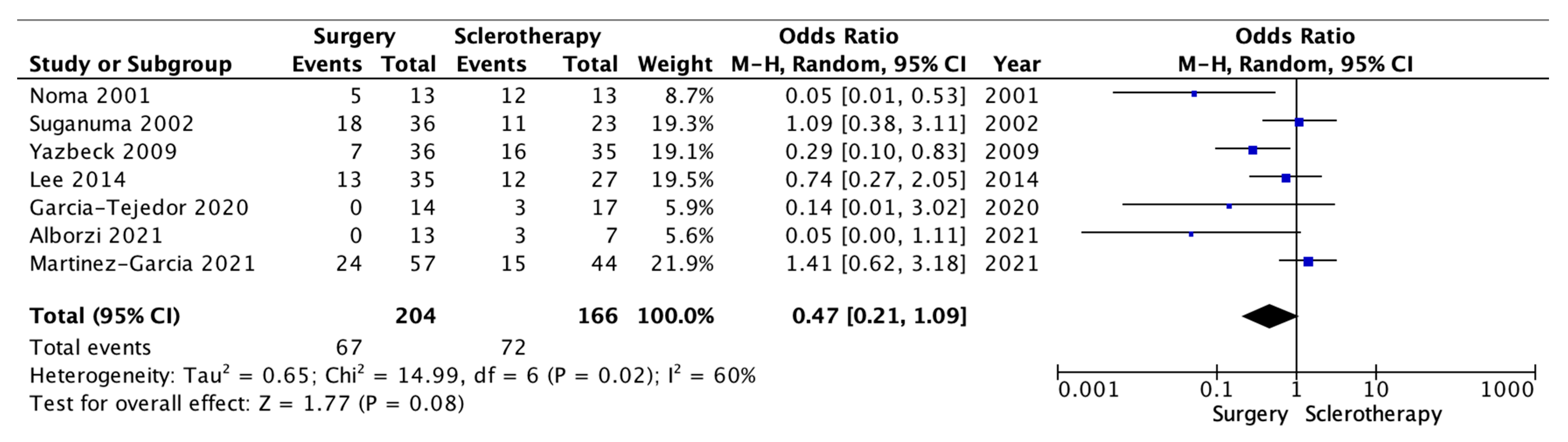
| Single-Arm Studies | ||||||
|---|---|---|---|---|---|---|
| Author, Year of Publication | Country | Study Type | Period of Enrollment | No. of Patients | Procedure | Substance |
| Chang et al., 1997 [17] | China | Prospective cohort | 1994–1996 | 32 | † US-guided sclerotherapy | Tetracycline 1%. Instilled 20% of cyst fluid volume and left in situ. |
| Mesogitis et al., 2000 [18] | Greece | Prospective cohort | ‡ N/A | 11 | US-guided sclerotherapy | Methotrexate 30 mg diluted in 3 mL saline solution |
| Koike et al., 2002 [19] | Japan | Retrospective cohort | 1996–1998 | 110 | US-guided sclerotherapy | Ethanol 50%. Instilled 100% of cyst fluid volume for 5 min. |
| Fisch et al., 2004 [20] | USA | Prospective cohort | N/A | 11 | US-guided sclerotherapy | Tetracycline 5%. Instilled volume 5–10 mL. |
| Agostini et al., 2006 [21] | France | Prospective cohort | 2002–2003 | 14 | Methotrexate sclerotherapy | Methotrexate 30 mg diluted in 3 mL saline solution |
| Ikuta et al., 2006 [22] | Japan | Prospective cohort | 1999–2005 | 18 | US-guided sclerotherapy | Ethanol 100% for 5 min. |
| Hsieh et al., 2008 [23] | Taiwan | Prospective cohort | 2002–2005 | 108 | US-guided sclerotherapy for 10 min (n = 78); retention sclerotherapy (n = 30) | Ethanol 95%. Instilled 80% of cyst fluid volume and ≤100 mL. |
| Gatta et al., 2010 [24] | Italy | Prospective cohort | 2006–2008 | 50 | US- or § LPS-guided sclerotherapy | Ethanol 95%. Instilled 100% of cyst fluid volume and left in situ. |
| André et al., 2011 [25] | Brazil | Prospective cohort pilot | 2007–2010 | 21 | US-guided sclerotherapy | Ethanol. Instilled 80% of cyst fluid volume and <60 mL for 5 min. |
| Shawki et al., 2011 [26] | Egypt | Randomized controlled trial | 2007–2010 | 93 | Methotrexate sclerotherapy (n = 93); aspiration (n = 95) | Methotrexate. 30 mg diluted in 3 mL normal saline solution. |
| Wang et al., 2011 [27] | China | Prospective cross-sectional | 2006–2008 | 132 | US-guided sclerotherapy for 10 min (n = 66); retention sclerotherapy (n = 66) | Ethanol 95%. Instilled 50% of cyst fluid volume. |
| Aflatoonian et al., 2013 [28] | Iran | Randomized controlled trial | 2011–2012 | 20 | US-guided sclerotherapy | Ethanol 98%. Instilled 80% of cyst fluid volume for 10 min. |
| Garcia-Tejedor et al., 2015 [29] | Spain | Prospective cohort | 2016–2018 | 25 | US-guided sclerotherapy | Ethanol. Instilled 66% of cyst fluid volume and <100 mL for 15 min. |
| Begum et al., 2015 [30] | Bangladesh | Prospective cohort | 2005–2013 | 53 | US-guided sclerotherapy | Ethanol 95%. Instilled and removed 75% of aspirated fluid. Re-instilled 5 to 10 mL and left in situ |
| Wang et al., 2015 [31] | China | Prospective cohort | 2010–2012 | 105 | US-guided sclerotherapy | Ethanol 95%. Instilled 50% of cyst fluid volume and <100 mL and left in situ. |
| Han et al., 2018 [32] | South Korea | Prospective cohort | 2015–2017 | 14 | Catheter-directed sclerotherapy | Ethanol 95%. Instilled 25% of cyst fluid volume and <100 mL for 20 min. |
| Aflatoonian et al., 2020 [33] | Iran | Retrospective cross-sectional | 2013–2017 | 53 | Sclerotherapy with retention of ethanol (n = 27); no retention (n = 16) | Ethanol 98%. Instilled 60% of cyst fluid volume for 10 min. |
| Miquel et al., 2020 [34] | France | Retrospective cohort | 2013–2017 | 37 | US-guided sclerotherapy | Ethanol 96%. Instilled 60% of cyst fluid volume and <60 mL for 10 min. |
| Huang et al., 2021 [35] | Taiwan | Retrospective cross-sectional | 2008–2018 | 124 | Sclerotherapy with retention of ethanol and CA-125 91 U/mL (n = 44); no retention and CA-125 91 U/mL (n = 80) | Ethanol 95%. Instilled variable 3–10 mL of cyst fluid volume for 1–3 min. |
| Lee et al., 2022 [36] | South Korea | Retrospective cohort | 2014–2021 | 18 | Catheter-directed sclerotherapy | Ethanol 99%. Instilled 25% of cyst fluid volume for 20 min. |
| Meng et al., 2022 [37] | China | Prospective cohort | 2020–2021 | 70 | US-guided pelvic artificial isolation with fluid | Ethanol 95%. Instilled 33% of cyst fluid volume and <60 mL for 1 min. |
| Comparative studies, included for meta-analysis | ||||||
| Noma et al., 2001 [38] | Japan | Retrospective cross-sectional | 1993–1998 | 100 | US-guided sclerotherapy (n = 74); laparoscopic cystectomy (n = 26) | Ethanol. Instilled 80% of cyst fluid volume and <100 mL. |
| Suganuma et al., 2002 [39] | Japan | Prospective cross-sectional | N/A | 59 | US-guided sclerotherapy (n = 23); laparoscopic cystectomy (n = 36) | Ethanol |
| Yazbeck et al., 2009 [40] | France | Prospective cross-sectional | 2004–2008 | 56 | US-guided sclerotherapy (n = 35); laparoscopic cystectomy (n = 36) | Ethanol 100%. Instilled 80% of cyst fluid volume and <60 mL for 10 min. |
| Lee et al., 2014 [41] | South Korea | Retrospective cohort | 2008–2012 | 65 | US-guided sclerotherapy (n = 29); surgical resection (n = 36) | Ethanol 20%. Instilled and flushed 80–90% of cyst fluid volume. |
| Garcia-Tejedor et al., 2020 [42] | Spain | Prospective cohort pilot | 2016–2018 | 31 | US-guided aspiration plus sclerotherapy (n = 17); laparoscopic cystectomy (n = 14) | Ethanol 100%. Instilled 66% of the cyst fluid volume and <100 mL for 15 min. |
| Alborzi et al., 2021 [43] | Iran | Prospective cross-sectional | 2013–2020 | 101 | US-guided sclerotherapy (n = 44); laparoscopic cystectomy (n = 57) | Ethanol 96%. Instilled 80% of the cyst fluid volume and left in situ. |
| Koo et al., 2021 [44] | South Korea | Randomized controlled trial | 2011–2019 | 71 | Catheter-directed sclerotherapy (n = 20); surgical excision (n = 51) | Ethanol 99%. Instilled 25% of the cyst fluid volume and <100 mL for 20 min. |
| Martinez-Garcia et al., 2021 [45] | Spain | Prospective cohort pilot | N/A | 40 | US-guided aspiration plus sclerotherapy (n = 16); laparoscopic cystectomy (n = 10) | Ethanol 100%. Instilled 66% of the cyst fluid volume and <100 mL for 15 min. |
| Single-Arm Studies | ||
|---|---|---|
| Authors, Year of Publication | Success Rate (%) | Recurrence Rate (%) |
| Chang et al., 1997 [17] | † N/A | 46.8 |
| Mesogitis et al., 2000 [18] | 81.8 | 61.9 |
| Koike et al., 2002 [19] | 100 | 13.3 |
| Fisch et al., 2004 [20] | 75.0 | 0.0 |
| Agostini et al., 2006 [21] | 100 | 28.6 |
| Ikuta et al., 2006 [22] | 100 | 11.1 |
| Hsieh et al., 2008 [23] | N/A | 26.9 |
| Gatta et al., 2010 [24] | 100 | 8.0 |
| André et al., 2011 [25] | N/A | N/A |
| Shawki et al., 2011 [26] | 86.0 | 19.3 |
| Wang et al., 2011 [27] | 92.4 | 0.0 |
| Aflatoonian et al., 2013 [28] | N/A | 20.0 |
| Garcia-Tejedor et al., 2015 [29] | 100 | 12.1 |
| Begum et al., 2015 [30] | 79.2 | 11.3 |
| Wang et al., 2015 [31] | 100 | 0.0 |
| Han et al., 2018 [32] | 100 | 0.0 |
| Aflatoonian et al., 2020 [33] | N/A | 44.1 |
| Miquel et al., 2020 [34] | 87.0 | 2.7 |
| Huang et al., 2021 [35] | N/A | 22.5 |
| Lee et al., 2022 [36] | 100 | 5.5 |
| Meng et al., 2022 [37] | 100 | N/A |
| Comparative studies | ||
| Noma et al., 2001 [38] | N/A | 14.9 |
| Suganuma et al., 2002 [39] | N/A | N/A |
| Yazbeck et al., 2009 [40] | 100 | 12.9 |
| Lee et al., 2014 [41] | N/A | N/A |
| Garcia-Tejedor et al., 2020 [42] | N/A | 5.9 |
| Alborzi et al., 2021 [43] | N/A | 34.1 |
| Koo et al., 2021 [44] | 100 | 0.0 |
| Martinez-Garcia et al., 2021 [45] | N/A | N/A |
| Single-Arm Studies | |
|---|---|
| Author, Year of Publication | Pregnancy Rate (%) |
| Chang et al., 1997 [17] | 34.7 |
| Mesogitis et al., 2000 [18] | † N/A |
| Koike et al., 2002 [19] | 41.8 |
| Fisch et al., 2004 [20] | 57.0 |
| Agostini et al., 2006 [21] | N/A |
| Ikuta et al., 2006 [22] | 33.3 |
| Hsieh et al., 2008 [23] | 8.1 |
| Gatta et al., 2010 [24] | 6.0 |
| André et al., 2011 [25] | 20.0 |
| Shawki et al., 2011 [26] | N/A |
| Wang et al., 2011 [27] | N/A |
| Aflatoonian et al., 2013 [28] | 33.3 |
| Garcia-Tejedor et al., 2015 [29] | 0.0 |
| Begum et al., 2015 [30] | 33.9 |
| Wang et al., 2015 [31] | N/A |
| Han et al., 2018 [32] | 100 |
| Aflatoonian et al., 2020 [33] | 39.5 |
| Miquel et al., 2020 [34] | 37.3 |
| Huang et al., 2021 [35] | 23.3 |
| Lee et al., 2022 [36] | N/A |
| Meng et al., 2022 [37] | N/A |
| Comparative studies | |
| Noma et al., 2001 [38] | 52.2 |
| Suganuma et al., 2002 [39] | 31.4 |
| Yazbeck et al., 2009 [40] | 55.2 |
| Lee et al., 2014 [41] | 44.4 |
| Garcia-Tejedor et al., 2020 [42] | 17.6 |
| Alborzi et al., 2021 [43] | 34.1 |
| Koo et al., 2021 [44] | N/A |
| Martinez-Garcia et al., 2021 [45] | 42.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronsini, C.; Iavarone, I.; Braca, E.; Vastarella, M.G.; De Franciscis, P.; Torella, M. The Efficiency of Sclerotherapy for the Management of Endometrioma: A Systematic Review and Meta-Analysis of Clinical and Fertility Outcomes. Medicina 2023, 59, 1643. https://doi.org/10.3390/medicina59091643
Ronsini C, Iavarone I, Braca E, Vastarella MG, De Franciscis P, Torella M. The Efficiency of Sclerotherapy for the Management of Endometrioma: A Systematic Review and Meta-Analysis of Clinical and Fertility Outcomes. Medicina. 2023; 59(9):1643. https://doi.org/10.3390/medicina59091643
Chicago/Turabian StyleRonsini, Carlo, Irene Iavarone, Eleonora Braca, Maria Giovanna Vastarella, Pasquale De Franciscis, and Marco Torella. 2023. "The Efficiency of Sclerotherapy for the Management of Endometrioma: A Systematic Review and Meta-Analysis of Clinical and Fertility Outcomes" Medicina 59, no. 9: 1643. https://doi.org/10.3390/medicina59091643
APA StyleRonsini, C., Iavarone, I., Braca, E., Vastarella, M. G., De Franciscis, P., & Torella, M. (2023). The Efficiency of Sclerotherapy for the Management of Endometrioma: A Systematic Review and Meta-Analysis of Clinical and Fertility Outcomes. Medicina, 59(9), 1643. https://doi.org/10.3390/medicina59091643








