The Influence of Platelet-Rich Fibrin on the Healing of Bone Defects after Harvesting Bone–Patellar Tendon–Bone Grafts
Abstract
1. Introduction
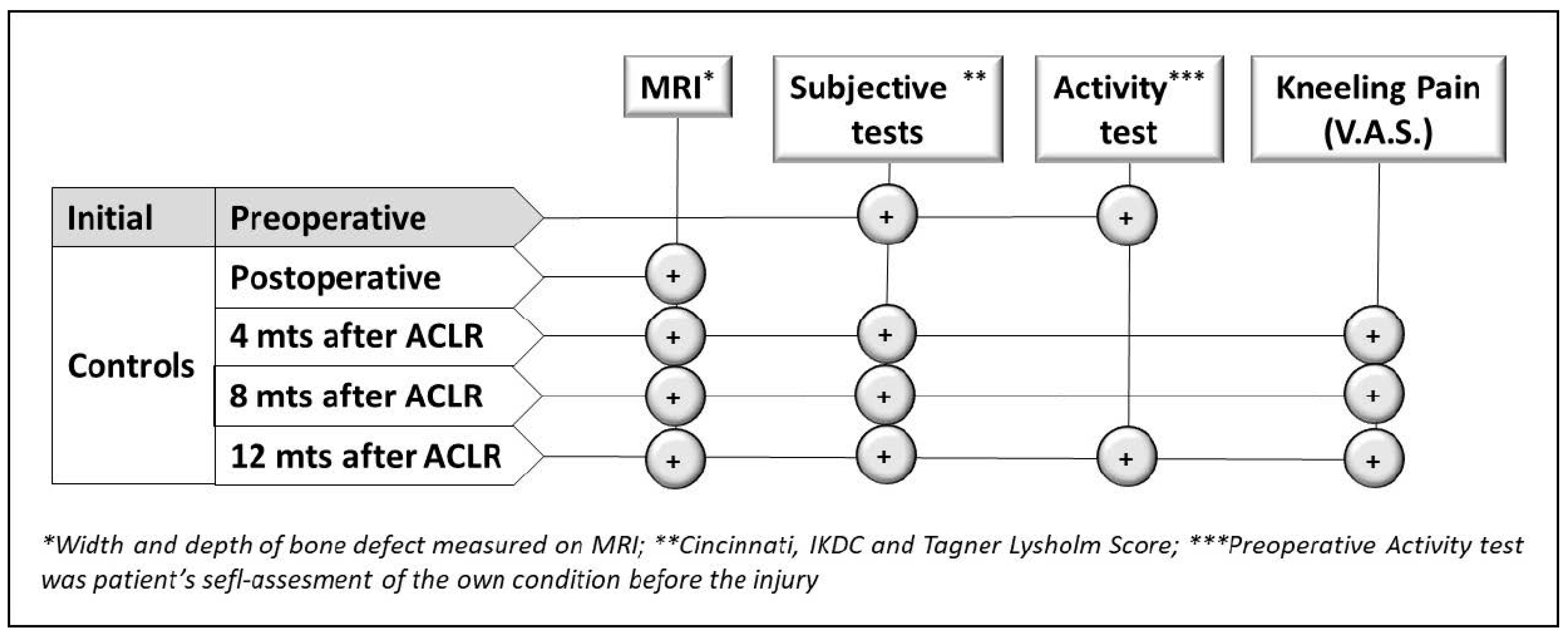
2. Materials and Methods
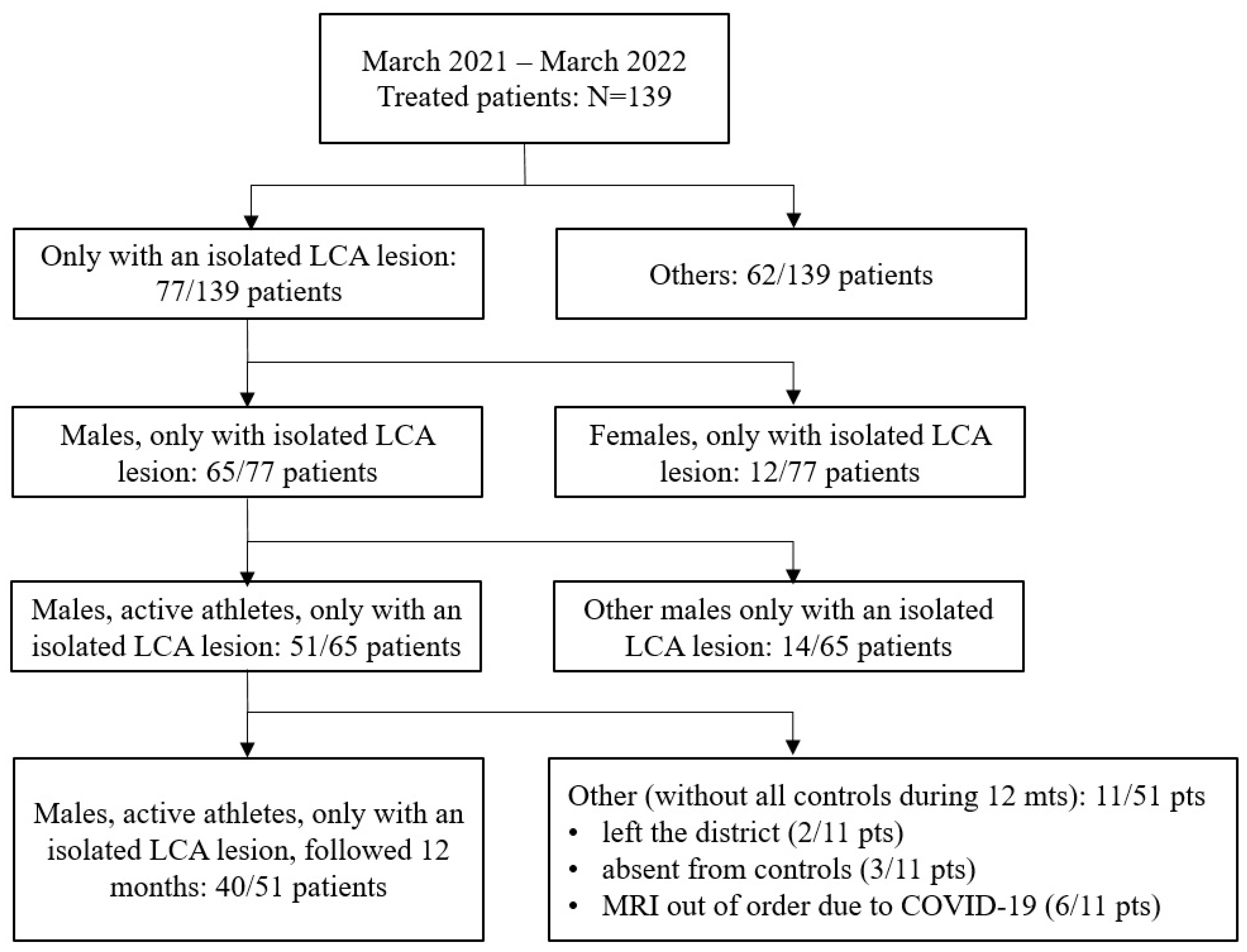
2.1. Study Background and Objective Limitations
2.2. Vivostat® PRF Preparation
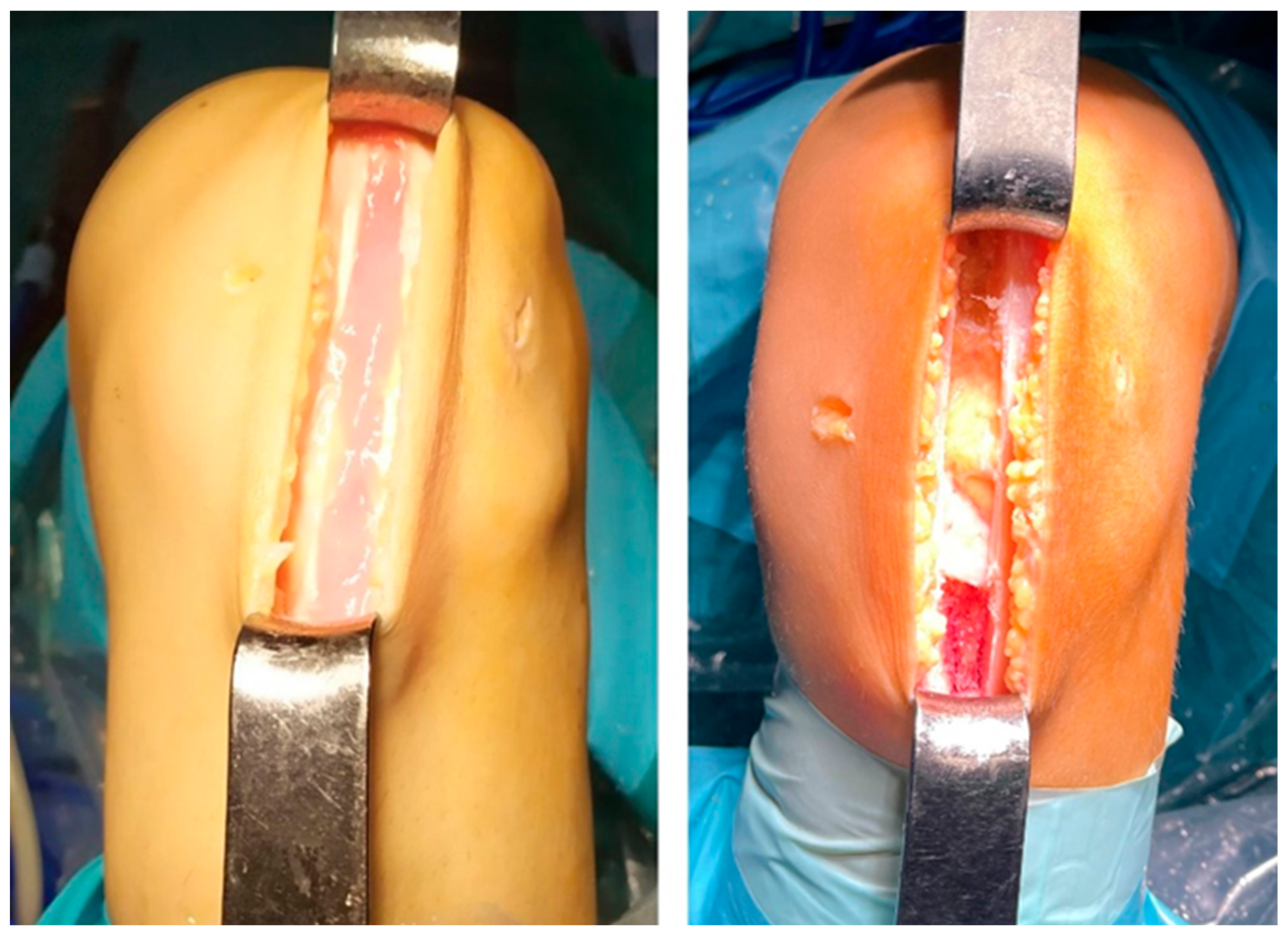
2.3. Surgical Technique and Postoperative Treatment
2.4. Magnetic Resonance Imaging (MRI)
- −
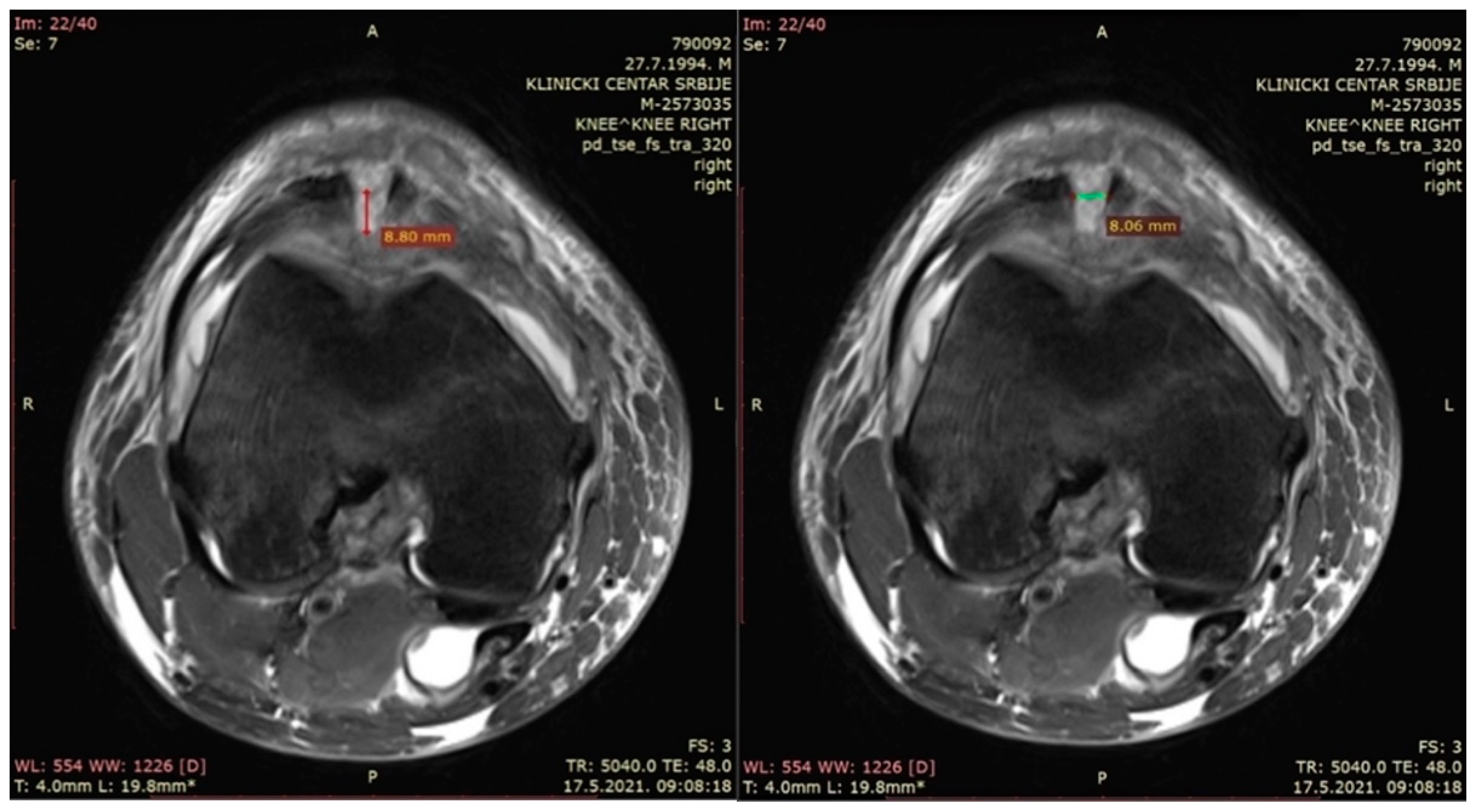
- The depth of the patellar bone defect (mm)—a variable used to assess the depth of the bone defect from the first section caudal to the top of the defect in a transverse plane in the t2_tse_sag sequence at the center of the defect, as defined using the sagittal plane (Figure 3—left).
- −
- The width of the patellar bone defect (mm)—a variable used to assess the width of the bone defect from the first section caudal to the top of the defect in a transverse plane in the pd_tse_fs_tra sequence (Figure 3—right).
2.5. Kneeling Test
2.6. Questionnaires and Evaluation of Donor Site Morbidity
- Knee functional questionnaires (Modified Cincinnati Rating System Questionnaire, Tegner Activity Level Scale, IKDC Subjective Knee Evaluation Form, and Tegner Lysholm Knee Scoring Scale).
- Subjective perception of kneeling pain (VAS: 0–10).
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Runer, A.; Keeling, L.; Wagala, N.; Nugraha, H.; Özbek, E.A.; Hughes, J.D.; Musahl, V. Current trends in graft choice for anterior cruciate ligament reconstruction—Part I: Anatomy, biomechanics, graft incorporation and fixation. J. Exp. Orthop. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Kartus, J.; Stener, S.; Lindahl, S.; Engström, B.; Eriksson, B.I.; Karlsson, J. Factors affecting donor-site morbidity after anterior cruciate ligament reconstruction using bone-patellar tendon-bone autografts. Knee Surg. Sports Traumatol. Arthrosc. 1997, 5, 222–228. [Google Scholar] [CrossRef]
- Cohen, S.B.; Flato, R.; Wascher, J.; Watson, R.; Salminen, M.; O’Brien, D.; Tjoumakaris, F.; Ciccotti, M. Incidence and Characterization of Hypoesthesia in the Distribution of the Infrapatellar Branch of the Saphenous Nerve after Anterior Cruciate Ligament Reconstruction: A Prospective Study of Patient-Reported Numbness. J. Knee Surg. 2018, 31, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Fares, A.; Hardy, A.; Bohu, Y.; Meyer, A.; Karam, K.; Lefevre, N. The impact of bone graft type used to fill bone defects in patients undergoing ACL reconstruction with bone-patellar tendon-bone (BPTB) autograft on kneeling, anterior knee pain and knee functional outcomes. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2023, 34, 181–190. [Google Scholar] [CrossRef]
- Tsuda, E.; Okamura, Y.; Ishibashi, Y.; Otsuka, H.; Toh, S. Techniques for reducing anterior knee symptoms after anterior cruciate ligament reconstruction using a bone-patellar tendon-bone autograft. Am. J. Sports Med. 2001, 29, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Beaufils, P.; Gaudot, F.; Drain, O.; Boisrenoult, P.; Pujol, N. Mini-invasive technique for bone patellar tendon bone harvesting: Its superiority in reducing anterior knee pain following ACL reconstruction. Curr. Rev. Musculoskelet. Med. 2011, 4, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gyulay, K.K.; Karászi, P.; Rédei, M.; Sólymos, P.; Schandl, K.; Lacza, Z.; Horváthy, D.B. Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases. Int. J. Mol. Sci. 2023, 24, 9232. [Google Scholar] [CrossRef]
- Sgardelis, P.; Naqvi, G.; Servant, C. Minimally Invasive Bone-Patellar Tendon-Bone Graft Harvest Is Associated with Less Frequent Anterior Knee Pain at Rest Than Hamstring Graft Harvest After Anterior Cruciate Ligament Reconstruction at the 1-Year Follow-Up. Arthrosc. Sports Med. Rehabil. 2023, 5, 100766. [Google Scholar] [CrossRef]
- Dhanakodi, N.; Thilak, J.; Varghese, J.; Menon, K.V.; Varma, H.; Tripathy, S.K. Ceramic Bone Graft Substitutes do not reduce donor-site morbidity in ACL reconstruction surgeries: A pilot study. SICOT-J 2019, 5, 14. [Google Scholar] [CrossRef]
- Kato, Y.; Chavez, J.; Yamada, S.; Hattori, S.; Takazawa, S.; Ohuchi, H. Beta-Tricalcium Phosphate Block for Donor Site Morbidity of the Patella in Anterior Cruciate Ligament Reconstruction Using Bone-Patellar Tendon-Bone Graft. Knee Surg. Relat. Res. 2019, 31, 113–119. [Google Scholar] [CrossRef]
- Walters, B.L.; Porter, D.A.; Hobart, S.J.; Bedford, B.B.; Hogan, D.E.; McHugh, M.M.; Klein, D.A.; Harousseau, K.; Nicholas, S.J. Effect of Intraoperative Platelet-Rich Plasma Treatment on Postoperative Donor Site Knee Pain in Patellar Tendon Autograft Anterior Cruciate Ligament Reconstruction: A Double-Blind Randomized Controlled Trial. Am. J. Sports Med. 2018, 46, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.M.; Demange, M.K.; Sobrado, M.F.; Rodrigues, M.B.; Pedrinelli, A.; Hernandez, A.J. Patellar Tendon Healing With Platelet-Rich Plasma: A Prospective Randomized Controlled Trial. Am. J. Sports Med. 2012, 40, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Grecu, A.F.; Reclaru, L.; Ardelean, L.C.; Nica, O.; Ciucă, E.M.; Ciurea, M.E. Platelet-Rich Fibrin and its Emerging Therapeutic Benefits for Musculoskeletal Injury Treatment. Med. Kaunas Lith. 2019, 55, 141. [Google Scholar] [CrossRef]
- Yonai, Y.; Lever, L.; Ben Natan, M.; Steinfeld, Y.; Seroguon, Y.; Berkovich, Y. Regenerative medicine in orthopedics—Updates and common uses. Harefuah 2022, 161, 443–447. [Google Scholar] [PubMed]
- Hudgens, J.L.; Sugg, K.B.; Grekin, J.A.; Gumucio, J.P.; Bedi, A.; Mendias, C.L. Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts. Am. J. Sports Med. 2016, 44, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, D.; Seghatchian, J.; Acker, J.P. Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus. Apher. Sci. 2019, 58, 102675. [Google Scholar] [CrossRef] [PubMed]
- Beyzadeoglu, T.; Pehlivanoglu, T.; Yildirim, K.; Buldu, H.; Tandogan, R.; Tuzun, U. Does the Application of Platelet-Rich Fibrin in Anterior Cruciate Ligament Reconstruction Enhance Graft Healing and Maturation? A Comparative MRI Study of 44 Cases. Orthop. J. Sports Med. 2020, 8, 2325967120902013. [Google Scholar] [CrossRef] [PubMed]
- Skarpas, G.A. Arthrozheal®, a Bioactive Fibrin Scaffold for Joint Cartilage, Tendon and Soft Tissue Lesions. Latest Results and Application Perspectives. Surg. Technol. Int. 2022, 41, sti41/1636. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Bayer, A.; Lammel, J.; Tohidnezhad, M.; Lippross, S.; Behrendt, P.; Klüter, T.; Pufe, T.; Cremer, J.; Jahr, H.; Rademacher, F.; et al. The Antimicrobial Peptide Human Beta-Defensin-3 Is Induced by Platelet-Released Growth Factors in Primary Keratinocytes. Mediat. Inflamm. 2017, 2017, 6157491. [Google Scholar] [CrossRef]
- Bayer, A.; Höntsch, G.; Kaschwich, M.; Dell, A.; Siggelkow, M.; Berndt, R.; Rusch, R.; Harder, J.; Gläser, R.; Cremer, J. Vivostat Platelet-Rich Fibrin® for Complicated or Chronic Wounds-A Pilot Study. Biomedicines 2020, 8, 276. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Smits, L.; Kotz, D.; Budé, L.; Spigt, M.; Serroyen, J.; Crutzen, R. A simple formula for the calculation of sample size in pilot studies. J. Clin. Epidemiol. 2015, 68, 1375–1379. [Google Scholar] [CrossRef]
- Cervellin, M.; de Girolamo, L.; Bait, C.; Denti, M.; Volpi, P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: A randomized, controlled clinical study. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2012, 20, 114–120. [Google Scholar] [CrossRef]
- Schandl, K.; Horváthy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csönge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. (SICOT) 2016, 40, 2097–2104. [Google Scholar] [CrossRef]
- Seijas, R.; Cuscó, X.; Sallent, A.; Serra, I.; Ares, O.; Cugat, R. Pain in donor site after BTB-ACL reconstruction with PRGF: A randomized trial. Arch. Orthop. Trauma Surg. 2016, 136, 829–835. [Google Scholar] [CrossRef]
- Di Matteo, B.; Loibl, M.; Andriolo, L.; Filardo, G.; Zellner, J.; Koch, M.; Angele, P. Biologic agents for anterior cruciate ligament healing: A systematic review. World J. Orthop. 2016, 7, 592–603. [Google Scholar] [CrossRef]
- Widner, M.; Dunleavy, M.; Lynch, S. Outcomes Following ACL Reconstruction Based on Graft Type: Are all Grafts Equivalent? Curr. Rev. Musculoskelet. Med. 2019, 12, 460–465. [Google Scholar] [CrossRef]
- Arnold, M.P.; Calcei, J.G.; Vogel, N.; Magnussen, R.A.; Clatworthy, M.; Spalding, T.; Campbell, J.D.; Bergfeld, J.A.; Sherman, S.L.; ACL Study Group ACL Study Group. Survey reveals the evolution of anterior cruciate ligament reconstruction graft choice over the past three decades. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2021, 29, 3871–3876. [Google Scholar] [CrossRef]
- Kovindha, K.; Ganokroj, P.; Lertwanich, P.; Vanadurongwan, B. Quantifying anterior knee pain during specific activities after using the bone-patellar tendon-bone graft for arthroscopic anterior cruciate ligament reconstruction. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2019, 15, 6–12. [Google Scholar] [CrossRef]
- Del Torto, M.; Enea, D.; Panfoli, N.; Filardo, G.; Pace, N.; Chiusaroli, M. Hamstrings anterior cruciate ligament reconstruction with and without platelet rich fibrin matrix. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2015, 23, 3614–3622. [Google Scholar] [CrossRef] [PubMed]
- Barié, A.; Sprinckstub, T.; Huber, J.; Jaber, A. Quadriceps tendon vs. patellar tendon autograft for ACL reconstruction using a hardware-free press-fit fixation technique: Comparable stability, function and return-to-sport level but less donor site morbidity in athletes after 10 years. Arch. Orthop. Trauma Surg. 2020, 140, 1465–1474. [Google Scholar] [CrossRef]
- Drogset, J.O.; Størset, K.H.; Nitteberg, T.M.; Gifstad, T. Clinical outcome after knee ligament reconstruction with tendon allografts. J. Exp. Orthop. 2021, 8, 11. [Google Scholar] [CrossRef]
- Seijas, R.; Ares, O.; Catala, J.; Alvarez-Diaz, P.; Cusco, X.; Cugat, R. Magnetic Resonance Imaging Evaluation of Patellar Tendon Graft Remodelling after Anterior Cruciate Ligament Reconstruction with or without Platelet-Rich Plasma. J. Orthop. Surg. 2013, 21, 10–14. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Vivostat | Standard | Test |
|---|---|---|---|---|
| Gender | ||||
| Male | 40 (100%) | 20 (100%) | 20 (100%) | - |
| Age (years) | ||||
| Mean (SD) | 27 (7.7) | 26.2 (8.2) | 27.8 (7.4) | ns * |
| Median (Range) | 24.5 (17–44) | 24 (17–42) | 25.5 (19–44) | |
| BMI (kg/m2) | ||||
| Mean (SD) | 25.9 (2.8) | 25.7 (2.5) | 26.1 (3.0) | ns * |
| Median (Range) | 25.1 (21.6–35.4) | 25.4 (21.6–31.2) | 25.1 (22.9–35.4) | |
| Length of time # (months) | ||||
| Mean (SD) | 14.8 (24.8) | 18.5 (32.9) | 11.2 (12.3) | ns * |
| Median (Range) | 7.5 (0.4–144) | 7 (0.4–144) | 8 (1–42) | |
| Operated knee | ||||
| Right | 15 (37.5%) | 5 (25%) | 10 (50%) | ns ** |
| Left | 25 (62.5%) | 15 (75%) | 10 (50%) | |
| Dominant leg injuries | ||||
| Right | 19 (47.5%) | 7 (35%) | 12 (60%) | ns ** |
| Left | 21 (52.5%) | 13 (65%) | 8 (40%) | |
| Total | 40 (100%) | 20 (100%) | 20 (100%) | - |
| Depth of Bone Defect (mm) | Total | Vivostat | Standard | Test |
|---|---|---|---|---|
| Postoperative values | ||||
| Mean (SD) | 6.7 (1.7) | 6.7 (1.9) | 6.7 (1.6) | ns * |
| Median (Range) | 6.9 (3.3–12.4) | 7.0 (3.3–12.4) | 6.68 (4.5–10.0) | |
| 4 months after surgery | ||||
| Mean (SD) | 4.4 (1.4) | 4.3 (1.3) | 4.4 (1.4) | ns * |
| Median (Range) | 4.5 (2.1–7.9) | 4.5 (2.1–6.7) | 4.5 (2.2–7.9) | |
| 8 months after surgery | ||||
| Mean (SD) | 3.4 (1.1) | 3.3 (1.1) | 3.4 (1.1) | ns * |
| Median (Range) | 3.5 (1.4–6.0) | 3.5 (1.4–6.0) | 3.5 (1.7–5.6) | |
| 12 months after surgery | ||||
| Mean (SD) | 2.9 (0.9) | 2.8 (1) | 2.9 (0.8) | ns * |
| Median (Range) | 3.0 (1.2–5.7) | 3.0 (1.2–5.7) | 2.9 (1.6–4.5) | |
| Test between controls | ||||
| All controls (Friedman Test) | ||||
| Postoperative vs. 4 # vs. 8 # vs. 12 # | p < 0.01 | p < 0.01 | p < 0.01 | - |
| Pair of controls (Wilcox Signed-Rank Test) | ||||
| Initial vs. 4 # | p ** < 0.0083 | p ** < 0.0083 | p ** < 0.0083 | - |
| Initial vs. 8 # | ||||
| Initial vs. 12 # | ||||
| 4 # vs. 8 # | ||||
| 4 # vs. 12 # | ||||
| 8 # vs. 12 # | ||||
| Total | 40 (100%) | 20 (100%) | 20 (100%) | - |
| Width of Bone Defect (mm) | Total | Vivostat | Standard | Test |
|---|---|---|---|---|
| Preoperative values | ||||
| Mean (SD) | 10.3 (2.0) | 10.0 (2.0) | 10.6 (2.0) | ns |
| Median (Range) | 10.3 (4.1–13.9) | 10.4 (5.5–13.6) | 10.3 (4.1–13.9) | |
| 4 months after surgery | ||||
| Mean (SD) | 8.1 (2.0) | 7.6 (2.0) | 8.6 (1.8) | ns |
| Median (Range) | 8.2 (2.5–11.8) | 7.6 (2.5–11.8) | 8.6 (3.8–11.7) | |
| 8 months after surgery | ||||
| Mean (SD) | 6.5 (1.8) | 5.8 (1.8) | 7.1 (1.5) | p < 0.05 |
| Median (Range) | 6.6 (2.0–9.5) | 5.4 (2.0–8.6) | 7.2 (3.5–9.5) | |
| 12 months after surgery | ||||
| Mean (SD) | 5.2 (1.5) | 4.6 (1.5) | 5.8 (1.2) | p < 0.05 |
| Median (Range) | 5.0 (1.2–7.9) | 4.4 (1.2–7.4) | 5.8 (3.3–7.9) | |
| Test between controls | ||||
| All controls (Friedman Test) | ||||
| Initial vs. 4 # vs. 8 # vs. 12 # | p < 0.01 | p < 0.01 | p < 0.01 | - |
| Pair of controls (Wilcoxon Signed-Rank Test) | ||||
| Initial vs. 4 # | p ** < 0.0083 | p ** < 0.0083 | p ** < 0.0083 | - |
| Initial vs. 8 # | ||||
| Initial vs. 12 # | ||||
| 4 # vs. 8 # | ||||
| 4 # vs. 12 # | ||||
| 8 # vs. 12 # | ||||
| Total | 40 (100%) | 20 (100%) | 20 (100%) | - |
| Type of Score | Total | Vivostat | Standard | Test * |
|---|---|---|---|---|
| Modified Cincinatti score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 65.5 (20.2) | 68.3 (18.5) | 62.7 (21.9) | ns |
| Median (Range) | 65 (22–100) | 68 (34–100) | 63 (22–91) | |
| 12 months after surgery | ||||
| Mean (SD) | 93.1 (8.2) | 92.4 (8.6) | 93.8 (8.0) | ns |
| Median (Range) | 96 (70–100) | 94.5 (75–100) | 96.8 (70–100) | |
| Wilcoxon Signed-Rank Test | ||||
| 12 months vs. initial | p < 0.01 | p < 0.01 | p < 0.01 | - |
| Activity score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 6.8 (2.6) | 6.8 (2.6) | 6.7 (2.7) | ns |
| Median (Range) | 7 (0–10) | 7 (2–10) | 7 (0–10) | |
| 12 months after surgery | ||||
| Mean (SD) | 6.6 (2.1) | 6.6 (2.1) | 6.6 (2.1) | ns |
| Median (Range) | 6 (3–10) | 6 (3–10) | 6.5 (3–10) | |
| Wilcoxon Signed-Rank Test | ||||
| 12 months vs. initial | ns | ns | ns | - |
| IKDC score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 57.8 (19.3) | 61.3 (18.3) | 54.2 (20.2) | ns |
| Median (Range) | 58.0 (17.2–93.1) | 64.4 (21.8–88.5) | 55.2 (17.2–93.1) | |
| 12 months after surgery | ||||
| Mean (SD) | 89.9 (10.3) | 89.2 (11.2) | 90.6 (9.5) | ns |
| Median (Range) | 90.8 (52.9–100) | 90.8 (52.9–100) | 91.4 (67.8–100) | |
| Wilcoxon Signed-Rank Test | ||||
| 12 months vs. initial | p < 0.01 | p < 0.01 | p < 0.01 | - |
| Tegner Lysholm score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 71.2 (21.2) | 74.8 (17.5) | 67.4 (24.2) | ns |
| Median (Range) | 77 (16–100) | 79 (39–100) | 76 (16–91) | |
| 12 months after surgery | ||||
| Mean (SD) | 93.5 (14.4) | 95.6 (5.4) | 91.4 (19.7) | ns |
| Median (Range) | 95 (10–100) | 97 (81–100) | 95 (10–100) | |
| Wilcoxon Signed-Rank Test | ||||
| 12 months vs. initial | p < 0.01 | p < 0.01 | p < 0.01 | - |
| Total | 40 (100%) | 20 (100%) | 20 (100%) | - |
| Bone Defect Dimensions | Pain Intensity (VAS) during the Kneeling Test (after Surgery) | ||
|---|---|---|---|
| 4 Months | 8 Months | 12 Months | |
| Depth of bone defect (mm) | |||
| Postoperative values | |||
| Spearman’s rho | rho = −0.10 | rho = −0.006 | rho = 0.06 |
| p-value | p = 0.52 | p = 0.97 | p = 0.70 |
| 4 months after surgery | |||
| Spearman’s rho | rho = −0.07 | rho = −0.01 | rho = −0.08 |
| p-value | p = 0.68 | p = 0.94 | p = 0.62 |
| 8 months after surgery | |||
| Spearman’s rho | - | rho = −0.02 | rho = −0.07 |
| p-value | p = 0.88 | p = 0.68 | |
| 12 months after surgery | |||
| Spearman’s rho | - | - | rho = −0.03 |
| p-value | p = 0.87 | ||
| Width of bone defect (mm) | |||
| Postoperative values | |||
| Spearman’s rho | rho = 0.15 | rho = 0.23 | rho = 0.17 |
| p-value | p = 0.33 | p = 0.15 | p = 0.28 |
| 4 months after surgery | |||
| Spearman’s rho | rho = 0.01 | rho = 0.24 | rho = 0.20 |
| p-value | p = 0.94 | p = 0.14 | p = 0.21 |
| 8 months after surgery | |||
| Spearman’s rho | - | rho = 0.18 | rho = 0.24 |
| p-value | p = 0.27 | p = 0.14 | |
| 12 months after surgery | |||
| Spearman’s rho | - | - | rho = 0.17 |
| p-value | p = 0.28 | ||
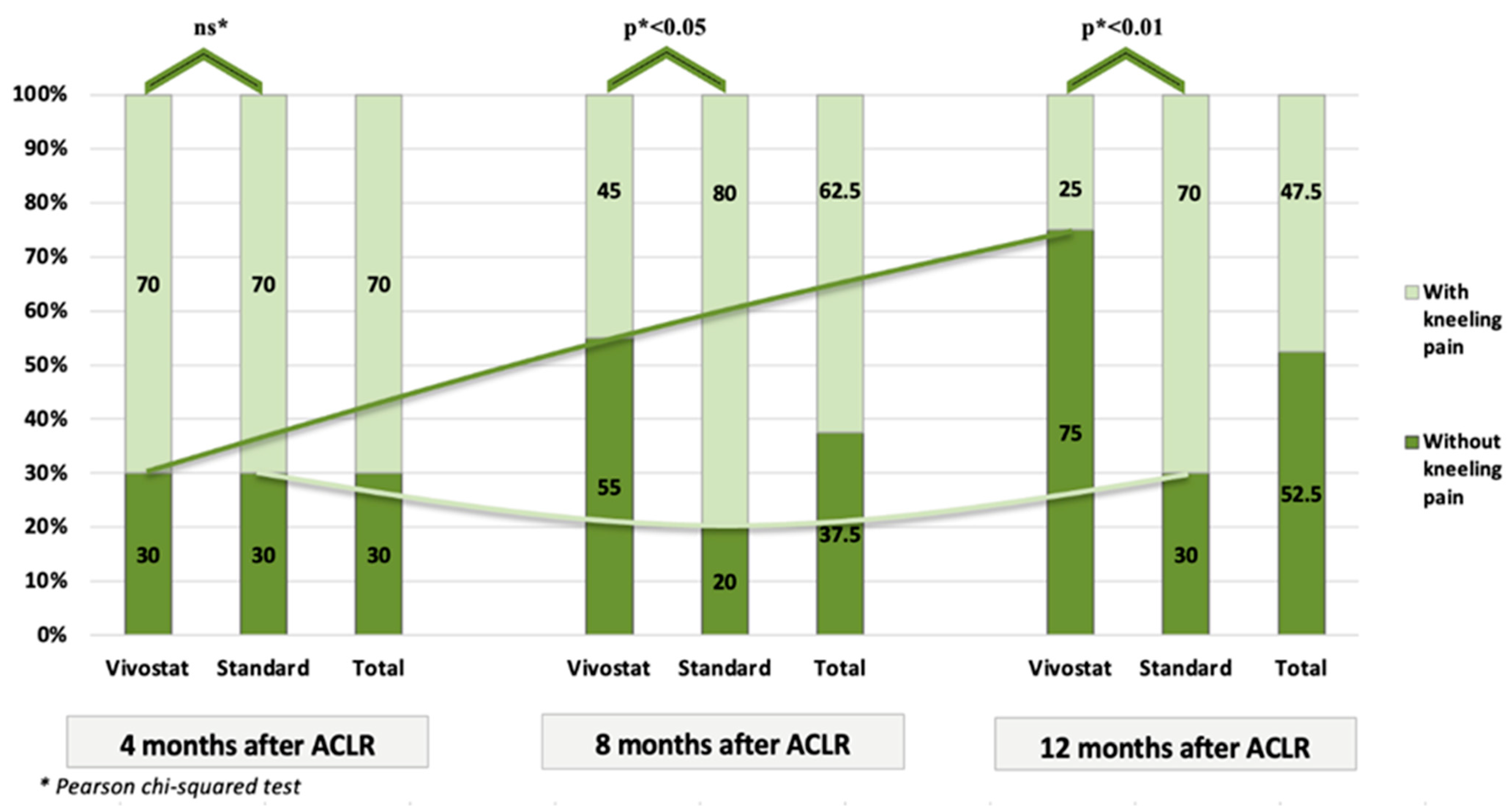
| Kneeling Pain (VAS) | Total | Vivostat | Standard | Test * |
|---|---|---|---|---|
| 4 months after surgery | ||||
| Mean (SD) | 1.7 (1.49) | 1.7 (1.53) | 1.7 (1.49) | ns |
| Median (Range) | 2 (0–5) | 2 (0–5) | 1.5 (0–4) | |
| 8 months after surgery | ||||
| Mean (SD) | 1.12 (1.14) | 0.75 (1.07) | 1.5 (1.1) | p < 0.05 |
| Median (Range) | 1 (0–4) | 0 (0–4) | 1 (0–3) | |
| 12 months after surgery | ||||
| Mean (SD) | 0.62 (0.81) | 0.35 (0.75) | 0.9 (0.79) | p < 0.01 |
| Median (Range) | 0 (0–3) | 0 (0–3) | 1 (0–3) | |
| Test between controls | ||||
| All controls (Friedman Test) | ||||
| 4 # vs. 8 # vs. 12 # | p < 0.01 | p < 0.01 | p < 0.01 | - |
| Pairs of controls (Wilcoxon Signed-Rank Test) | ||||
| 4 # vs. 8 # | p ** < 0.0167 | p ** < 0.0167 | ns ** | - |
| 4 # vs. 12 # | p ** < 0.0167 | p ** < 0.0167 | p ** < 0.0167 | - |
| 8 # vs. 12 # | p ** < 0.0167 | p ** < 0.0167 | p ** < 0.0167 | - |
| Total | 40 (100%) | 20 (100%) | 20 (100%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanovic, D.; Vukman, P.; Gavrilovic, D.; Begovic, N.; Stijak, L.; Sreckovic, S.; Kadija, M. The Influence of Platelet-Rich Fibrin on the Healing of Bone Defects after Harvesting Bone–Patellar Tendon–Bone Grafts. Medicina 2024, 60, 154. https://doi.org/10.3390/medicina60010154
Milovanovic D, Vukman P, Gavrilovic D, Begovic N, Stijak L, Sreckovic S, Kadija M. The Influence of Platelet-Rich Fibrin on the Healing of Bone Defects after Harvesting Bone–Patellar Tendon–Bone Grafts. Medicina. 2024; 60(1):154. https://doi.org/10.3390/medicina60010154
Chicago/Turabian StyleMilovanovic, Darko, Petar Vukman, Dusica Gavrilovic, Ninoslav Begovic, Lazar Stijak, Svetlana Sreckovic, and Marko Kadija. 2024. "The Influence of Platelet-Rich Fibrin on the Healing of Bone Defects after Harvesting Bone–Patellar Tendon–Bone Grafts" Medicina 60, no. 1: 154. https://doi.org/10.3390/medicina60010154
APA StyleMilovanovic, D., Vukman, P., Gavrilovic, D., Begovic, N., Stijak, L., Sreckovic, S., & Kadija, M. (2024). The Influence of Platelet-Rich Fibrin on the Healing of Bone Defects after Harvesting Bone–Patellar Tendon–Bone Grafts. Medicina, 60(1), 154. https://doi.org/10.3390/medicina60010154






