Mind the Gap: A Questionnaire on the Distance between Diagnostic Advances and Clinical Practice in Skin Cancer Treatment
Abstract
1. Introduction
- − Adhesive patch biopsy (tape stripping mRNA);
- − EIS (electrical impedance spectroscopy);
- − Multispectral imaging;
- − High-frequency ultrasonography (HFUS);
- − Optical coherence tomography (OCT);
- − Reflectance confocal microscopy (RCM);
- − Artificial intelligence (AI) and computer analysis.
2. Materials and Methods
3. Results
3.1. General Results
3.2. Knowledge of Diagnostic Instruments
- Multispectral imaging: Just 6.9% (n = 2) of our cohort knew about this technology and none of them applied it (n = 0) (Table 3).
- Confocal reflectance microscopy (RCM): Only 55.2% (n = 16) of our cohort knew about this technology and only 20.7% (n = 6) applied it (Table 3).
- Artificial intelligence (AI) and computer analysis: 72.4% (n = 21) of our cohort knew about this technology and only 13.8% (n = 4) applied it (Table 3).
4. Discussion
| Author | Year | Journal | Methodology | Techniques |
|---|---|---|---|---|
| Lassau et al. [33] | 1997 | Radiographics | Original Article | HFUS |
| Moncrieff et al. [22] | 2002 | Br J Dermatol | Original Article | MI |
| Bessoud et al. [12] | 2003 | Ultrasound Med Biol | Original Article | HFUS |
| Glickman et al. [42] | 2003 | Skin Res Technol | Original Article | EIS |
| Ruocco et al. [43] | 2004 | Dermatol Surg | Review Article | RCM |
| Har-shai et al. [44] | 2005 | Plast Reconstr Surg | Multicenter Study | EIS |
| Machet et al. [45] | 2009 | Ultrasound Med Biol | Review Article | HFUS |
| Wachsman et al. [19] | 2011 | Br J Dermatol | Original Article | APB |
| Monheit et al. [11] | 2011 | Arch Dermatol | Original Article | MI |
| Crisan et al. [31] | 2013 | Arch Dermatol Res | Original Article | HFUS |
| Gerami et al. [10] | 2014 | J Am Acad Dermatol | Original Article | APB |
| Malvehy et al. [5] | 2014 | Br J Dermatol | Clinical Trial | MI |
| Meyer et al. [46] | 2014 | Br J Dermatol | Original Article | HFUS, OCT |
| Longo et al. [47] | 2014 | J Am Acad Dermatol | Original Article | RCM |
| March et al. [8] | 2015 | J Am Acad Dermatol | Review Article | EIS, MI |
| Markowitz et al. [13] | 2015 | J Clin Aesthet Dermatol | Original Article | OCT |
| Gambichler et al. [14] | 2015 | J Eur Acad Dermatol Venereol | Original Article | OCT |
| Olsen et al. [34] | 2016 | Photodiagn Photodyn Ther | Original Article | OCT |
| Que et al. [15] | 2016 | Dermatol Clin | Original Article | RCM |
| Borsari et al. [16] | 2016 | JAMA Dermatol | Original Article | RCM |
| Guilera et al. [48] | 2016 | Dermatol Clin | Review Article | RCM |
| Ferris et al. [17] | 2017 | JAMA Dermatol | Original Article | APB |
| Gerami et al. [18] | 2017 | J Am Acad Dermatol | Original Article | APB |
| Welzel et al. [28] | 2017 | J Dtsch Dermatol Ges | Review Article | EIS, OCT, RCM, MI |
| Braun et al. [29] | 2017 | Dermatol Clin | Original Article | EIS |
| Niculescu et al. [49] | 2017 | Photodiagn Photodyn Ther | Original Article | OCT |
| Heibel et al. [7] | 2020 | Am J Clin Dermatol | Review Article | APB; EIS, MI; HFUS; OCT; RCM |
| Chu et al. [39] | 2020 | Front Med | Review Article | AI |
| Pathania et al. [25] | 2022 | J Cosmet Dermatol | Review Article | APB; EIS, MI; HFUS; OCT; RCM: AI |
| Owida et al. [50] | 2022 | J Skin Cancer | Review Article | MI; HUFS; OCT; RCM |
| Thomsen et al. [9] | 2023 | Skin Res Technol | Systematic Review | APB |
4.1. Skin Cancer Units
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urban, K.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, G.R. The global burden of skin cancer: A longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int. 2021, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer Today, International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence (accessed on 25 September 2023).
- ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-All$2-All$4-1,2$3-27$6-0,85$5-2022,2022$7-7$CEstByCountry$X0_8-3$X0_19-AE27$X0_20-No$CEstBySexByCountry$X1_8-3$X1_19-AE27$X1_-1-1$CEstByIndiByCountry$X2_8-3$X2_19-AE27$X2_20-No$CEstRelative$X3_8-3$X3_9-AE27$X3_19-AE27$CEstByCountryTable$X4_19-AE27 (accessed on 12 September 2023).
- American Cancer Society. Facts & Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023; Available online: https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html#:~:text=About%2097%2C610%20new%20melanomas%20will,5%2C420%20men%20and%202%2C570%20women (accessed on 13 September 2023).
- Malvehy, J.; Hauschild, A.; Curiel-Lewandrowski, C.; Mohr, P.; Hofmann-Wellenhof, R.; Motley, R.; Berking, C.; Grossman, D.; Paoli, J.; Loquai, C.; et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: An international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br. J. Dermatol. 2014, 171, 1099–1107. [Google Scholar] [CrossRef]
- Townsend, T. Diagnosis and management of melanoma in a rural general practice. J. Prim. Health Care 2018, 10, 207–209. [Google Scholar] [CrossRef]
- Heibel, H.D.; Hooey, L.; Cockerell, C.J. A Review of Noninvasive Techniques for Skin Cancer Detection in Dermatology. Am. J. Clin. Dermatol. 2020, 21, 513–524. [Google Scholar] [CrossRef] [PubMed]
- March, J.; Hand, M.; Grossman, D. Practical application of new technologies for melanoma diagnosis: Part I. Noninvasive approaches. J. Am. Acad. Dermatol. 2015, 72, 929–941, quiz 941–942; Erratum in J. Am. Acad. Dermatol. 2015, 73, 720. [Google Scholar] [CrossRef]
- Thomsen, I.M.N.; Heerfordt, I.M.; Karmisholt, K.E.; Mogensen, M. Detection of cutaneous malignant melanoma by tape stripping of pigmented skin lesions—A systematic review. Skin Res. Technol. 2023, 29, e13286. [Google Scholar] [CrossRef] [PubMed]
- Gerami, P.; Alsobrook JP 2nd Palmer, T.J.; Robin, H.S. Development of a novel noninvasive adhesive patch test for the evaluation of pigmented lesions of the skin. J. Am. Acad. Dermatol. 2014, 71, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Monheit, G.; Cognetta, A.B.; Ferris, L.; Rabinovitz, H.; Gross, K.; Martini, M.; Grichnik, J.M.; Mihm, M.; Prieto, V.G.; Googe, P.; et al. The performance of MelaFind: A prospective multicenter study. Arch. Dermatol. 2011, 147, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Bessoud, B.; Lassau, N.; Koscielny, S.; Longvert, C.; Avril, M.F.; Duvillard, P.; Rouffiac, V.; Leclère, J.; Roche, A. High-frequency sonography and color Doppler in the management of pigmented skin lesions. Ultrasound. Med. Biol. 2003, 29, 875–879. [Google Scholar] [CrossRef]
- Markowitz, O.; Schwartz, M.; Feldman, E.; Bienenfeld, A.; Bieber, A.K.; Ellis, J.; Alapati, U.; Lebwohl, M.; Siegel, D.M. Evaluation of Optical Coherence Tomography as a Means of Identifying Earlier Stage Basal Cell Carcinomas while Reducing the Use of Diagnostic Biopsy. J. Clin. Aesthet. Dermatol. 2015, 8, 14–20. [Google Scholar]
- Gambichler, T.; Schmid-Wendtner, M.H.; Plura, I.; Kampilafkos, P.; Stücker, M.; Berking, C.; Maier, T. A multicentre pilot study investigating high-definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.; Grant-Kels, J.M.; Rabinovitz, H.S.; Oliviero, M.; Scope, A. Application of Handheld Confocal Microscopy for Skin Cancer Diagnosis: Advantages and Limitations Compared with the Wide-Probe Confocal. Dermatol. Clin. 2016, 34, 469–475. [Google Scholar] [CrossRef]
- Borsari, S.; Pampena, R.; Lallas, A.; Kyrgidis, A.; Moscarella, E.; Benati, E.; Raucci, M.; Pellacani, G.; Zalaudek, I.; Argenziano, G.; et al. Clinical Indications for Use of Reflectance Confocal Microscopy for Skin Cancer Diagnosis. JAMA Dermatol. 2016, 152, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.K.; Jansen, B.; Ho, J.; Busam, K.J.; Gross, K.; Hansen, D.D.; Alsobrook, J.P.; Yao, Z.; Peck, G.L.; Gerami, P. Utility of a Noninvasive 2-Gene Molecular Assay for Cutaneous Melanoma and Effect on the Decision to Biopsy. JAMA Dermatol. 2017, 153, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Gerami, P.; Yao, Z.; Polsky, D.; Jansen, B.; Busam, K.; Ho, J.; Martini, M.; Ferris, L.K. Development and validation of a noninvasive 2-gene molecular assay for cutaneous melanoma. J. Am. Acad. Dermatol. 2017, 76, 114–120.e2. [Google Scholar] [CrossRef]
- Wachsman, W.; Morhenn, V.; Palmer, T.; Walls, L.; Hata, T.; Zalla, J.; Scheinberg, R.; Sofen, H.; Mraz, S.; Gross, K.; et al. Noninvasive genomic detection of melanoma. Br. J. Dermatol. 2011, 164, 797–806. [Google Scholar] [CrossRef]
- Tran, K.T.; Wright, N.A.; Cockerell, C.J. Biopsy of the pigmented lesion--when and how. J. Am. Acad. Dermatol. 2008, 59, 852–871. [Google Scholar] [CrossRef]
- Wassef, C.; Rao, B.K. Uses of non-invasive imaging in the diagnosis of skin cancer: An overview of the currently available modalities. Int. J. Dermatol. 2013, 52, 1481–1489. [Google Scholar] [CrossRef]
- Moncrieff, M.; Cotton, S.; Claridge, E.; Hall, P. Spectrophotometric intracutaneous analysis: A new technique for imaging pigmented skin lesions. Br. J. Dermatol. 2002, 146, 448–457. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME Expression in Melanocytic Tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef]
- Hornberger, J.; Siegel, D.M. Economic Analysis of a Noninvasive Molecular Pathologic Assay for Pigmented Skin Lesions. JAMA Dermatol. 2018, 154, 1025–1031. [Google Scholar] [CrossRef]
- Pathania, Y.S.; Apalla, Z.; Salerni, G.; Patil, A.; Grabbe, S.; Goldust, M. Non-invasive diagnostic techniques in pigmentary skin disorders and skin cancer. J. Cosmet. Dermatol. 2022, 21, 444–450. [Google Scholar] [CrossRef]
- Ferris, L.K.; Gerami, P.; Skelsey, M.K.; Peck, G.; Hren, C.; Gorman, C.; Frumento, T.; Siegel, D.M. Real-world performance and utility of a noninvasive gene expression assay to evaluate melanoma risk in pigmented lesions. Melanoma Res. 2018, 28, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, R.M.; Prado, G.; Mirsky, R.S.; Rigel, D.S. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J. Am. Acad. Dermatol. 2019, 80, 285–287. [Google Scholar] [CrossRef]
- Welzel, J.; Schuh, S. Noninvasive diagnosis in dermatology. J. Dtsch. Dermatol. Ges. 2017, 15, 999–1016. [Google Scholar] [CrossRef]
- Braun, R.P.; Mangana, J.; Goldinger, S.; French, L.; Dummer, R.; Marghoob, A.A. Electrical Impedance Spectroscopy in Skin Cancer Diagnosis. Dermatol. Clin. 2017, 35, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Aberg, P.; Nicander, I.; Holmgren, U.; Geladi, P.; Ollmar, S. Assessment of skin lesions and skin cancer using simple electrical impedance indices. Skin Res. Technol. 2003, 9, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Crisan, D.; Sannino, G.; Lupsor, M.; Badea, R.; Amzica, F. Ultrasonographic staging of cutaneous malignant tumors: An ultrasonographic depth index. Arch. Dermatol. Res. 2013, 305, 305–313. [Google Scholar] [CrossRef]
- Crisan, D.; Wortsman, X.; Alfageme, F.; Catalano, O.; Badea, A.; Scharffetter-Kochanek, K.; Sindrilaru, A.; Crisan, M. Ultrasonography in dermatologic surgery: Revealing the unseen for improved surgical planning. J. Dtsch. Dermatol. Ges. 2022, 20, 913–926. [Google Scholar] [CrossRef]
- Lassau, N.; Spatz, A.; Avril, M.F.; Tardivon, A.; Margulis, A.; Mamelle, G.; Vanel, D.; Leclere, J. Value of high-frequency US for preoperative assessment of skin tumors. Radiographics 1997, 17, 1559–1565. [Google Scholar] [CrossRef]
- Olsen, J.; Themstrup, L.; De Carvalho, N.; Mogensen, M.; Pellacani, G.; Jemec, G.B. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagn. Photodyn. Ther. 2016, 16, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.L.M.; Suppa, M.; Pellacani, G.; Marneffe, A.; Miyamoto, M.; Alarcon, I.; Ruini, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Jemec, G.B.E.; et al. High-definition optical coherence tomography algorithm for discrimination of basal cell carcinoma from clinical BCC imitators and differentiation between common subtypes. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1771. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.L.M.; Marneffe, A.; Suppa, M.; Miyamoto, M.; Alarcon, I.; Hofmann-Wellenhof, R.; Malvehy, J.; Pellacani, G.; Del Marmol, V. High-definition optical coherence tomography algorithm for the discrimination of actinic keratosis from normal skin and from squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1606–1615. [Google Scholar] [CrossRef]
- Haroon, A.; Shafi, S.; Rao, B.K. Using Reflectance Confocal Microscopy in Skin Cancer Diagnosis. Dermatol. Clin. 2017, 35, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Marghoob, A.A. Discriminating Nevi from Melanomas: Clues and Pitfalls. Dermatol. Clin. 2016, 34, 395–409. [Google Scholar] [CrossRef]
- Chu, Y.S.; An, H.G.; Oh, B.H.; Yang, S. Artificial Intelligence in Cutaneous Oncology. Front. Med. 2020, 7, 318. [Google Scholar] [CrossRef]
- Yee, J.; Rosendahl, C.; Aoude, L.G. The role of artificial intelligence and convolutional neural networks in the management of melanoma: A clinical, pathological, and radiological perspective. Melanoma Res. 2023. [Google Scholar] [CrossRef]
- Giavina Bianchi, M.; Santos, A.; Cordioli, E. Dermatologists’ perceptions on the utility and limitations of teledermatology after examining 55,000 lesions. J. Telemed. Telecare 2021, 27, 166–173. [Google Scholar] [CrossRef]
- Glickman, Y.A.; Filo, O.; David, M.; Yayon, A.; Topaz, M.; Zamir, B.; Ginzburg, A.; Rozenman, D.; Kenan, G. Electrical impedance scanning: A new approach to skin cancer diagnosis. Skin Res. Technol. 2003, 9, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, E.; Argenziano, G.; Pellacani, G.; Seidenari, S. Noninvasive imaging of skin tumors. Dermatol. Surg. 2004, 30 Pt 2, 301–310. [Google Scholar] [CrossRef]
- Har-Shai, Y.; Glickman, Y.A.; Siller, G.; McLeod, R.; Topaz, M.; Howe, C.; Ginzburg, A.; Zamir, B.; Filo, O.; Kenan, G.; et al. Electrical impedance scanning for melanoma diagnosis: A validation study. Plast. Reconstr. Surg. 2005, 116, 782–790. [Google Scholar] [CrossRef]
- Machet, L.; Belot, V.; Naouri, M.; Boka, M.; Mourtada, Y.; Giraudeau, B.; Laure, B.; Perrinaud, A.; Machet, M.C.; Vaillant, L. Preoperative measurement of thickness of cutaneous melanoma using high-resolution 20 MHz ultrasound imaging: A monocenter prospective study and systematic review of the literature. Ultrasound. Med. Biol. 2009, 35, 1411–1420. [Google Scholar] [CrossRef]
- Meyer, N.; Lauwers-Cances, V.; Lourari, S.; Laurent, J.; Konstantinou, M.P.; Lagarde, J.M.; Krief, B.; Batatia, H.; Lamant, L.; Paul, C. High-frequency ultrasonography but not 930-nm optical coherence tomography reliably evaluates melanoma thickness in vivo: A prospective validation study. Br. J. Dermatol. 2014, 171, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Lallas, A.; Kyrgidis, A.; Rabinovitz, H.; Moscarella, E.; Ciardo, S.; Zalaudek, I.; Oliviero, M.; Losi, A.; Gonzalez, S.; et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J. Am. Acad. Dermatol. 2014, 71, 716–724.e1. [Google Scholar] [CrossRef]
- Guilera, J.M.; Barreiro Capurro, A.; Carrera Alvárez, C.; Puig Sardá, S. The Role of Reflectance Confocal Microscopy in Clinical Trials for Tumor Monitoring. Dermatol. Clin. 2016, 34, 519–526. [Google Scholar] [CrossRef]
- Niculescu, L.; Bierhoff, E.; Hartmann, D.; Ruzicka, T.; Berking, C.; Braunmühl, T.V. Optical coherence tomography imaging of basal cell carcinoma undergoing photodynamic therapy: A pilot study. Photodiagn. Photodyn. Ther. 2017, 18, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Owida, H.A. Developments and Clinical Applications of Noninvasive Optical Technologies for Skin Cancer Diagnosis. J. Skin Cancer. 2022, 2022, 9218847. [Google Scholar] [CrossRef]
- Marino, M.L.; Carrera, C.; Marchetti, M.A.; Marghoob, A.A. Practice Gaps in Dermatology: Melanocytic Lesions and Melanoma. Dermatol. Clin. 2016, 34, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Losco, L.; Bolletta, A.; Pierazzi, D.M.; Spadoni, D.; Cuomo, R.; Marcasciano, M.; Cavalieri, E.; Roxo, A.C.; Ciamarra, P.; Cantisani, C.; et al. Reconstruction of the Nose: Management of Nasal Cutaneous Defects According to Aesthetic Subunit and Defect Size. A Review. Medicina 2020, 56, 639. [Google Scholar] [CrossRef]
- Bolletta, A.; Losco, L.; Pozzi, M.; Schettino, M.; Cigna, E. A Retrospective Study on Single-Stage Reconstruction of the Ear following Skin Cancer Excision in Elderly Patients. J. Clin. Med. 2022, 11, 838. [Google Scholar] [CrossRef]
- Paolino, G.; Cardone, M.; Didona, D.; Moliterni, E.; Losco, L.; Corsetti, P.; Schipani, G.; Lopez, T.; Calvieri, S.; Bottoni, U. Prognostic factors in head and neck melanoma according to facial aesthetic units. G. Ital. Dermatol. Venereol. 2020, 155, 41–45. [Google Scholar] [CrossRef]
- Lo Torto, F.; Redi, U.; Cigna, E.; Losco, L.; Marcasciano, M.; Casella, D.; Ciudad, P.; Ribuffo, D. Nasal Reconstruction with Two Stages versus Three Stages Forehead Fap: What is Better for Patients with High Vascular Risk? J. Craniofac. Surg. 2020, 31, e57–e60. [Google Scholar] [CrossRef] [PubMed]
- Govshievich, A.; Bauder, A.; Kovach, S.J.; Levin, L.S. Aesthetic Considerations in Extremity Salvage and Reconstruction. Plast. Reconstr. Surg. 2023, 151, 679e–687e. [Google Scholar] [CrossRef]
- Kenkel, J.M. Introducing Aesthetic Breast Reconstruction and Aesthetic Breast Surgery. Aesthetic Surg. J. 2023, 43, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, H.S. A novel approach to teaching dermatology and plastic surgery in a combined module for undergraduate medical students. Adv. Med. Educ. Pract. 2019, 10, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Kim, J.B. Integration of plastic surgery into the undergraduate medical curriculum: The Norwich model and experience. Int. J. Med. Educ. 2012, 3, 14–16. [Google Scholar] [CrossRef]
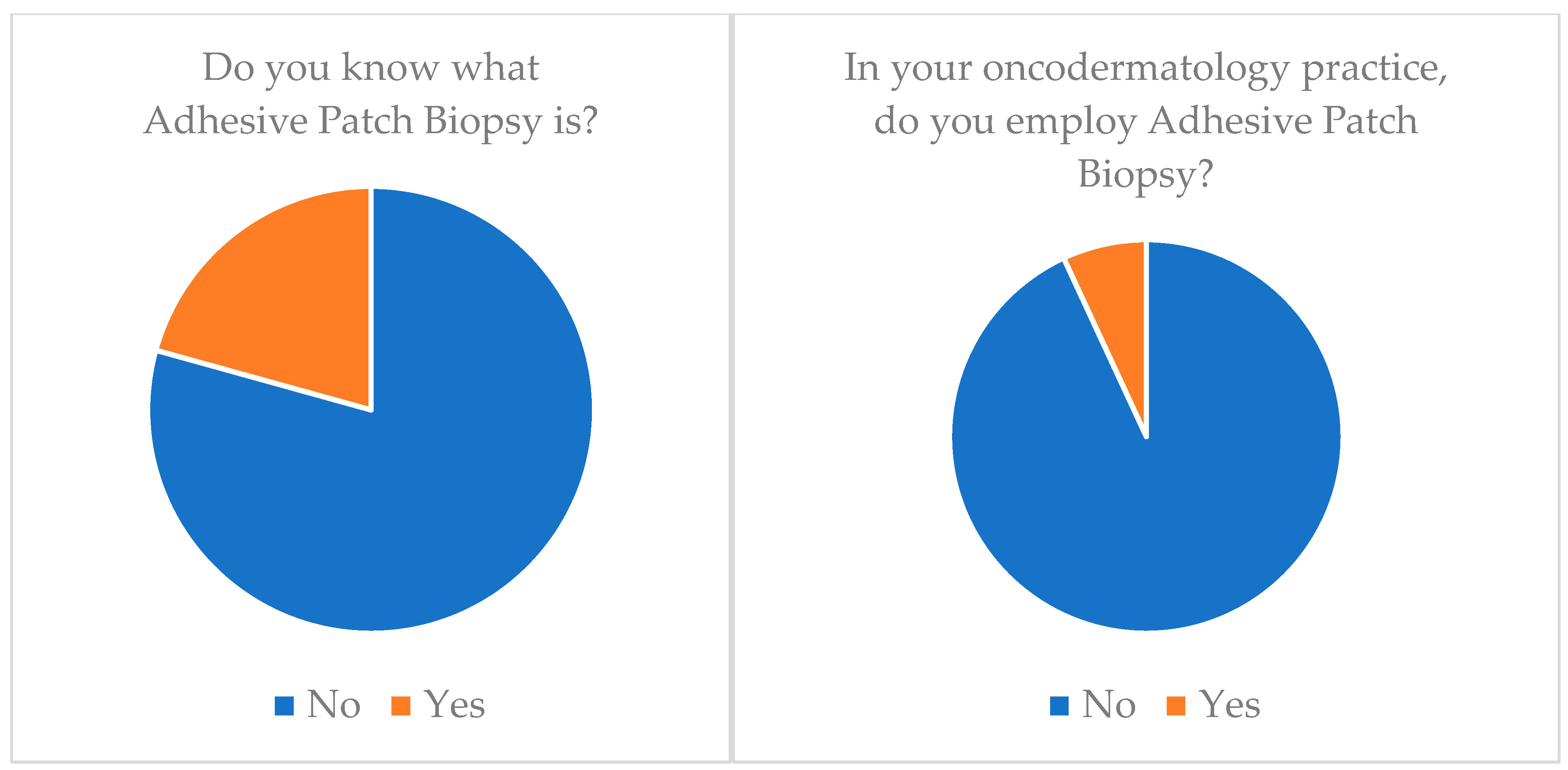


| Technique | Summary Indication |
|---|---|
| Adhesive Patch Biopsy (APB) | Early melanoma diagnostic determination (in association with clinic and dermoscopy) and prognostic information. |
| Electrical Impedance Spectroscopy (EIS) | Improves diagnostic ability of MSC and NMSC (in association with clinic and dermoscopy). |
| Multispectral Imaging | Complementary tool in the diagnostic definition of melanocytic lesions. |
| High-Frequency Ultrasonography (HFUS) | Useful in the diagnosis of surgical margins and determination of lesion depth. |
| Optical Coherence Tomography (OCT) | Very sensitive and specific instrument in the diagnostic determination of BCC. Useful in determining surgical margins and monitoring nonsurgical treatments. |
| Reflectance Confocal Microscopy (RCM) | Allows in vivo visualization of tissue at very high resolution. Allows determination of lesion margins and monitoring of nonsurgical treatments over time. |
| Computer-Assisted Diagnosis and Artificial Intelligence (AI) | Advanced methods with possibility of automation and standardization of the diagnostic process. Possible application on smartphones. |
| Inclusion Criteria |
|---|
| Presence of a dermatology unit and a plastic surgery unit |
| Treatment of all malignant skin cancers except sarcomas |
| Complete responses to the questionnaire |
| I Know It (%) | It Is Employed (%) | |
|---|---|---|
| Adhesive Patch Biopsy (APB) | 20.7 | 6.9 |
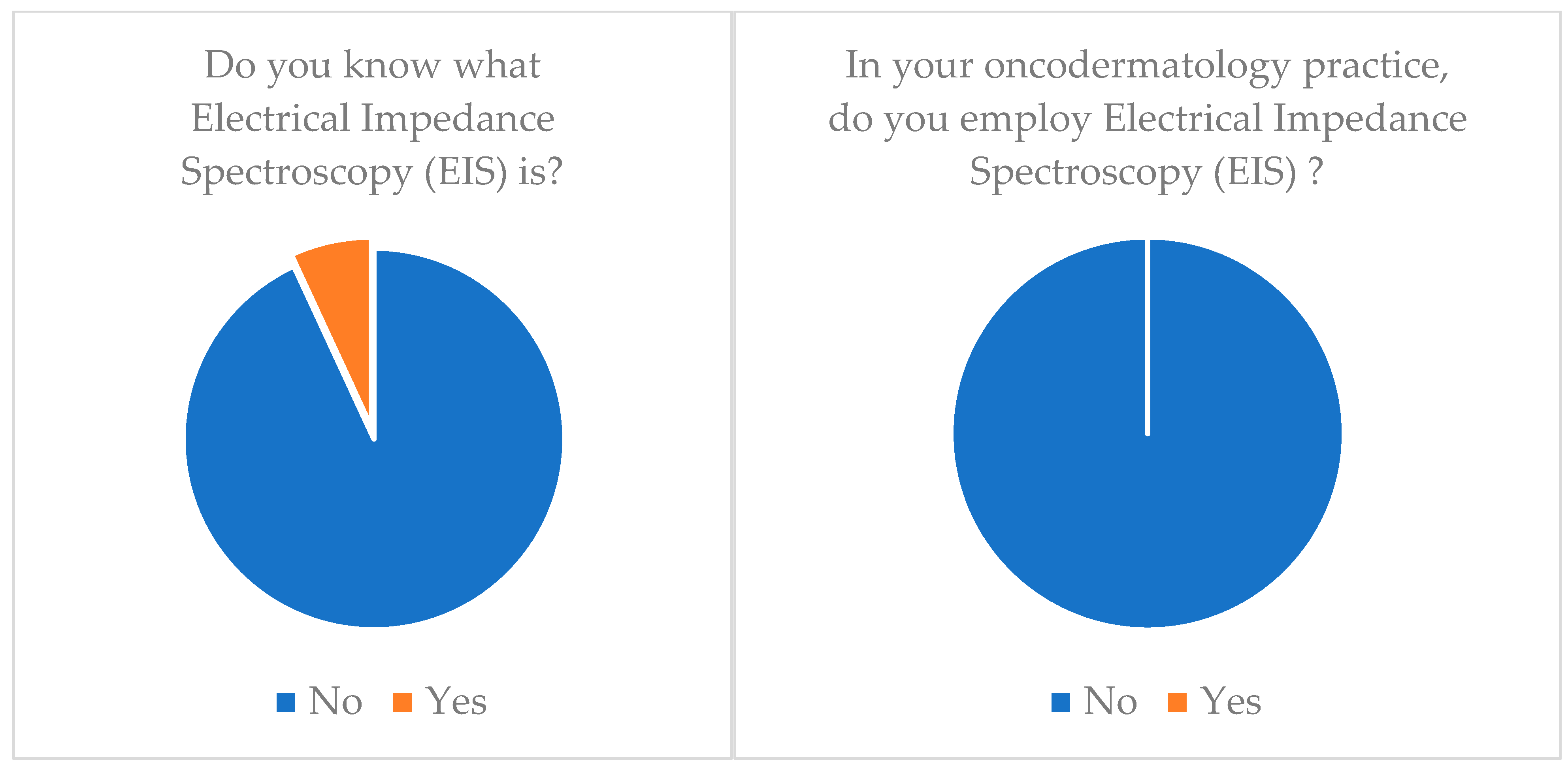
| Electrical Impedance Spectroscopy (EIS) | 6.9 | 0 |
| Multispectral Imaging | 6.9 | 0 |
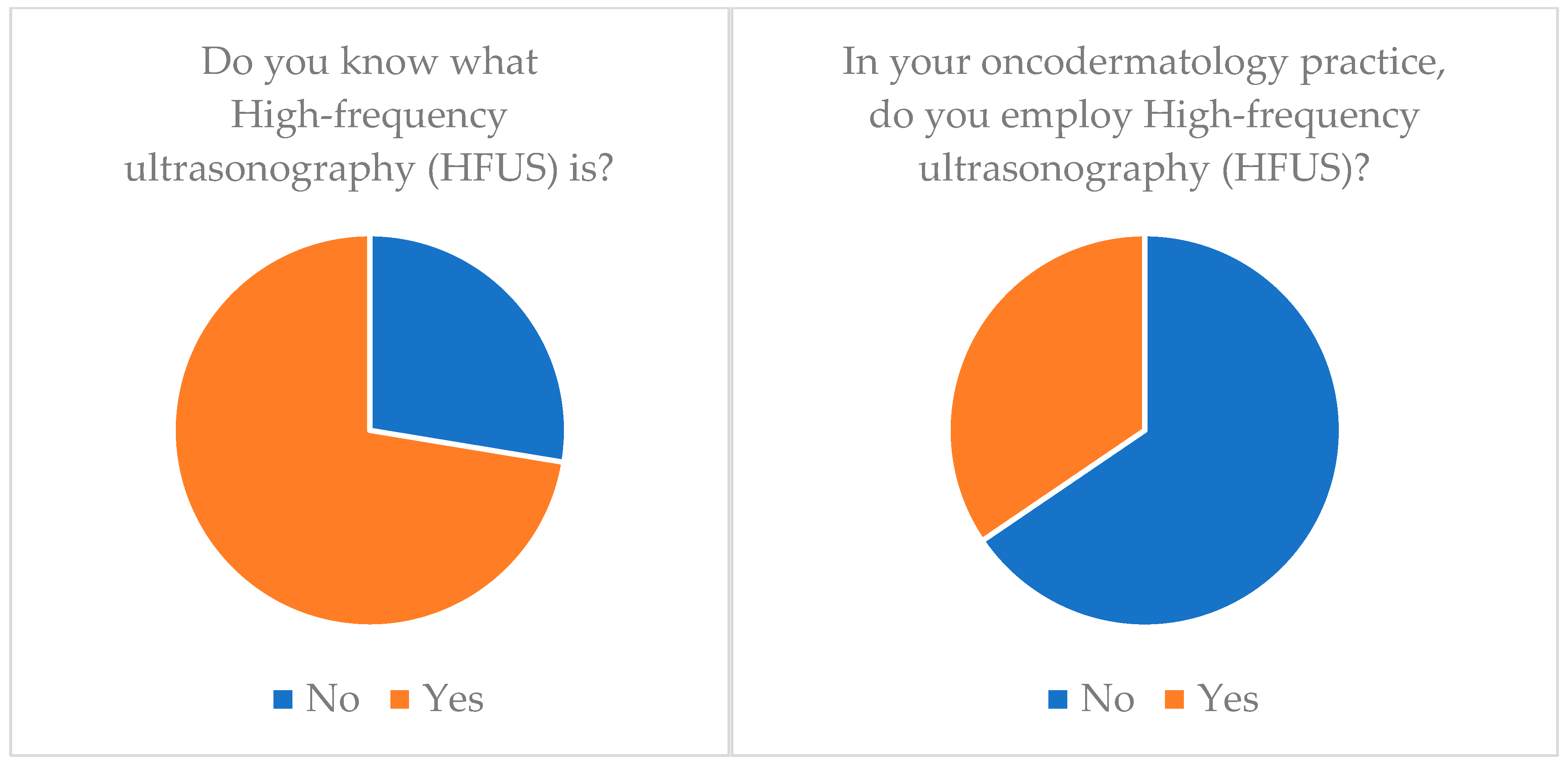
| High-Frequency Ultrasonography (HFUS) | 72.4 | 34.5 |
| Optical Coherence Tomography (OCT) | 55.2 | 20.7 |
| Reflectance Confocal Microscopy (RCM) | 55.2 | 20.7 |
| Computer-Assisted Diagnosis and Artificial Intelligence (AI) | 72.4 | 13.8 |
| Technique | Advantages | Limits | Sensibility | Specificity |
|---|---|---|---|---|
| Adhesive Patch Biopsy (APB) | Good sensitivity and specificity in the diagnosis of MSC. Can avoid unnecessary biopsies. Provides information on the genetic pattern of the lesion. | Do not use on bleeding or ulcerated lesions. Do not use on mucous membranes or palms of hands and feet. Some melanomas do not express the genes tested. | 68.8–100% (Thomse [9]) - 91% (Gerami [10]) | 69.1–100% (Thomsen [9]) - 67% (Gerami [10])) |
| Electrical Impedance Spectroscopy (EIS) | Good sensitivity. Useful for monitoring lesions over time and diagnosing early melanomas, especially in patients with numerous pigmented neoformations. | Low specificity. Not usable in bleeding or ulcerated lesions. Many false positives in the presence of seborrheic keratoses. Efficacy and values vary depending on the thickness of the skin in the region analyzed. | MSC 96.6% (Malvehy [5]) | MSC 34.4% (Malvehy [5]) |
| Multispectral Imaging (MI) | Good sensitivity. Useful as a diagnostic aid in non-specialized settings. | Low specificity. Not sufficient to make diagnosis. Does not seem suitable for diagnosis of NMSC. | 98.3% (Monheit [11]) | 9.9% (Monheit [11]) |
| High-Frequency Ultrasonography (HFUS) | Good sensitivity. Useful in determining lesion depth. Useful in in vivo determination of lesions and in determining surgical margins. | Intermediate specificity. It is an operator-dependent method, difficult to estimate in cases of very thin or very thick tumors. | MSC 83–100% (Bessoud [12]) | MSC 32% (Bessoud [12]) |
| Optical Coherence Tomography (OCT) | Good sensitivity and specificity. Useful in defining surgical margins and monitoring noninvasive therapies. Useful in the diagnosis of BCC. | Lower resolution than RCM, does not reach a level of cellular resolution. Possible misdiagnosis in case of amelanocytic melanoma. | BCC 92.9% (Markowitz [13]) - MSC 74.1% (Gambichler [14]) | BCC 80.0% (Markowitz [13]) - MSC 92.4% (Gambichler [14]) |
| Reflectance Confocal Microscopy (RCM) | Good sensitivity and specificity. Provides very high-resolution images in vivo. Useful for determining margins and distinguishing different lesions based on specific patterns. | Depth of fabric examined limited. Elevated cost. Reduced efficacy in ulceration, inflammation, hyperpigmentation, and hyperkeratosis. | MSC 91–100% (Que [15]) - BCC 85–97% (Que [15]) - All skin cancer 95.3% (Borsari [16]) | 68–98% (Que [15]) - 89–99% (Que [15]) - All skin cancer 83.5% (Borsari [16]) |
| Computer-Assisted Diagnosis and Artificial Intelligence (AI) | Broad potential, with possibilities for automating diagnostic processes, a field still largely unknown. | Field still unknown, difficulty in data standardization and deviation of results generated from real-world scenarios. | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diluiso, G.; Pozzi, M.; Liso, F.G.; Mendes, V.M.; Hannouille, J.; Losco, L.; Bolletta, A.; Cigna, E.; Schettino, M. Mind the Gap: A Questionnaire on the Distance between Diagnostic Advances and Clinical Practice in Skin Cancer Treatment. Medicina 2024, 60, 155. https://doi.org/10.3390/medicina60010155
Diluiso G, Pozzi M, Liso FG, Mendes VM, Hannouille J, Losco L, Bolletta A, Cigna E, Schettino M. Mind the Gap: A Questionnaire on the Distance between Diagnostic Advances and Clinical Practice in Skin Cancer Treatment. Medicina. 2024; 60(1):155. https://doi.org/10.3390/medicina60010155
Chicago/Turabian StyleDiluiso, Giuseppe, Mirco Pozzi, Flavio Giulio Liso, Vanessa Marron Mendes, Jenna Hannouille, Luigi Losco, Alberto Bolletta, Emanuele Cigna, and Michela Schettino. 2024. "Mind the Gap: A Questionnaire on the Distance between Diagnostic Advances and Clinical Practice in Skin Cancer Treatment" Medicina 60, no. 1: 155. https://doi.org/10.3390/medicina60010155
APA StyleDiluiso, G., Pozzi, M., Liso, F. G., Mendes, V. M., Hannouille, J., Losco, L., Bolletta, A., Cigna, E., & Schettino, M. (2024). Mind the Gap: A Questionnaire on the Distance between Diagnostic Advances and Clinical Practice in Skin Cancer Treatment. Medicina, 60(1), 155. https://doi.org/10.3390/medicina60010155







