Gingival Manifestations in Oral Chronic Autoimmune Bullous Diseases: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Inclusion/Exclusion Criteria
2.2. Statistical Analysis
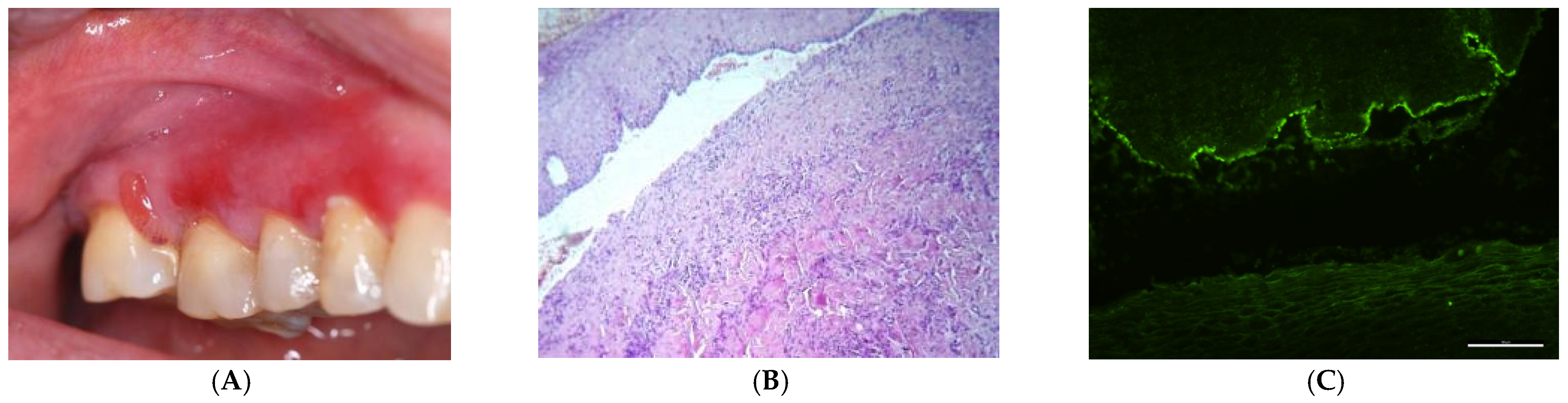
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sciuca, A.M.; Toader, M.P.; Stelea, C.G.; Maftei, G.A.; Ciurcanu, O.E.; Stefanescu, O.M.; Onofrei, B.-A.; Popa, C. Desquamative Gingivitis in the Context of Autoimmune Bullous Dermatoses and Lichen Planus—Challenges in the Diagnosis and Treatment. Diagnostics 2022, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Rees, T.D. Diagnosis and management of desquamative gingivitis. In Gingival Diseases—Their Aetiology, Prevention and Treatment. Fotinos Panagakos, Robin Davies Rijeka; InTech: Rijeka, Croatia, 2011; pp. 171–188. [Google Scholar]
- Karagoz, G.; Bektas-Kayhan, K.; Unur, M. Desquamative Gingivitis: A Review. J. Istanb. Univ. Fac. Dent. 2016, 50, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, M.D.; Fortuna, G.; Leuci, S.; Ruoppo, E.; Marasca, F.; Matarasso, S. Nikolsky’s Sign on the Gingival Mucosa: A Clinical Tool for Oral Health Practitioners. J. Periodontol. 2008, 79, 2241–2246. [Google Scholar] [CrossRef]
- Nisengard, R.J. Diagnosis and Management of Desquamative Gingivitis. Periodontal Insights 1995, 2, 4. [Google Scholar]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions—Introduction and Key Changes from the 1999 Classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Holmstrup, P.; Plemons, J.; Meyle, J. Non-Plaque-Induced Gingival Diseases. J. Periodontol. 2018, 89, S28–S45. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Fortuna, G.; Aria, M.; Mignogna, M.D. Autoimmune Mucocutaneous Blistering Diseases in the South of Italy: A 25-Year Retrospective Study on 169 Patients. J. Oral Pathol. Med. 2020, 49, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Leuci, S.; Ruoppo, E.; Adamo, D.; Calabria, E.; Mignogna, M.D. Oral Autoimmune Vesicobullous Diseases: Classification, Clinical Presentations, Molecular Mechanisms, Diagnostic Algorithms, and Management. Periodontology 2019, 80, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Lamberts, A.; Diercks, G.F.H.; Pas, H.H.; Meijer, J.M.; Bolling, M.C.; Horváth, B. Oral Lesions in Autoimmune Bullous Diseases: An Overview of Clinical Characteristics and Diagnostic Algorithm. Am. J. Clin. Dermatol. 2019, 20, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Amber, K.T.; Murrell, D.F.; Schmidt, E.; Joly, P.; Borradori, L. Autoimmune Subepidermal Bullous Diseases of the Skin and Mucosae: Clinical Features, Diagnosis, and Management. Clin. Rev. Allergy Immunol. 2018, 54, 26–51. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Pellegrini, M.; Li Vigni, G.; Pulicari, F.; Spadari, F. Desquamative Gingivitis, Oral Hygiene, and Autoimmune Oral Diseases: A Scoping Review. Appl. Sci. 2023, 13, 10535. [Google Scholar] [CrossRef]
- Pollmann, R.; Schmidt, T.; Eming, R.; Hertl, M. Pemphigus: A Comprehensive Review on Pathogenesis, Clinical Presentation and Novel Therapeutic Approaches. Clin. Rev. Allergy Immunol. 2018, 54, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Maglie, R.; Eming, R.; Hertl, M. Pemphigus: Current and Future Therapeutic Strategies. Front. Immunol. 2019, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Alramadhan, S.A.; Islam, M.N. Vesiculobullous Lesions of the Oral Cavity. Oral Maxillofac. Surg. Clin. 2023, 35, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Fania, L.; Didona, B.; Eming, R.; Hertl, M.; Di Zenzo, G. Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. Int. J. Mol. Sci. 2020, 21, 2178. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Lamberts, A.; Borradori, L.; Alberti-Violetti, S.; Barry, R.J.; Caproni, M.; Carey, B.; Carrozzo, M.; Caux, F.; Cianchini, G.; et al. European Guidelines (S3) on Diagnosis and Management of Mucous Membrane Pemphigoid, Initiated by the European Academy of Dermatology and Venereology—Part I. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1750–1764. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.; Sakka, N.; Artsi, O.; Trau, H.; Barzilai, A. Diagnosis and Classification of Autoimmune Blistering Diseases. Autoimmun. Rev. 2014, 13, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, M.D.; Lo Muzio, L.; Bucci, E. Clinical Features of Gingival Pemphigus Vulgaris. J. Clin. Periodontol. 2001, 28, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.R.; Pindborg, J.J.; Bezroukov, V.; Infirri, J.S. Guide to Epidemiology and Diagnosis of Oral Mucosal Diseases and Conditions. Community Dent. Oral Epidemiol. 1980, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Paes De Almeida, O.; Porter, S.R.; Gilkes JJ, H. Pemphigus Vulgaris: The Manifestations and Long-Term Management of 55 Patients with Oral Lesions. Br. J. Dermatol. 1999, 140, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Villa, A.; Saavedra, A.; Treister, N.; Woo, S.-B. Oral Mucous Membrane Pemphigoid and Pemphigus Vulgaris—A Retrospective Two-Center Cohort Study. Oral Dis. 2017, 23, 498–504. [Google Scholar] [CrossRef]
- Alshami, M.L.; Aswad, F.; Abdullah, B. A Clinical and Demographic Analysis of Oral Pemphigus Vulgaris: A Retrospective Cross-Sectional Study from 2001 to 2021. Health Sci. Rep. 2022, 5, e832. [Google Scholar] [CrossRef]
- Feliciani, C.; Joly, P.; Jonkman, M.F.; Zambruno, G.; Zillikens, D.; Ioannides, D.; Kowalewski, C.; Jedlickova, H.; Kárpáti, S.; Marinovic, B.; et al. Management of Bullous Pemphigoid: The European Dermatology Forum Consensus in Collaboration with the European Academy of Dermatology and Venereology. Br. J. Dermatol. 2015, 172, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Schmidt, E.; Zillikens, D.; Ludwig, R.J.; Kridin, K. Immunological Features and Factors Associated with Mucocutaneous Bullous Pemphigoid—A Retrospective Cohort Study. J. Dtsch. Dermatol. Ges. 2021, 19, 1289–1295. [Google Scholar] [CrossRef]
- Esmaili, N.; Hallaji, Z.; Soori, T.; Davatchi, C.C. Bullous Pemphigoid in Iranian Patients: A Descriptive Study on 122 Cases. Dir. Open Access J. 2012, 50, 335–338. [Google Scholar]
- Budimir, J.; Lugović Mihić, L.; Šitum, M.; Bulat, V.; Peršić, S.; Tomljanović-Veselski, M. Oral Lesions in Patients with Pemphigus Vulgaris and Bullous Pemphigoid. PubMed 2008, 47, 13–18. [Google Scholar]
- Daltaban, Ö.; Özçentik, A.; Akman Karakaş, A.; Üstün, K.; Hatipoğlu, M.; Uzun, S. Clinical Presentation and Diagnostic Delay in Pemphigus Vulgaris: A Prospective Study from Turkey. J. Oral Pathol. Med. 2020, 49, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Laskaris, G.; Sklavounou, A.; Stratigos, J. Bullous Pemphigoid, Cicatricial Pemphigoid, and Pemphigus Vulgaris. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 656–662. [Google Scholar] [CrossRef]
- Buajeeb, W.; Pimolbutr, K.; Panpradit, N.; Okuma, N. Oral Mucous Membrane Pemphigoid in a Group of Thai Patients—A 15-Year Retrospective Study. J. Dent. Sci. 2022, 17, 1009–1017. [Google Scholar] [CrossRef]
- Petruzzi, M.; della Vella, F.; Squicciarini, N.; Lilli, D.; Campus, G.; Piazzolla, G.; Lucchese, A.; van der Waal, I. Diagnostic Delay in Autoimmune Oral Diseases. Oral Dis. 2023, 29, 2614–2623. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Taimeh, D.; Al Khawaldeh, H.; Sawair, F. Diagnostic Patterns and Delays in Autoimmune Blistering Diseases of the Mouth: A Cross-Sectional Study. Oral Dis. 2018, 24, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Rees, T.D.; Niwa, H.; Kuyama, K.; Iijima, M.; Imamura, R.; Kato, T.; Doi, K.; Yamamoto, H.; Ito, T. Desquamative Gingivitis. Insights Var. Asp. Oral Health 2017, 2–27. [Google Scholar] [CrossRef]
- Leao, J.; Ingafou, M.; Khan, A.; Scully, C.; Porter, S. Desquamative Gingivitis: Retrospective Analysis of Disease Associations of a Large Cohort. Oral Dis. 2008, 14, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Broccoletti, R.; Sciannameo, V.; Scully, C. A Practical Clinical Recording System for Cases of Desquamative Gingivitis. Br. J. Dermatol. 2017, 177, 299–301. [Google Scholar] [CrossRef]
- Sklavounou, A.; Laskaris, G. Frequency of Desquamative Gingivitis in Skin Diseases. Oral Surg. Oral Med. Oral Pathol. 1983, 56, 141–144. [Google Scholar] [CrossRef]
- Jascholt, I.; Lai, O.; Zillikens, D.; Kasperkiewicz, M. Periodontitis in Oral Pemphigus and Pemphigoid: A Systematic Review of Published Studies. J. Am. Acad. Dermatol. 2017, 76, 975–978.e3. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Subepithelial Autoimmune Diseases Group (%) | Intraepithelial Autoimmune Diseases Group (%) | p Value | |
|---|---|---|---|
| Total | 34 (38.63%) | 54 (61.36%) | |
| Gender | |||
| Female | 28 (82.35%) | 41 (74.54%) | p = 0.475 * |
| Male | 6 (17.65%) | 13 (23.64%) | |
| Age (mean) | 66 years | 54.88 years | |
| SD ± 10.21 | SD ± 15.3 | ||
| Range | 27–83 years | 23–86 years | |
| Level of education | |||
| Primary | 14 (41.17%) | 16 (29.62%) | p = 0.193 * |
| Secondary | 7 (20.58%) | 21 (38.89%) | |
| Tertiary | 13 (38.23%) | 17 (31.48%) | |
| Smoking status | |||
| Non-smoker | 26 (76.47%) | 39 (72.22%) | p = 0.659 * |
| Smoker and former smoker | 8 (23.52%) | 15 (27.77%) | |
| Environment | |||
| Urban | 31 (91.17%) | 43 (79.62%) | p = 0.149 * |
| Rural | 3 (8.82%) | 11 (20.37%) | |
| Most frequent associated medical and surgical diseases | |||
| Appendicectomy | 5 (14.70%) | 14 (25.92%) | |
| Cholecystectomy | 6 (17.64%) | 5 (9.25%) | |
| Diabetes mellitus | 3 (8.82%) | 4 (7.40%) | |
| Hypertension | 6 (17.65%) | 11 (20.37%) | |
| Ischemic heart disease | 8 (23.52%) | 7 (12.96%) | |
| Another autoimmune disease | 10 (29.41%) | 4 (7.40%) | |
| Duration of symptoms (months) mean ± SD | 20.32 ± 41.04 | 6.07 ± 8.37 | p = 0.015 ** |
| Direct immunofluorescence findings | |||
| IgG | 29 (85.29%) | 35 (64.81%) | |
| C3 | 17 (50%) | 28 (51.85%) | |
| IgA | 9 (26.47%) | 11 (20.37%) | |
| Fibrinogen | 9 (26.47%) | 8 (14.81%) | |
| IgM | 1 (2.94%) | 8 (14.81%) |
| Subepithelial Autoimmune Diseases Group No. (%) | Intraepithelial Autoimmune Diseases Group No. (%) | p Value | |
|---|---|---|---|
| Gingiva | 27 (79.41%) | 15 (27.77%) | p < 0.00001 * |
| Tongue-dorsal surface | 4 (11.76%) | 5 (9.25%) | p = 0.729 ** |
| Tongue-ventral surface | 1 (2.94%) | 11 (20.37%) | p = 0.252 * |
| Tongue-margins | 0 (%) | 4 (7.40%) | p = 0.155 ** |
| Floor of the mouth | 3 (8.82%) | 5 (9.25%) | p = 0.999 ** |
| Lips | 1 (2.94%) | 6 (11.11%) | p = 0.241 ** |
| Labial mucosa | 5 (14.70%) | 9 (16.67%) | p = 0.999 ** |
| Hard palate | 6 (17.65%) | 2 (3.70%) | p = 0.048 ** |
| Soft palate | 11 (32.35%) | 25 (46.29%) | p = 0.032 * |
| Buccal mucosa | 14 (41.17%) | 45 (83.33%) | p < 0.0001 * |
| Retromolar region | 1 (2.94%) | 9 (16.67%) | p = 0.081 ** |
| Tonsillar pillar | 3 (8.82%) | 15 (27.77%) | p = 0.055 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parlatescu, I.; Tovaru, S.; Tofan, C.; Perlea, P.; Milanesi, E.; Dobre, M.; Mihai, L.L. Gingival Manifestations in Oral Chronic Autoimmune Bullous Diseases: A Retrospective Study. Medicina 2024, 60, 167. https://doi.org/10.3390/medicina60010167
Parlatescu I, Tovaru S, Tofan C, Perlea P, Milanesi E, Dobre M, Mihai LL. Gingival Manifestations in Oral Chronic Autoimmune Bullous Diseases: A Retrospective Study. Medicina. 2024; 60(1):167. https://doi.org/10.3390/medicina60010167
Chicago/Turabian StyleParlatescu, Ioanina, Serban Tovaru, Cristina Tofan, Paula Perlea, Elena Milanesi, Maria Dobre, and Laurenta Lelia Mihai. 2024. "Gingival Manifestations in Oral Chronic Autoimmune Bullous Diseases: A Retrospective Study" Medicina 60, no. 1: 167. https://doi.org/10.3390/medicina60010167
APA StyleParlatescu, I., Tovaru, S., Tofan, C., Perlea, P., Milanesi, E., Dobre, M., & Mihai, L. L. (2024). Gingival Manifestations in Oral Chronic Autoimmune Bullous Diseases: A Retrospective Study. Medicina, 60(1), 167. https://doi.org/10.3390/medicina60010167







