New Spinal Shortening Technique for Tethered Cord Syndrome: A Technical Note
Abstract
:1. Introduction
2. Case 1: 31-Year-Old Male, Tethered Cord Syndrome
2.1. Patient History
2.2. Physical Examination
2.3. Preoperative Imaging
2.4. Surgery
2.4.1. Anterior Discectomy
2.4.2. Posterior Osteotomy
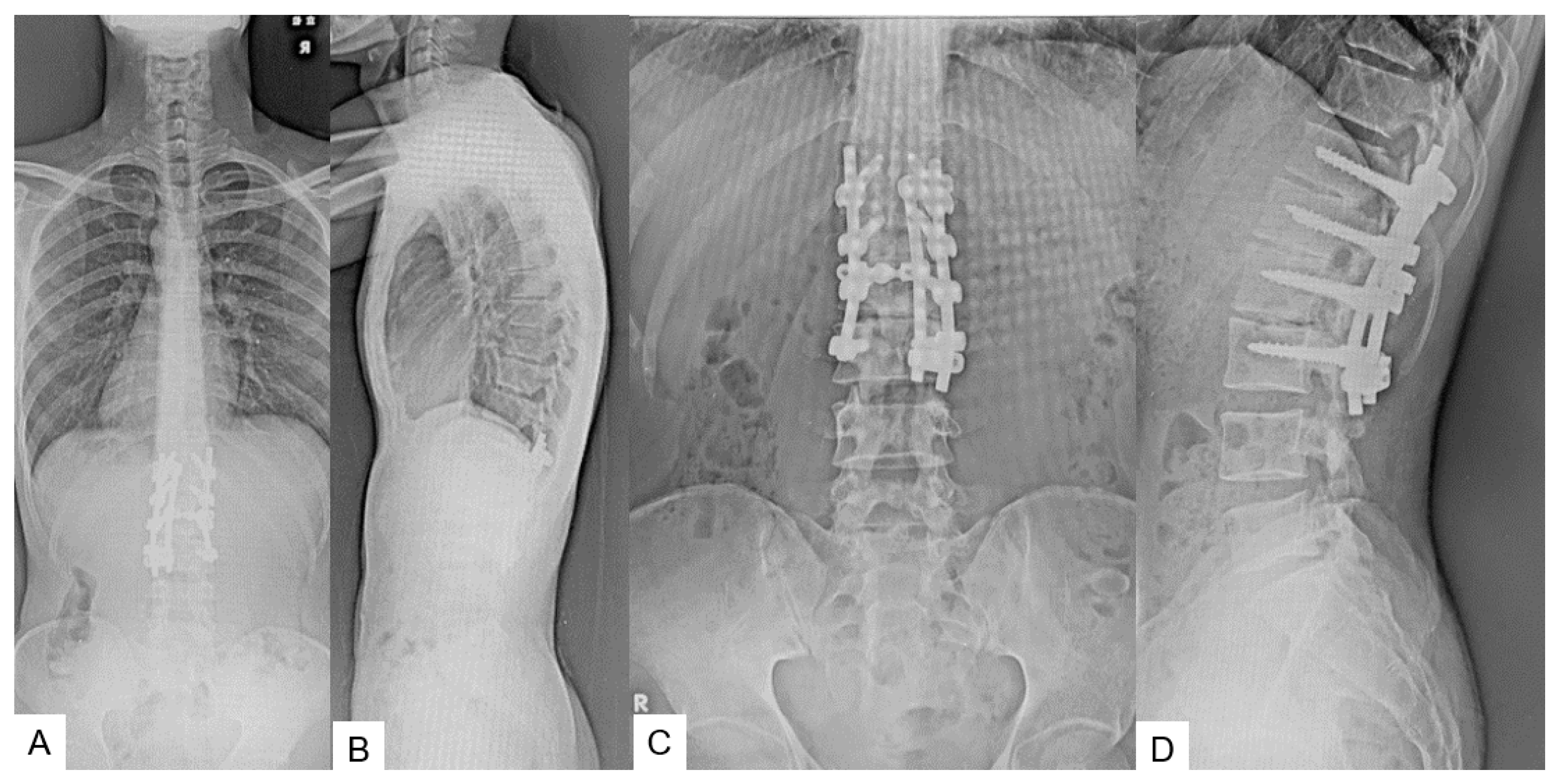
2.5. Postoperative Images
2.6. One Year Follow-Up
3. Case 2: 33-Year-Old Male, Tethered Cord Syndrome, Conventional Technique
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, P.H. Congenital intraspinal lipomas: Anatomic considerations and surgical treatment. Child’s Brain 1982, 9, 37–47. [Google Scholar] [PubMed]
- Yamada, S.; Knerium, D.S.; Mandybur, G.M.; Schultz, R.L.; Yamada, B.S. Pathophysiology of tethered cord syndrome and other complex factors. Neurol. Res. 2004, 26, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Wilberger, J.E., Jr. Tethered cord syndrome in adults. J. Neurosurg. 1982, 57, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.J.; Tubbs, R.S.; Oakes, W.J. Tethered cord syndrome in children: A review. Neurosurg. Focus 2007, 23, E2. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Stapleton, C.J.; Moldavskiy, P.; Koski, T.R.; Ondra, S.L.; Gokaslan, Z.L.; Kuntz, C. Posterior vertebral column subtraction osteotomy for the treatment of tethered cord syndrome: Review of the literature and clinical outcomes of all cases reported to date. Neurosurg. Focus 2010, 29, E6. [Google Scholar] [CrossRef]
- Archibeck, M.J.; Smith, J.T.; Carroll, K.L.; Davitt, J.S.; Stevens, P.M. Surgical release of tethered spinal cord: Survivorship analysis and orthopedic outcome. J. Pediatr. Orthop. 1997, 17, 773–776. [Google Scholar] [CrossRef]
- Filler, A.G.; Britton, J.A.; Uttley, D.; Marsh, H.T. Adult postrepair myelomeningocoele and tethered cord syndrome: Good surgical outcome after abrupt neurological decline. Br. J. Neurosurg. 1995, 9, 659–666. [Google Scholar]
- Samuels, R.; McGirt, M.J.; Attenello, F.J.; Garcés Ambrossi, G.L.; Singh, N.; Solakoglu, C.; Weingart, J.D.; Carson, B.S.; Jallo, G.I. Incidence of symptomatic retethering after surgical management of pediatric tethered cord syndrome with or without duraplasty. Child’s Nerv. Syst. 2009, 25, 1085–1089. [Google Scholar] [CrossRef]
- Herman, J.M.; McLone, D.G.; Storrs, B.B.; Dauser, R.C. Analysis of 153 patients with myelomeningocele or spinal lipoma reoperated upon for a tethered cord. Presentation, management and outcome. Pediatr. Neurosurg. 1993, 19, 243–249. [Google Scholar] [CrossRef]
- Maher, C.O.; Goumnerova, L.; Madsen, J.R.; Proctor, M.; Scott, R.M. Outcome following multiple repeated spinal cord untethering operations. J. Neurosurg. 2007, 106 (Suppl. S6), 434–438. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Abe, E.; Suzuki, T.; Kido, T.; Chiba, M.; Shimada, Y. Spine-shortening vertebral osteotomy for tethered cord syndrome: Report of three cases. Spine 2009, 34, E823–E825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chang, C.-C.; Mummaneni, P.V.; Yuan, C.; Dhall, S.; Jian, F.; Gupta, N.; Chou, D. Spinal column shortening versus revision detethering for recurrent adult tethered cord syndrome: A preliminary comparison of perioperative and clinical outcomes. J. Neurosurg. Spine 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, J.D.; Lenke, L.G.; Bridwell, K.H.; Sehn, J.K.; Milby, A.H.; Bumpass, D.; Crawford, C.H., 3rd; O’Shaughnessy, B.A.; Buchowski, J.M.; Chang, M.S.; et al. Major complications and comparison between 3-column osteotomy techniques in 105 consecutive spinal deformity procedures. Spine 2012, 37, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Garceau, G.J. The filum terminale syndrome. J. Bone Jt. Surg. 1953, 35, 711–716. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Hendrick, B.; Humphreys, R.P. The tethered spinal cord its protean manifestations, diagnosis and surgical correction. Child’s Brain 1976, 2, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Zinke, D.E.; Sanders, D. Pathophysiology of “tethered cord syndrome”. J. Neurosurg. 1981, 54, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, N.; Subramaniam, B.; Gnanaseelan, T.; Rathinam, R.; Thiruthavadoss, A.; Shivapathasundram, G.; Stoodley, M.A.; Cornips, E.M.; Vereijken, I.M.; Beuls, E.A.; et al. Tethered cord syndrome in children with anorectal. J. Neurosurg. 2000, 92, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Warder, D.E.; Oakes, W.J. Tethered cord syndrome and the conus in a normal position. Neurosurgery 1993, 33, 374–378. [Google Scholar]
- O’Connor, K.P.; Smitherman, A.D.; Milton, C.K.; Palejwala, A.H.; Lu, V.M.; Johnston, S.E.; Homburg, H.; Zhao, D.; Martin, M.D. Surgical Treatment of Tethered Cord Syndrome in Adults: A Systematic Review and Meta-Analysis. World Neurosurg. 2020, 137, 221–241. [Google Scholar] [CrossRef]
- Morimoto, K.; Takemoto, O.; Wakayama, A. Spinal lipomas in children—Surgical management and long-term follow-up. Pediatr. Neurosurg. 2005, 41, 84–87. [Google Scholar] [CrossRef]
- Kokubun, S.; Ozawa, H.; Aizawa, T.; Ly, N.M.; Tanaka, Y. Spine-shortening osteotomy for patients with tethered cord syndrome caused by lipomyelomeningocele. J. Neurosurg. Spine 2011, 15, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ruparel, S.; Oda, Y.; Fujiwara, Y.; Shama, S.; Uotani, K.; Arataki, S.; Yamauchi, T.; Sake, N. C-arm-Free Simultaneous OLIF51 and Percutaneous Pedicle Screw Fixation in a Single Lateral Position. J. Vis. Exp. 2022, 16, 187–192. [Google Scholar]
- Li, B.; Guo, R.; Jiang, X.; Wu, J.; Zhang, D.; Yang, C.; Zhao, Q.; Zhang, C.; Yan, H.; Wang, Z.; et al. Posterior wedge osteotomy assisted by O-arm navigation for treating ankylosing spondylitis with thoracolumbar fractures: An early clinical evaluation. Ann. Palliat. Med. 2021, 10, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.M.; Nguyen, S.; Streitmatter, S.W.; Sherrod, B.A.; Hardy, J.; Cole, K.L.; Gamblin, A.S.; Bisson, E.F.; Mazur, M.D.; Dailey, A.T. O-Arm Accuracy and Radiation Exposure in Adult Deformity Surgery. World Neurosurg. 2023, 171, 440–446. [Google Scholar] [CrossRef]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Sonawane, S.; Arataki, S.; Fujiwara, Y.; Taoka, T.; Uotani, K.; Oda, Y.; Shinohara, K. New Spinal Shortening Technique for Tethered Cord Syndrome: A Technical Note. Medicina 2024, 60, 20. https://doi.org/10.3390/medicina60010020
Tanaka M, Sonawane S, Arataki S, Fujiwara Y, Taoka T, Uotani K, Oda Y, Shinohara K. New Spinal Shortening Technique for Tethered Cord Syndrome: A Technical Note. Medicina. 2024; 60(1):20. https://doi.org/10.3390/medicina60010020
Chicago/Turabian StyleTanaka, Masato, Sumeet Sonawane, Shinya Arataki, Yoshihiro Fujiwara, Takuya Taoka, Koji Uotani, Yoshiaki Oda, and Kensuke Shinohara. 2024. "New Spinal Shortening Technique for Tethered Cord Syndrome: A Technical Note" Medicina 60, no. 1: 20. https://doi.org/10.3390/medicina60010020
APA StyleTanaka, M., Sonawane, S., Arataki, S., Fujiwara, Y., Taoka, T., Uotani, K., Oda, Y., & Shinohara, K. (2024). New Spinal Shortening Technique for Tethered Cord Syndrome: A Technical Note. Medicina, 60(1), 20. https://doi.org/10.3390/medicina60010020







